| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 14, Number 5, October 2024, pages 226-239
A Meta-Analysis on the Prevalence and Risk of Gestational Diabetes Mellitus in the Context of COVID-19
aResearch and Development, Vedanadhi, Salem-636115, Tamil Nadu, India
bCoimbatore Medical College and Hospital, Tamil Nadu, India
cEmail:
Manuscript submitted July 25, 2024, accepted September 20, 2024, published online October 26, 2024
Short title: GDM in the Context of COVID-19
doi: https://doi.org/10.14740/jem1020
| Abstract | ▴Top |
Background: The objectives of this study are: 1) to compare the prevalence of gestational diabetes mellitus (GDM) among pandemic and pre-pandemic cohorts, 2) to evaluate the risk of GDM among pregnant women who tested positive and negative for coronavirus disease 2019 (COVID-19), and 3) to evaluate the risk of COVID-19 among pregnant women diagnosed with and without GDM.
Methods: A literature search was carried out in PubMed and Cochrane library databases with relevant keywords from its inception till March 2024. Observational studies that 1) evaluated the prevalence of GDM during pandemic and pre-pandemic period, and 2) investigated the GDM and COVID-19 status among pregnant women were included.
Results: The analysis revealed that the prevalence of GDM was significantly increased by 17% (odds ratio (OR), 1.17; 95% confidence interval (CI), 1.12 to 1.23; P < 0.00001) during the pandemic period compared to pre-pandemic period and the odds of pregnant women with GDM tested positive for COVID-19 were 1.28-fold greater (OR, 1.28; 95% CI, 1.13 to 1.44; P < 0.0001) than the odds of pregnant women with GDM tested negative for COVID-19. However, the analysis also revealed that pregnant women with COVID-19 were less likely (OR, 0.02; 95% CI, 0.01 to 0.02; P < 0.00001) to be diagnosed with GDM when compared to pregnant women with COVID-19 and without GDM.
Conclusion: The present study suggests that GDM acts as a risk factor for COVID-19 infection among pregnant women. This might be due to the hypothesis that altered sense of taste is associated among pregnant women with GDM and COVID-19 due to the taste receptor polymorphisms which regulates the innate immunity downstream signaling. However, molecular studies were needed to validate this hypothesis and evaluate the therapeutic role of taste receptors in the management of COVID-19 and GDM.
Keywords: Gestational diabetes mellitus; SARS-CoV-2; Coronavirus disease 2019; Meta-analysis; Long COVID
| Introduction | ▴Top |
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), declared as a global pandemic by World Health Organization (WHO) on March 11, 2020, caused adverse effects and disrupted global economy, health-care systems, leading to long COVID symptoms such as taste dysfunction and dysregulation of immune system and immunological memory [1-3]. Several studies have observed the indirect impact of the pandemic on maternal health-care services such as reduced hospital visits for routine and unscheduled pregnancy care, limited physical activity due to the pandemic lockdowns and adverse pregnancy outcomes [1-4]. Evidence from the previous observational studies reported that there was an increased prevalence of gestational diabetes mellitus (GDM) among pregnant women during the pandemic period along with the rising risk factors of obesity/overweight, reduced outdoor physical activities, dietary, behavioral (mental health problems), lifestyle changes and taste dysfunction during the COVID-19 pandemic period [4, 5]. Interestingly, studies also demonstrated that there is a significant prevalence of taste dysfunction among individuals with confirmed COVID-19 [6, 7] and the type 2 taste receptor R family member 38 (TAS2R38) proline-alanine-valine (PAV) taster allele was significantly associated with COVID-19 [8]. Previous studies hypothesized that single nucleotide polymorphisms (SNPs) in extra-oral type 2 taste receptors (TAS2Rs) found in respiratory tract regulate innate immunity through facilitating the release of anti-viral nitric oxide (NO) which causes intra-cellular damage of microbes, removal of pathogens by increasing ciliary beat frequency and muco-ciliary clearance [9-11]. Further, NO release from the ciliated epithelial cells prevents viral RNA replication by inhibiting the binding of spike proteins of coronavirus with angiotensin converting enzyme 2 [9-11]. In addition, studies showed that adverse pregnancy outcomes such as gestational diabetes mellitus (GDM) might be influenced by maternal immune dysregulation and GDM also influences neonatal innate immunity [5, 12]. Although chloroquine (or hydroxychloroquine), an agonist of TAS2Rs (bitter taste receptors) was used for a brief period in the management of COVID-19, it was discontinued due to its potential adverse events [10, 11, 13]. Moreover, our previous analysis has suggested that SNPs in taste receptor genes such as TAS2R subtypes (type 2 taste receptor R family member 9 (TAS2R9)), transient receptor potential cation channel subfamily M member 5 (TRPM5) were significantly associated with increased risk of GDM susceptibility and pregnant women with GDM had an impaired sweet, salt and bitter taste stimuli perception due to SNPs in taste receptor genes [5]. Further, expression of TRPM5 and TAS2R9 in pancreatic beta cells potentially regulates glucose and insulin homeostasis [5]. However, the meta-analyses published in 2021 were conducted with studies published until January 2021 and reported inconsistent results regarding the association between the COVID-19 pandemic and GDM [1-3]. Hence, the objectives of this study are 1) to compare the prevalence of GDM among pandemic and pre-pandemic cohorts, 2) to evaluate the risk of GDM among the pregnant women who tested positive and negative for COVID-19, and 3) to evaluate the risk of COVID-19 among pregnant women diagnosed with and without GDM, with additional studies published until March 2024.
| Material and Methods | ▴Top |
This meta-analysis was registered in International Prospective Register of Systematic Reviews (PROSPERO) (Reg. No. CRD42024521805) and prepared following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. Institutional review board/ethical clearance is not applicable as this is a meta-analysis conducted from the previously published observational studies.
Search strategy
A literature search was carried out in PubMed and Cochrane library databases with keywords such as “(severe acute respiratory syndrome coronavirus 2) OR (SARS-CoV-2) OR (coronavirus disease 2019) OR (COVID-19) AND (gestational diabetes mellitus)” from its inception till March 2024. Further citation searches were carried out to identify relevant studies. Observational studies that 1) evaluated the prevalence of GDM during pandemic and pre-pandemic period and 2) investigated the GDM and COVID-19 status among pregnant women were included. Articles that are not relevant to our study, not in English language, case reports, not have sufficient data and without control groups, review articles, editorials, erratums, book chapters, guidelines and research letters were excluded. The screening of title and abstract was carried out to identify the relevant articles. Further, the full texts of the identified studies were reviewed for inclusion based on the inclusion criteria and further the patient/population, intervention, comparison, outcomes (PICO) inclusion criteria were listed in the Supplementary Material 1 (www.jofem.org). The definitions of pandemic, pre-pandemic cohorts, COVID-19 and GDM diagnostic criteria were based on the respective included studies.
Data extraction and quality assessment
Data extraction was carried out in excel spreadsheet. Data regarding the study design, population, number of pregnant women diagnosed with GDM during the pandemic and pre-pandemic period, number of pregnant women with confirmed COVID-19 infection diagnosed with and without GDM, number of pregnant women with GDM tested positive and negative for COVID-19 were extracted from the respective studies wherever applicable. Quality assessment of the included studies was carried out using the Newcastle-Ottawa scale (NOS) for case-control and cohort studies. NOS comprises three domains: selection domain, comparability domain and outcome/exposure domain. The studies were categorized into good quality (defined as three or four stars in the selection domain, one or two stars in the comparability domain, two or three stars in the outcome and three or four stars in the exposure domain), fair quality (defined as two stars in the selection domain, one or two stars in the comparability domain, two or three stars in the outcome domain and two stars in the exposure domain), and poor quality (defined as zero or one star in the selection domain, zero stars in the comparability domain and zero or one star in the outcome/exposure domain) according to Agency for Health Research and Quality (AHRQ) standards.
Statistical analysis
Statistical analysis was done in RevMan software (Version 5.4; Cochrane collaboration). Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were estimated from random effects meta-analysis models and P-value less than 0.05 was considered statistically significant. Heterogeneity was assessed by the I2 statistic and Chi-squared test, where a P value of less than 0.1 was considered statistically significant. Publication bias was assessed by visual inspection of funnel plot and leave-one out sensitivity analysis.
| Results | ▴Top |
Of the 21,178 records identified through database searches, 63 studies were included in the meta-analysis. Figure 1 depicts the process of selection of articles. Across the included studies representing more than 18 countries, a total of 164,578 pregnant women diagnosed with GDM among 2,452,025 pregnant women during the COVID-19 pandemic and 212,952 pregnant women diagnosed with GDM among 3,145,914 pregnant women during the pre-pandemic period were reported. Further, among 21,254 pregnant women with confirmed COVID-19 infection, 2,591 pregnant women diagnosed with GDM were compared with 18,663 pregnant women without GDM. In addition, the study also included 1,142 pregnant women tested positive for COVID-19 among 8,886 pregnant women with GDM and 106,405 pregnant women tested negative for COVID-19 among the 880,342 pregnant women with GDM. The characteristics of the included studies are shown in Table 1 [4, 14-75] and Supplementary Table 1 (www.jofem.org).
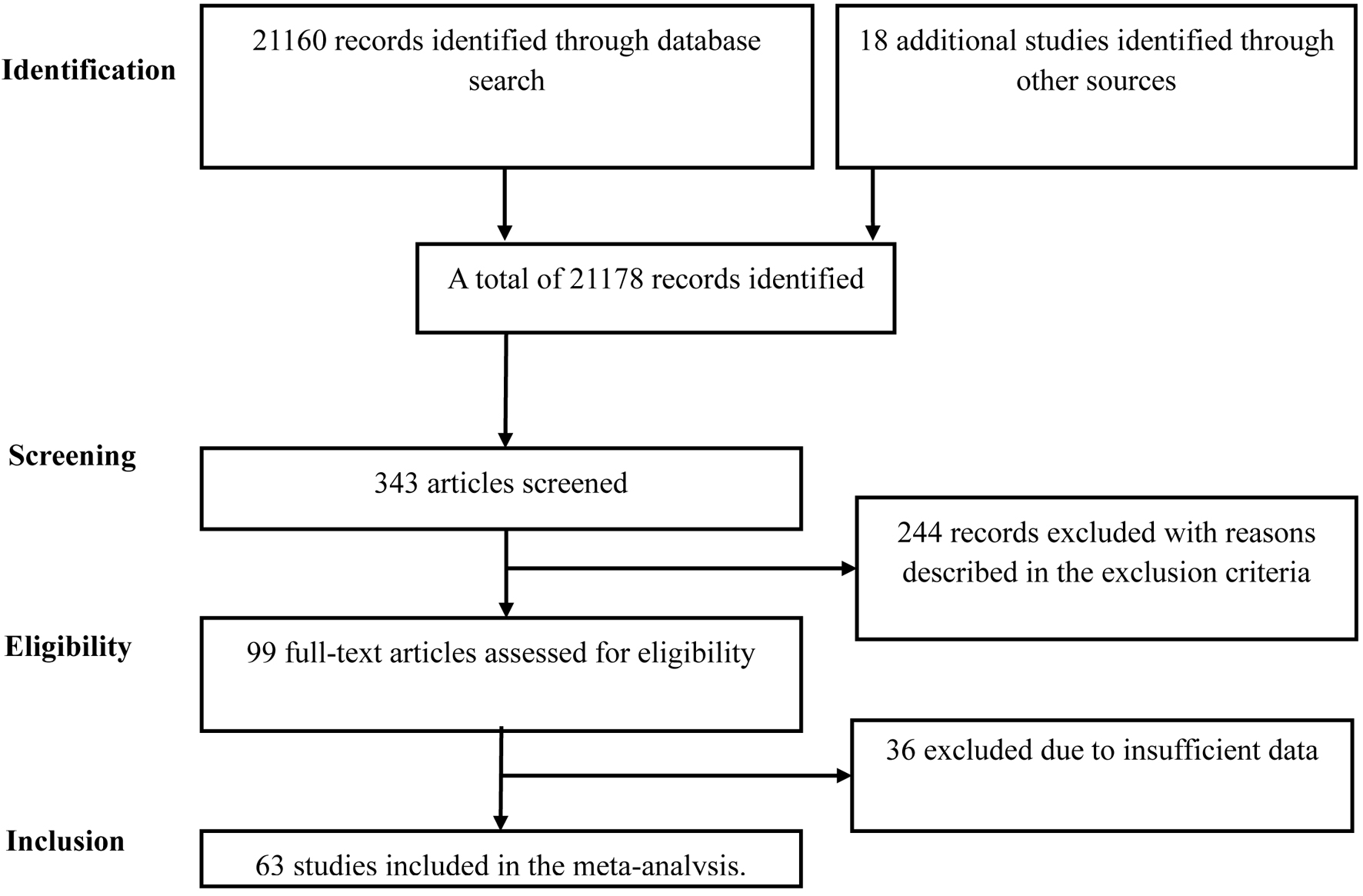 Click for large image | Figure 1. Flowchart summarizing the selection process. |
 Click to view | Table 1. Characteristics of Studies Included in the Meta-Analysis |
Comparison of GDM prevalence during pandemic and pre-pandemic period
Twenty-six studies comprising 5,597,939 pregnant women categorized into pandemic cohort and pre-pandemic cohort were included in our meta-analysis. The random effects meta-analysis model revealed that the prevalence of GDM was significantly increased by 17% during the COVID-19 pandemic period when compared to the pre-pandemic period (OR, 1.17; 95% CI, 1.12 to 1.23; P < 0.00001) with heterogeneity of I2 = 96% (P < 0.00001) as shown in Figure 2. Methodological quality assessment of the included studies by NOS suggested an overall good quality for the 26 studies and visual inspection of the funnel plot shows low publication bias (Supplementary Figure 1, www.jofem.org). The fixed effects meta-analysis model (OR, 1.15; 95% CI, 1.14 to 1.16; P < 0.00001) and the leave-one out sensitivity analysis (ORs were in the range of 1.16 to 1.19) showed no significant difference from the overall effect measure estimated from the random effects meta-analysis model (Supplementary Table 2, www.jofem.org).
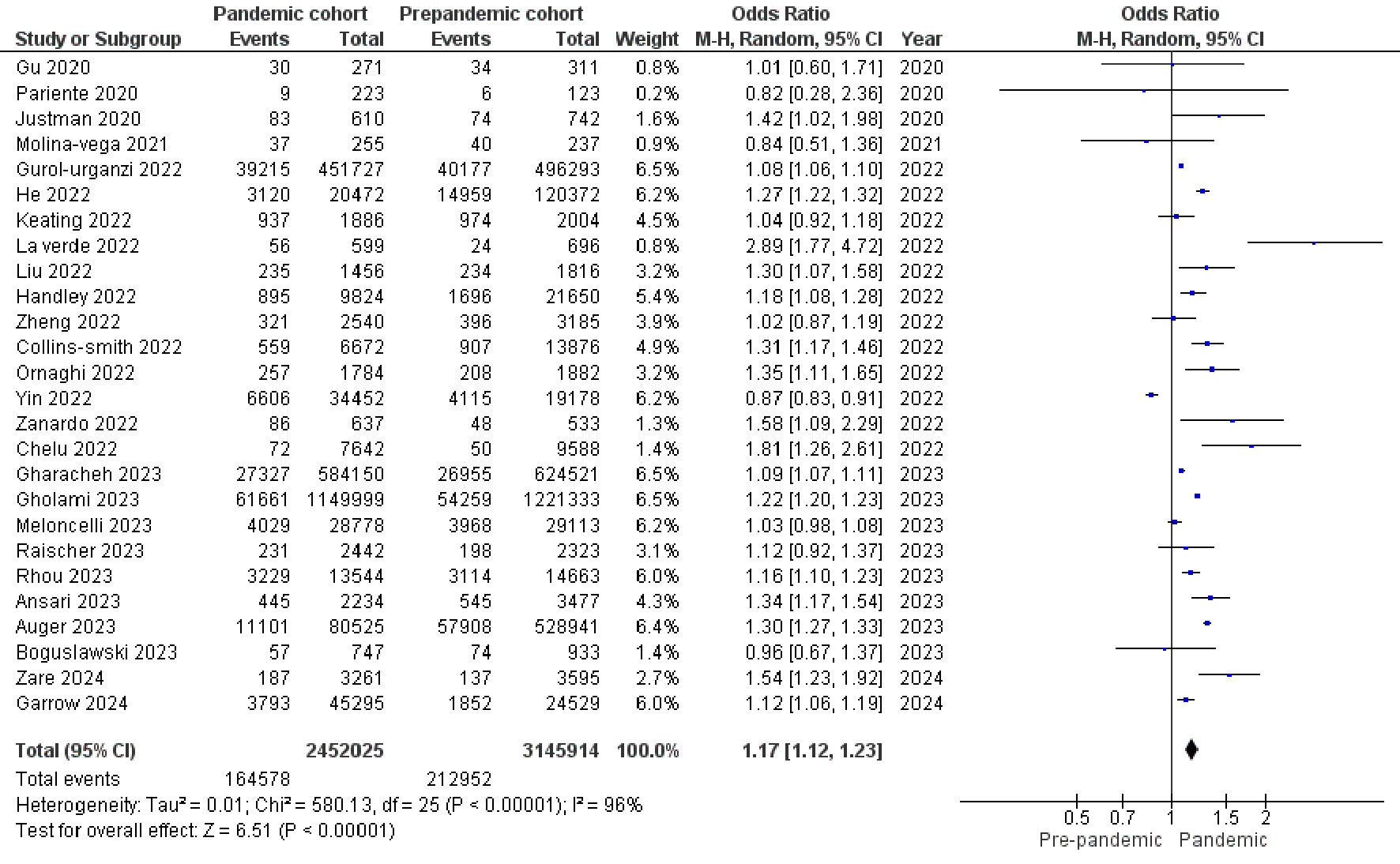 Click for large image | Figure 2. Forest plot shows the random effects meta-analysis model comparing the prevalence of GDM among pregnant women during pandemic and pre-pandemic period. COVID-19: coronavirus disease 2019; GDM: gestational diabetes mellitus. |
COVID-19 as a risk factor for GDM
Thirty-seven studies comprising 21,254 pregnant women confirmed with COVID-19 infection were included in the meta-analysis. The random effects meta-analysis models revealed that the odds of pregnant women with COVID-19 infection associated with GDM were less compared to pregnant women with COVID-19 infection associated without GDM (OR, 0.02; 95% CI, 0.01 to 0.02; P < 0.00001) with heterogeneity of I2 = 97% (P < 0.00001) as shown in Figure 3. Methodological quality assessment of included studies by NOS suggested that four studies were of poor quality, one was of fair quality and remaining 32 studies were of good quality. Visual inspection of funnel plot shows low publication bias (Supplementary Figure 2, www.jofem.org). The stratification analysis by eliminating the poor-quality studies (OR = 0.02), fixed effects meta-analysis model (OR, 0.02; 95% CI, 0.02 to 0.03; P < 0.00001) and the leave-one out sensitivity analysis (OR = 0.02) showed no significant difference from the overall effect measure estimated from the random effects meta-analysis model (Supplementary Table 2, www.jofem.org). However, limitations such as small differences and high heterogeneity need to be considered.
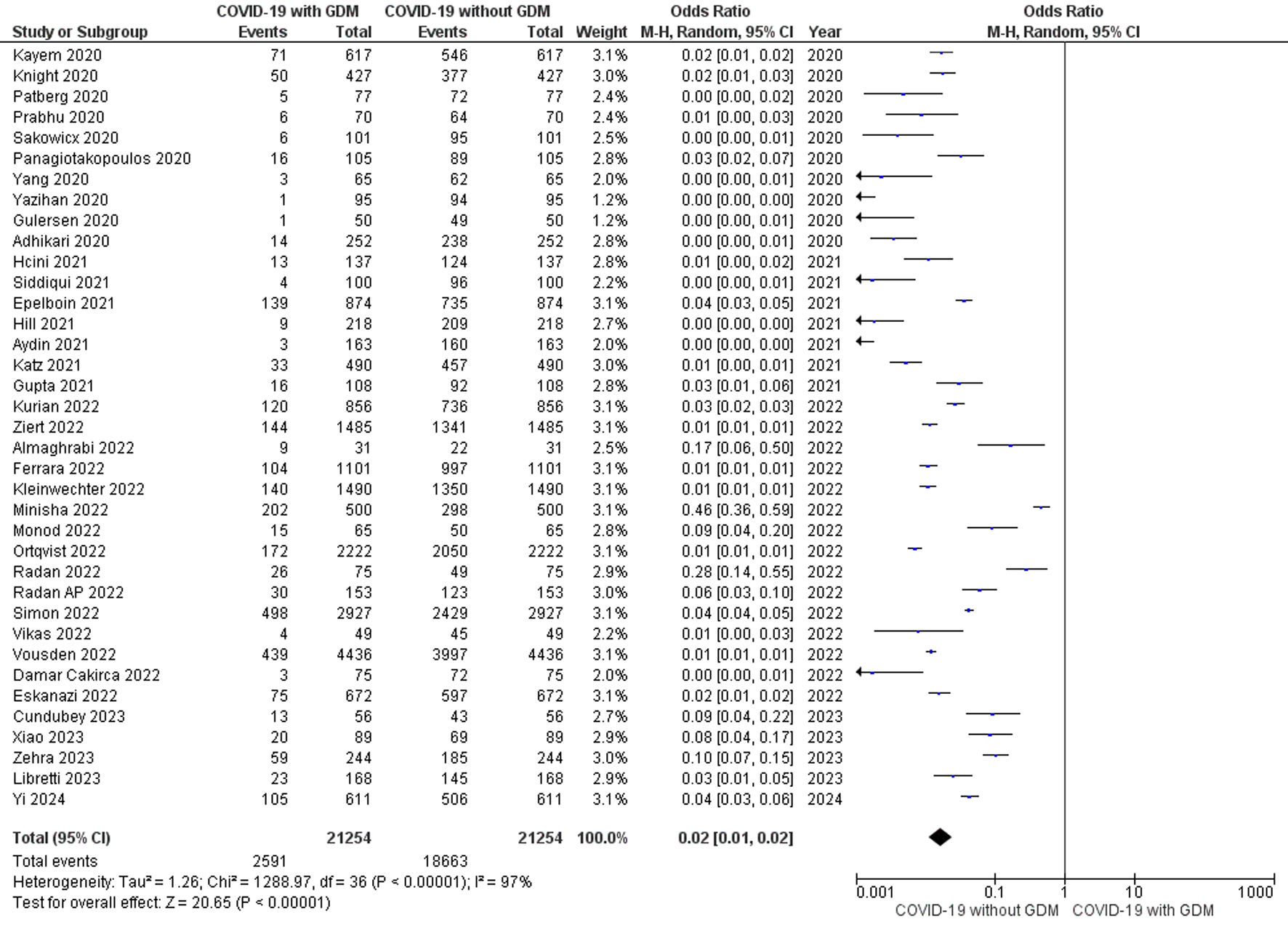 Click for large image | Figure 3. Forest plot shows the random effects meta-analysis model evaluating the risk of COVID-19 for the diagnosis of GDM among pregnant women diagnosed with GDM and without GDM. COVID-19: coronavirus disease 2019; GDM: gestational diabetes mellitus. |
GDM as a risk factor for COVID-19
Twenty-one studies comprising 889,228 pregnant women with GDM tested positive and negative for COVID-19 were included in the meta-analysis. The random effects model showed that the odds of pregnant women with GDM diagnosed as positive for COVID-19 were 1.28-fold greater than the odds of pregnant women with GDM tested negative for COVID-19 (OR, 1.28; 95% CI, 1.13 to 1.44; P < 0.0001) with heterogeneity of I2 = 35% (P = 0.06) as shown in Figure 4. Methodological quality assessment of the included studies by NOS suggested an overall good quality and visual inspection of the funnel plot shows low publication bias (Supplementary Figure 3, www.jofem.org). The fixed effects meta-analysis model (OR, 1.31; 95% CI, 1.23 to 1.40; P < 0.00001) and the leave-one out sensitivity analysis (ORs were in the range of 1.24 to 1.30) showed no significant difference from the overall effect measure estimated from the random effects meta-analysis model (Supplementary Table 2, www.jofem.org).
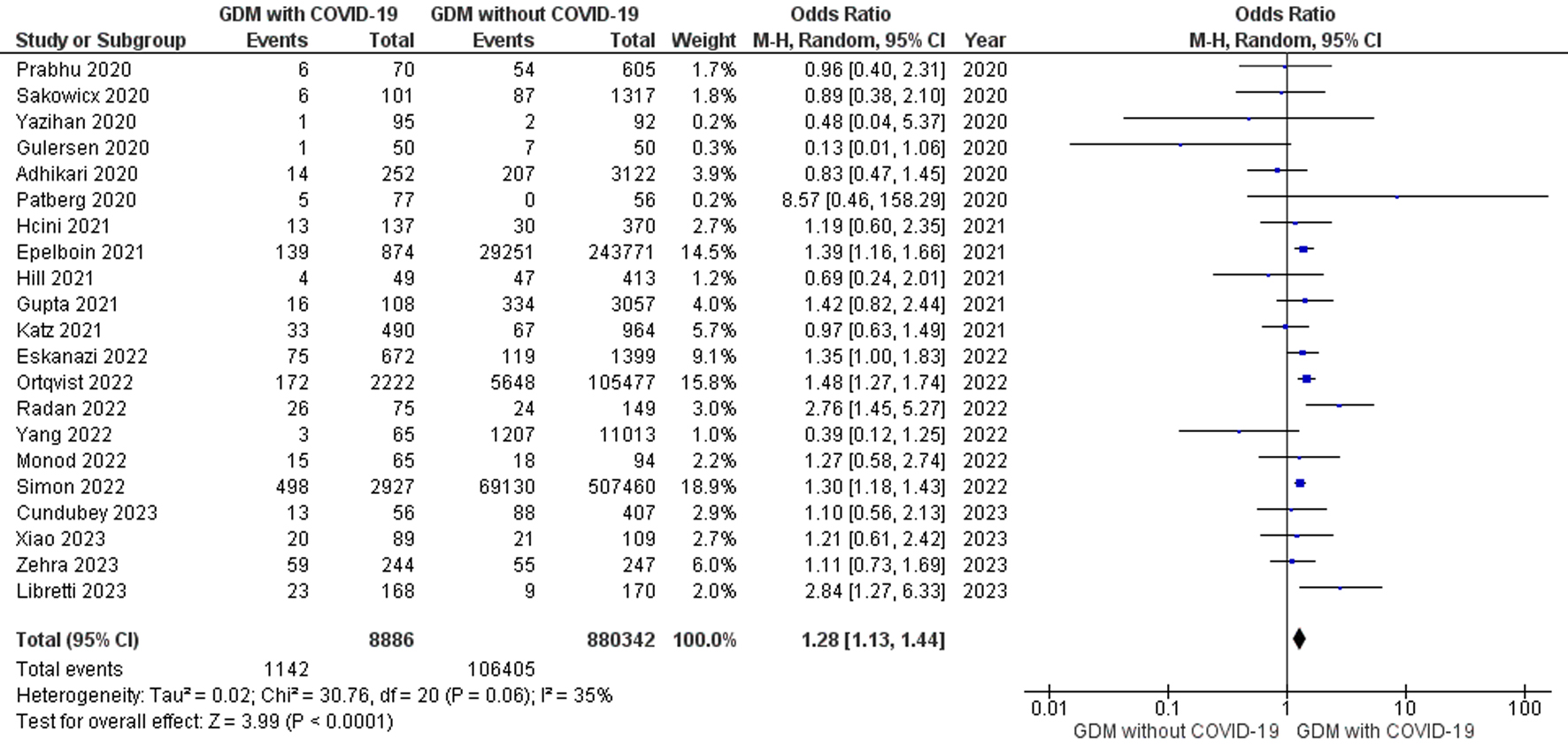 Click for large image | Figure 4. Forest plot shows the random effects meta-analysis model evaluating the risk of GDM for the diagnosis of COVID-19 among pregnant women tested positive and negative for COVID-19. COVID-19: coronavirus disease 2019; GDM: gestational diabetes mellitus. |
| Discussion | ▴Top |
The meta-analysis showed that the prevalence of GDM has increased by 17% during the pandemic period compared to pre-pandemic period and the odds of pregnant women with GDM tested positive for COVID-19 were 1.28-fold greater than the odds of pregnant women with GDM tested negative for COVID-19. These findings suggest that pregnant women with GDM have increased risk of COVID-19 infections among GDM cohorts (pregnant women diagnosed with GDM) which is consistent with the results reported by our previous meta-analysis [76-78] and contradictory to the results reported by the previous meta-analysis [1-3]. Further, this is in consistent with the previous observational studies that the prevalence of GDM was increased during the pandemic period compared to the pre-pandemic period [33, 34] and contradictory to the overall decreased prevalence of GDM from 2019 to 2021, although the prevalence of GDM increased during the most severe period of the pandemic [27]. The increased odds of pregnant women with GDM tested positive for COVID-19 were consistent with the previous observational studies that the occurrence of GDM was significantly higher among pregnant women with COVID-19 compared to pregnant women without COVID-19 [64, 67]. This might be due to the lockdown restrictions, restricted physical activity, maternal stress, dietary changes, behavioral changes and taste dysfunction associated with COVID-19. However, the results also showed that the odds of pregnant women with confirmed COVID-19 infection associated with GDM were significantly less compared to pregnant women with COVID-19 infection associated without GDM among COVID-19 cohorts (pregnant women tested positive for COVID-19). This is contradictory to the previous reports that pregnant women who are overweight or obese and with GDM were more likely to have severe COVID-19 infection and more likely to be tested positive for COVID-19 infection respectively [64, 69]. This might be due to the decreased frequency of visits to the hospitals and outdoor activities due to fear of infections and lockdown restrictions [1-4].
Further, stratification analysis by regions specified by WHO showed that the prevalence of GDM was significantly increased among regions such as Europe and Central Asia, Middle East and North Africa, and North America during the pandemic period (Supplementary Figure 4, www.jofem.org). In addition, stratification analysis by incomes specified by WHO revealed that the prevalence of GDM was significantly increased among lower middle income countries and high income countries but not in upper middle income countries during the pandemic period (Supplementary Figure 5, www.jofem.org). These might be due to the reports that socio-economic determinants of health play a pivotal role in the increased prevalence of GDM [4, 16]. In addition, stratification analysis for cohort studies showed significant association but no significant association was found in case-control studies (Supplementary Figures 6, 7, www.jofem.org).
Regarding COVID-19 as a risk factor for GDM, stratification analysis by cohort studies, case-control studies, WHO regions and incomes was consistent with the finding that pregnant women with COVID-19 were less likely to be diagnosed with GDM among COVID-19 cohorts (Supplementary Figures 8-12, www.jofem.org).
Regarding GDM as a risk factor for COVID-19, stratification analysis by WHO regions showed that the odds of pregnant women with GDM tested positive for COVID-19 were significantly higher among regions such as Europe and Central Asia when compared to pregnant women with GDM tested negative for COVID-19 but no significant association was found among East Asia and Pacific, North America, and South Asia (Supplementary Figure 13, www.jofem.org). Stratification analysis by incomes specified by WHO revealed that the odds of pregnant women tested positive for COVID-19 were significantly higher in high income countries when compared to pregnant women with GDM tested negative for COVID-19 but no significant association was found among lower middle income countries and upper middle income countries (Supplementary Figure 14, www.jofem.org). In addition, stratification analysis for cohort studies showed significant association but no significant association was found in case-control studies (Supplementary Figures 15, 16, www.jofem.org).
In summary, the underlying mechanism behind the increased prevalence of GDM and its associated risk with COVID-19 infection was not exactly known, but the available evidence suggests that disruption of maternity health-care services, reduced health seeking behavior due to the fear of COVID-19 infection or restricted visitations in hospitals due to COVID-19 burden, unequal distribution of tele-health services and nutrition counseling, perceived maternal psychological stress due to financial burdens and social isolation, physical inactivity, dietary and behavioral lifestyle changes might have contributed to the increased prevalence of GDM during the COVID-19 pandemic [1-4, 16]. The increased risk of COVID-19 among pregnant women with GDM (GDM cohorts) might be explained by the hypothesis that taste dysfunction (one of the long COVID pathology) and altered taste perception towards impaired sweet, salt and fat taste stimuli downstream signaling among pregnant women with GDM due to SNPs in taste receptors such as TAS2R subtypes and TRPM5 correlates with the impaired innate immunity regulated by the taste receptor polymorphisms against respiratory infections such as COVID-19 [9-12]. Thus, the increased prevalence of gestational diabetes among pregnant women during the COVID-19 pandemic might be related to the taste dysfunction due to COVID-19 infection [10-12]. However, molecular studies were needed to validate this hypothesis and evaluate the role of taste receptor polymorphisms and its downstream signaling pathways in the management of COVID-19 and GDM.
Strengths and limitations
Some limitations need to be considered when interpreting these results such as high heterogeneity, retrospective nature of the included studies, different definitions of the study period, lack of stratification analysis regarding asymptomatic COVID-19, severity and timing of COVID-19 infection, timing of GDM diagnosis, different diagnostic criteria for GDM, gestational weight gain, maternal comorbidities, social determinants of health and the literature search was restricted to English language. The high heterogeneity might be due to the different consideration of pandemic and pre-pandemic period among the included studies, different diagnostic criteria for GDM, different region and study population. Additionally, sensitivity analysis showed no credible differences from the overall effect measures (Supplementary Table 2, www.jofem.org). The information regarding the missing data due to reasons specified in the respective included studies were incorporated into the assessment of overall quality of studies by NOS and stratification analysis by excluding the overall poor and fair quality of studies reveals no significant difference from the overall effect measures and several stratification analysis based on different regions, incomes, case-control and cohort studies were shown in the Supplementary Table 2 and Supplementary Figures 4-16 (www.jofem.org). Nevertheless, the present study provides a comprehensive analysis regarding the impact of COVID-19 pandemic on pregnant women with GDM as a full text article compared to the abstracts presented by the author in International Diabetic Federation Congress 2023. Further, the current evidence could aid clinicians and policymakers in the development of more efficient strategies and modify implementation of programs in combating the risk of GDM among pregnant women during future potential pandemics.
Conclusion
The results suggest that there was an increased prevalence of GDM during the COVID-19 pandemic period compared to pre-pandemic period and there was an increased odds of pregnant women with GDM tested positive for COVID-19. This suggests that dysregulated immune mechanisms among pregnant women with GDM might predispose to COVID-19 infections. The etiology might be the taste receptor polymorphisms or other molecular mechanisms yet to be hypothesized which regulates the innate immunity against respiratory infections such as COVID-19. However, the results also suggest that GDM was less likely to be diagnosed among pregnant women with COVID-19 infection. Moreover, molecular studies were needed to evaluate the therapeutic role of taste receptors in the management of COVID-19 and GDM. The present analysis highlights the need for future studies to evaluate the pathophysiological mechanisms of COVID-19 and the impact of associated risk factors on pregnant women with GDM.
| Supplementary Material | ▴Top |
Suppl 1. PICO definitions for Inclusion criteria.
Supplementary Table 1. Characteristics of participants of the included studies.
Supplementary Table 2. Leave-one out sensitivity analysis.
Supplementary Figure 1. Funnel plot (random (A) and fixed (B) effects models) for the comparison of GDM prevalence during pandemic and pre-pandemic cohorts.
Supplementary Figure 2. Funnel plots (random (A) and fixed (B) effects models) regarding COVID-19 as a risk factor for GDM.
Supplementary Figure 3. Funnel plot (random (A) and fixed (B) effects models) regarding GDM as a risk factor for COVID-19.
Supplementary Figure 4. Forest plot shows the random effects meta-analysis model comparing the prevalence of GDM during pandemic and pre-pandemic period stratified by WHO specified regions.
Supplementary Figure 5. Forest plot shows the random effects meta-analysis model comparing the prevalence of GDM during pandemic and pre-pandemic period stratified by WHO specified countries based on income levels and economies.
Supplementary Figure 6. Forest plot shows the random effects meta-analysis model comparing the prevalence of GDM during pandemic and pre-pandemic period stratified by including only cohort studies.
Supplementary Figure 7. Forest plot shows the random effects meta-analysis model comparing the prevalence of GDM during pandemic and pre-pandemic period stratified by Case-control studies only.
Supplementary Figure 8. Forest plot shows the random effects meta-analysis model evaluating the risk of COVID-19 for the diagnosis of GDM among pregnant women stratified by WHO specified regions. The study Eskanazi et. al., 2022 excluded as it involves 18 countries.
Supplementary Figure 9. Forest plot shows the random effects meta-analysis model evaluating the risk of COVID-19 for the diagnosis of GDM among pregnant women stratified by WHO specified countries with different incomes and economies. The study Eskanazi et. al., 2022 excluded as it involves 18 countries.
Supplementary Figure 10. Forest plot shows the random effects meta-analysis model evaluating the risk of COVID-19 for the diagnosis of GDM among pregnant women stratified by cohort studies only.
Supplementary Figure 11. Forest plot shows the random effects meta-analysis model evaluating the risk of COVID-19 for the diagnosis of GDM among pregnant women stratified by case-control studies only.
Supplementary Figure 12. Forest plot shows the random effects meta-analysis model evaluating the risk of COVID-19 for the diagnosis of GDM among pregnant women stratified by overall high quality studies only.
Supplementary Figure 13. Forest plot shows the random effects meta-analysis model evaluating the risk of GDM for the diagnosis of COVID-19 among pregnant women stratified by WHO specified regions. The study Eskanazi et. al., 2022 excluded as it involves 18 countries.
Supplementary Figure 14. Forest plot shows the random effects meta-analysis model evaluating the risk of GDM for the diagnosis of COVID-19 among pregnant women stratified by WHO specified countries with different incomes and economies. The study Eskanazi et. al., 2022 excluded as it involves 18 countries.
Supplementary Figure 15. Forest plot shows the random effects meta-analysis model evaluating the risk of GDM for the diagnosis of COVID-19 among pregnant women stratified by cohort studies only.
Supplementary Figure 16. Forest plot shows the random effects meta-analysis model evaluating the risk of GDM for the diagnosis of COVID-19 among pregnant women stratified by case-control studies only.
Acknowledgments
I am deeply grateful to Dr. Siva Kumar, Head of the Department, General Medicine, Coimbatore Medical College and Hospital for his support and provided ample time during my internship period for the preparation of this manuscript and extend my thanks to other professors and my colleagues in Coimbatore Medical College and Hospital for their support during my absence in the internship period. I would also like to acknowledge the reviewers for their constructive comments, the authors of the included studies and their study participants.
Financial Disclosure
The author declares that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
The author has no relevant financial or non-financial interests to disclose. The author has no competing interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization, study selection, study screening, quality assessment, data extraction, statistical analysis, original draft, revisions and preparation of final version for submission of the manuscript were carried out by the author Vishnu Shivam.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AHRQ: Agency for Health Research and Quality; 95% CI: 95% confidence interval; COVID-19: coronavirus disease 2019; GDM: gestational diabetes mellitus; OR: odds ratio; PAV: proline-alanine-valine; PICO: patient/population, intervention, comparison, outcomes; PROSPERO: International Prospective Register of Systematic Reviews; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TAS2Rs: type 2 taste receptors; TAS2R38: type 2 taste receptor R family member 38; TAS2R9: type 2 taste receptor R family member 9; TRPM5: transient receptor potential cation channel subfamily M member 5; WHO: World Health Organization
| References | ▴Top |
- Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, O'Brien P, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(6):e759-e772.
doi pubmed pmc - Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S, Bose D, Alimohammadi S, Basirjafari S, et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev Med Virol. 2021;31(5):1-16.
doi pubmed pmc - Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16):E540-E548.
doi pubmed pmc - Garrow J, Fan I, Lilly C, Lefeber C, Barone Gibbs B, Lefeber T, John C, et al. The COVID-19 pandemic and its impact on the development of gestational diabetes mellitus (GDM) in West Virginia. Diabetes Res Clin Pract. 2024;208:111126.
doi pubmed - Shivam V, Gillies CL, Goff LM, Zaccardi F, Khunti K. Taste perception genomics in gestational diabetes mellitus: A systematic review. Diabetes Obes Metab. 2024;26(4):1544-1547.
doi pubmed - Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, Lin C, et al. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chem Senses. 2023;48:bjad043.
doi pubmed pmc - Saniasiaya J, Islam MA, Abdullah B. Prevalence and characteristics of taste disorders in cases of COVID-19: a meta-analysis of 29,349 patients. Otolaryngol Head Neck Surg. 2021;165(1):33-42.
doi pubmed - Parsa S, Mogharab V, Ebrahimi M, Ahmadi SR, Shahi B, Mehramiz NJ, Foroughian M, et al. COVID-19 as a worldwide selective event and bitter taste receptor polymorphisms: An ecological correlational study. Int J Biol Macromol. 2021;177:204-210.
doi pubmed pmc - Taha MA, Hall CA, Shortess CJ, Rathbone RF, Barham HP. Treatment protocol for COVID-19 based on T2R phenotype. Viruses. 2021;13(3):503.
doi pubmed pmc - Watanabe LM, Pires IF, Noronha NY, Pinhel MAS, Nonino CB. The influence of bitter-taste receptor (TAS2R) expression in pharmacological response to Chloroquine in obese patients with COVID-19. Clinics (Sao Paulo). 2020;75:e2181.
doi pubmed pmc - Manivannan E, Karthikeyan C, Moorthy N, Chaturvedi SC. The rise and fall of chloroquine/hydroxychloroquine as compassionate therapy of COVID-19. Front Pharmacol. 2021;12:584940.
doi pubmed pmc - Yanai S, Tokuhara D, Tachibana D, Saito M, Sakashita Y, Shintaku H, Koyama M. Diabetic pregnancy activates the innate immune response through TLR5 or TLR1/2 on neonatal monocyte. J Reprod Immunol. 2016;117:17-23.
doi pubmed - Gould S, Norris SL. Contested effects and chaotic policies: the 2020 story of (hydroxy) chloroquine for treating COVID-19. Cochrane Database Syst Rev. 2021;3(3):ED000151.
doi pubmed pmc - Gu XX, Chen K, Yu H, Liang GY, Chen H, Shen Y. How to prevent in-hospital COVID-19 infection and reassure women about the safety of pregnancy: Experience from an obstetric center in China. J Int Med Res. 2020;48(7):300060520939337.
doi pubmed pmc - Justman N, Shahak G, Gutzeit O, Ben Zvi D, Ginsberg Y, Solt I, Vitner D, et al. Lockdown with a Price: The impact of the COVID-19 pandemic on prenatal care and perinatal outcomes in a tertiary care center. Isr Med Assoc J. 2020;22(9):533-537.
pubmed - Pariente G, Wissotzky Broder O, Sheiner E, Lanxner Battat T, Mazor E, Yaniv Salem S, Kosef T, et al. Risk for probable post-partum depression among women during the COVID-19 pandemic. Arch Womens Ment Health. 2020;23(6):767-773.
doi pubmed pmc - Molina-Vega M, Gutierrez-Repiso C, Lima-Rubio F, Suarez-Arana M, Linares-Pineda TM, Cobos Diaz A, Tinahones FJ, et al. Impact of the gestational diabetes diagnostic criteria during the pandemic: an observational study. J Clin Med. 2021;10(21):4904.
doi pubmed pmc - Chelu S, Bernad E, Craina M, Neamtu R, Mocanu AG, Vernic C, Chiriac VD, et al. Prevalence of gestational diabetes in preCOVID-19 and COVID-19 years and its impact on pregnancy: a 5-year retrospective study. Diagnostics (Basel). 2022;12(5):1241.
doi pubmed pmc - Collins-Smith A, Prasannan L, Shan W, Dori E, Katzow M, Blitz MJ. Effect of lockdown period of COVID-19 pandemic on maternal weight gain, gestational diabetes, and newborn birth weight. Am J Perinatol. 2024;41(S01):e584-e593.
doi pubmed pmc - Gurol-Urganci I, Waite L, Webster K, Jardine J, Carroll F, Dunn G, Fremeaux A, et al. Obstetric interventions and pregnancy outcomes during the COVID-19 pandemic in England: A nationwide cohort study. PLoS Med. 2022;19(1):e1003884.
doi pubmed pmc - Handley SC, Ledyard R, Lundsberg LS, Passarella M, Yang N, Son M, McKenney K, et al. Changes in prenatal testing during the COVID-19 pandemic. Front Pediatr. 2022;10:1064039.
doi pubmed pmc - He Z, Lv Y, Zheng S, Pu Y, Lin Q, Zhou H, Dong M, et al. Association of COVID-19 lockdown with gestational diabetes mellitus. Front Endocrinol (Lausanne). 2022;13:824245.
doi pubmed pmc - Keating N, Carpenter K, McCarthy K, Coveney C, McAuliffe F, Mahony R, Walsh J, et al. Clinical outcomes following a change in gestational diabetes mellitus diagnostic criteria due to the COVID-19 pandemic: a case-control study. Int J Environ Res Public Health. 2022;19(3):1884.
doi pubmed pmc - La Verde M, Torella M, Riemma G, Narciso G, Iavarone I, Gliubizzi L, Palma M, et al. Incidence of gestational diabetes mellitus before and after the Covid-19 lockdown: A retrospective cohort study. J Obstet Gynaecol Res. 2022;48(5):1126-1131.
doi pubmed pmc - Liu Y, Dai M, Tang S. Effect of initial COVID-19 outbreak during first trimester on pregnancy outcome in Wuxi, China. BMC Pregnancy Childbirth. 2022;22(1):54.
doi pubmed pmc - Ornaghi S, Fumagalli S, Guinea Montalvo CK, Beretta G, Invernizzi F, Nespoli A, Vergani P. Indirect impact of SARS-CoV-2 pandemic on pregnancy and childbirth outcomes: a nine-month long experience from a university center in Lombardy. Int J Gynaecol Obstet. 2022;156(3):466-474.
doi pubmed pmc - Yin B, Wu K, Hu L, Zheng W, Zheng Y, Duan X, Zhu B. Gestational diabetes mellitus in the COVID-19 pandemic: a retrospective study from Hangzhou, China. J Diabetes. 2022;14(10):711-720.
doi pubmed pmc - Zanardo V, Tortora D, Sandri A, Severino L, Mesirca P, Straface G. COVID-19 pandemic: impact on gestational diabetes mellitus prevalence. Diabetes Res Clin Pract. 2022;183:109149.
doi pubmed pmc - Zheng W, Wang J, Zhang K, Liu C, Zhang L, Liang X, Zhang L, et al. Maternal and infant outcomes in women with and without gestational diabetes mellitus in the COVID-19 era in China: Lessons learned. Front Endocrinol (Lausanne). 2022;13:982493.
doi pubmed pmc - Ansari H, Amini Z, Madreseh E. The effect of Coronavirus disease pandemic on maternal and neonatal health: A cohort study from Isfahan, Iran. Int J Reprod Biomed. 2023;21(2):159-166.
doi pubmed pmc - Auger N, Wei SQ, Dayan N, Ukah UV, Quach C, Lewin A, Healy-Profitos J, et al. Impact of COVID-19 on rates of gestational diabetes in a North American pandemic epicenter. Acta Diabetol. 2023;60(2):257-264.
doi pubmed pmc - Boguslawski SM, Joseph NT, Stanhope KK, Ti AJ, Geary FH, Boulet SL. Impact of the COVID-19 pandemic on prenatal care utilization at a public hospital. Am J Perinatol. 2023;40(13):1484-1494.
doi pubmed - Gharacheh M, Kalan ME, Khalili N, Ranjbar F. An increase in cesarean section rate during the first wave of COVID-19 pandemic in Iran. BMC Public Health. 2023;23(1):936.
doi pubmed pmc - Gholami R, Borumandnia N, Kalhori E, Taheri M, Khodakarami N. The impact of covid-19 pandemic on pregnancy outcome. BMC Pregnancy Childbirth. 2023;23(1):811.
doi pubmed pmc - Meloncelli NJ, Barnett AG, Cameron CM, McIntyre D, Callaway LK, d'Emden MC, de Jersey SJ. Gestational diabetes mellitus screening and diagnosis criteria before and during the COVID-19 pandemic: a retrospective pre-post study. Med J Aust. 2023;219(10):467-474.
doi pubmed - Benyamini Raischer H, Garmi G, Malchi D, Nachshon AA, Inbar S, Romano S, Salim R. Impact of COVID-19 mandatory lockdown on maternal gestational weight gain and neonatal macrosomia rate at an academic medical center in Israel. J Matern Fetal Neonatal Med. 2023;36(1):2204391.
doi pubmed - Rhou YJJ, Elhindi J, Melov SJ, Cheung NW, Pasupathy D, Western Sydney C-PSG. Indirect effects of the COVID-19 pandemic on risk of gestational diabetes and factors contributing to increased risk in a multiethnic population: a retrospective cohort study. BMC Pregnancy Childbirth. 2023;23(1):341.
doi pubmed pmc - Zare F, Karimi A, Daliri S. Complications in pregnant women and newborns before and during the COVID-19 pandemic. Iran J Nurs Midwifery Res. 2024;29(1):91-97.
doi pubmed pmc - Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, Spong CY. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3(11):e2029256.
doi pubmed pmc - Gulersen M, Prasannan L, Tam Tam H, Metz CN, Rochelson B, Meirowitz N, Shan W, et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM. 2020;2(4):100211.
doi pubmed pmc - Kayem G, Lecarpentier E, Deruelle P, Bretelle F, Azria E, Blanc J, Bohec C, et al. A snapshot of the COVID-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49(7):101826.
doi pubmed pmc - Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, O'Brien P, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
doi pubmed pmc - Panagiotakopoulos L, Myers TR, Gee J, Lipkind HS, Kharbanda EO, Ryan DS, Williams JTB, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics - Eight U.S. Health Care Centers, March 1-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1355-1359.
doi pubmed pmc - Patberg ET, Adams T, Rekawek P, Vahanian SA, Akerman M, Hernandez A, Rapkiewicz AV, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224(4):382.e1-18.
doi pubmed pmc - Prabhu M, Cagino K, Matthews KC, Friedlander RL, Glynn SM, Kubiak JM, Yang YJ, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127(12):1548-1556.
doi pubmed pmc - Sakowicz A, Ayala AE, Ukeje CC, Witting CS, Grobman WA, Miller ES. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM. 2020;2(4):100198.
doi pubmed pmc - Yang R, Mei H, Zheng T, Fu Q, Zhang Y, Buka S, Yao X, et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18(1):330.
doi pubmed pmc - Yazihan N, Tanacan A, Erol SA, Anuk AT, Sinaci S, Biriken D, Keskin HL, et al. Comparison of VEGF-A values between pregnant women with COVID-19 and healthy pregnancies and its association with composite adverse outcomes. J Med Virol. 2021;93(4):2204-2209.
doi pubmed - Aydin GA, Unal S, Ozsoy HGT. The effect of gestational age at the time of diagnosis on adverse pregnancy outcomes in women with COVID-19. J Obstet Gynaecol Res. 2021;47(12):4232-4240.
doi pubmed pmc - Epelboin S, Labrosse J, De Mouzon J, Fauque P, Gervoise-Boyer MJ, Levy R, Sermondade N, et al. Obstetrical outcomes and maternal morbidities associated with COVID-19 in pregnant women in France: A national retrospective cohort study. PLoS Med. 2021;18(11):e1003857.
doi pubmed pmc - Gupta P, Kumar S, Sharma SS. SARS-CoV-2 prevalence and maternal-perinatal outcomes among pregnant women admitted for delivery: Experience from COVID-19-dedicated maternity hospital in Jammu, Jammu and Kashmir (India). J Med Virol. 2021;93(9):5505-5514.
doi pubmed pmc - Hcini N, Maamri F, Picone O, Carod JF, Lambert V, Mathieu M, Carles G, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: A single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021;257:11-18.
doi pubmed pmc - Hill J, Patrick HS, Ananth CV, O'Brien D, Spernal S, Horgan R, Brandt JS, et al. Obstetrical outcomes and follow-up for patients with asymptomatic COVID-19 at delivery: a multicenter prospective cohort study. Am J Obstet Gynecol MFM. 2021;3(6):100454.
doi pubmed pmc - Katz D, Bateman BT, Kjaer K, Turner DP, Spence NZ, Habib AS, George RB, et al. The society for obstetric anesthesia and perinatology coronavirus disease 2019 registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome coronavirus-2 outbreak in the United States. Anesth Analg. 2021;133(2):462-473.
doi pubmed - Siddiqui S, Najam R. Pregnancy outcomes with COVID-19 lessons learned from the pandemic. Cureus. 2021;13(7):e16358.
doi pubmed pmc - Almaghrabi R, Shaiba LA, Babic I, Abdelbaky M, Aljuhani SI, Omer M, Abdelmaksoud HA, et al. Possible vertical transmission of corona virus disease 19 (COVID-19) from infected pregnant mothers to neonates: a multicenter study. J Matern Fetal Neonatal Med. 2022;35(25):9558-9567.
doi pubmed - Damar Cakirca T, Torun A, Hamidanoglu M, Portakal RD, Olcen M, Cakirca G, Haksever M. COVID-19 infection in pregnancy: a single center experience with 75 cases. Ginekol Pol. 2022;93(5):410-415.
doi pubmed - Eskenazi B, Rauch S, Iurlaro E, Gunier RB, Rego A, Gravett MG, Cavoretto PI, et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol. 2022;227(1):74.e1-16.
doi pubmed pmc - Ferrara A, Hedderson MM, Zhu Y, Avalos LA, Kuzniewicz MW, Myers LC, Ngo AL, et al. Perinatal complications in individuals in california with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. 2022;182(5):503-512.
doi pubmed pmc - Kleinwechter HJ, Weber KS, Mingers N, Ramsauer B, Schaefer-Graf UM, Groten T, Kuschel B, et al. Gestational diabetes mellitus and COVID-19: results from the COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS). Am J Obstet Gynecol. 2022;227(4):631.e1-19.
doi pubmed pmc - Kurian S, Mathews M, Reshmi VP, Divakaran B, Ajith S. Impact of diabetes on the severity of COVID-19 infection in pregnant women - A single-center descriptive study. Diabetes Metab Syndr. 2022;16(1):102362.
doi pubmed pmc - Minisha F, Farrell T, Abuyaqoub S, Abdel Rahim A, Ahmed H, Omer M, Abraham M, et al. Maternal risk factors of COVID-19-affected pregnancies: A comparative analysis of symptomatic and asymptomatic COVID-19 from the Q-PRECIOUS registry. Qatar Med J. 2022;2022(4):52.
doi pubmed pmc - Monod C, Kotzaeridi G, Eppel D, Linder T, Bozkurt L, Hosli I, Gobl CS, et al. Assessment of glucose levels in pregnant women with history of COVID-19 in a case-control study. Front Physiol. 2022;13:988361.
doi pubmed pmc - Ortqvist AK, Dahlqwist E, Magnus MC, Ljung R, Jonsson J, Aronsson B, Pasternak B, et al. COVID-19 vaccination in pregnant women in Sweden and Norway. Vaccine. 2022;40(33):4686-4692.
doi pubmed pmc - Radan AP, Fluri MM, Nirgianakis K, Mosimann B, Schlatter B, Raio L, Surbek D. Gestational diabetes is associated with SARS-CoV-2 infection during pregnancy: A case-control study. Diabetes Metab. 2022;48(4):101351.
doi pubmed pmc - Radan AP, Baud D, Favre G, Papadia A, Surbek D, Baumann M, Raio L. Low placental weight and altered metabolic scaling after severe acute respiratory syndrome coronavirus type 2 infection during pregnancy: a prospective multicentric study. Clin Microbiol Infect. 2022;28(5):718-722.
doi pubmed pmc - Simon E, Gouyon JB, Cottenet J, Bechraoui-Quantin S, Rozenberg P, Mariet AS, Quantin C. Impact of SARS-CoV-2 infection on risk of prematurity, birthweight and obstetric complications: A multivariate analysis from a nationwide, population-based retrospective cohort study. BJOG. 2022;129(7):1084-1094.
doi pubmed pmc - N V, St K, R M, Shenoy V, Patil N. Clinical radiological and biochemical profile of moderate to severe COVID-19 pregnant females and its correlation with clinical outcome. J Assoc Physicians India. 2022;70(4):11-12.
pubmed - Vousden N, Ramakrishnan R, Bunch K, Morris E, Simpson N, Gale C, O'Brien P, et al. Management and implications of severe COVID-19 in pregnancy in the UK: data from the UK Obstetric Surveillance System national cohort. Acta Obstet Gynecol Scand. 2022;101(4):461-470.
doi pubmed pmc - Ziert Y, Abou-Dakn M, Backes C, Banz-Jansen C, Bock N, Bohlmann M, Engelbrecht C, et al. Maternal and neonatal outcomes of pregnancies with COVID-19 after medically assisted reproduction: results from the prospective COVID-19-Related Obstetrical and Neonatal Outcome Study. Am J Obstet Gynecol. 2022;227(3):495.e1-11.
doi pubmed pmc - Cundubey CR, Ak M, Demir B, Cam S. Effects of COVID-19 Infection on the Oral Glucose Tolerance Test Results in Pregnancy. Cureus. 2023;15(10):e46404.
doi pubmed pmc - Libretti A, Troia L, Cappello AM, Casarotti C, D'Amato AT, Dallarda G, Ghio M, et al. Pregnancy and neonatal outcomes of SARS-CoV-2 infection discovered at the time of delivery: a tertiary center experience in North Italy. J Perinat Med. 2024;52(2):215-221.
doi pubmed - Xiao H, Chen C, Huang S, Zhang W, Cai S, Hou X, Luo Y, et al. Effects of novel coronavirus Omicron variant infection on pregnancy outcomes: a retrospective cohort study from Guangzhou. Front Med (Lausanne). 2023;10:1256080.
doi pubmed pmc - Zehra SM, Parkar S, Kazi Z, Pethani A, Malik A, Mirza A, Abro F, et al. Impact of COVID-19 on feto-maternal and neonatal health in Karachi, Pakistan, A retrospective cohort study. PLOS Glob Public Health. 2023;3(8):e0002139.
doi pubmed pmc - Yi J, Chen L, Meng X, Chen Y. The impact of gestational weeks of Coronavirus disease 2019 (COVID-19) infection on perinatal outcomes. Reprod Health. 2024;21(1):31.
doi pubmed pmc - Shivam V, Senathipathi V, Vinayagam P. IDF23-0102 A meta-analysis on the association of gestational diabetes mellitus with COVID-19 infection. Diabetes Research and Clinical Practice. 2024;209:111273.
doi - Shivam V, Vinayagam P, Senathipathi V. IDF23-0112 Gestational diabetes as a risk factor for COVID-19 infection: A meta-analysis. Diabetes Research and Clinical Practice. 2024;209:111140.
doi - Shivam V, Vinayagam P, Senathipathi V, Murugesan S. IDF23-0101 Ameta-analysis on the prevalence of gestational diabetes mellitus during COVID-19 pandemic and pre-pandemic period. Diabetes Research and Clinical Practice. 2024;209:111272.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
