| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 13, Number 4, November 2023, pages 144-152
Acute Effect of COVID-19 Vaccination on Glycemic Profile in Patients With Type 1 Diabetes Mellitus
Khaled Hani Aburisheha, g , Mohammed Khalid Alkhalifaha, b, Mohammad Fahad AlKheraijic, Saleh Ibrahim Alwaland, Mohammed Jamal Anabie, Amjad Salem Alshammarif, Amani Ayyed Aldhewailaa
aUniversity Diabetes Center, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
bDepartment of Family Medicine and Polyclinics, King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia
cFirst Health Cluster (C1), Ministry of Health, Riyadh, Saudi Arabia
dPrince Sultan Military Medical City - Family & Community Medicine Department, Riyadh, Saudi Arabia
eKing Abdulaziz University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
fKing Abdulaziz Specialist Hospital, Aljouf, Saudi Arabia
gCorresponding Author: Khaled Hani Aburisheh, University Diabetes Center, King Saud University Medical City, King Saud University, PO Box 11472, Riyadh 7805, Saudi Arabia
Manuscript submitted August 7, 2023, accepted August 28, 2023, published online October 21, 2023
Short title: COVID-19 Vaccine Effect on Glycemic Profile
doi: https://doi.org/10.14740/jem894
| Abstract | ▴Top |
Background: Coronavirus disease 2019 (COVID-19) affected the whole world socially, economically, and medically. People with diabetes mellitus could have higher rates of morbidity and mortality if infected by the virus. New-onset diabetes and diabetic emergencies were, in some cases, first identified after the COVID-19 vaccine. In this study, we aimed to evaluate the acute effect of COVID-19 vaccination on the glycemic parameters of patients with type 1 diabetes mellitus (T1DM).
Methods: This was a retrospective observational study that included patients with T1DM older than 14 years old using Freestyle libre sensors and vaccinated with the COVID-19 vaccine. Data were collected from patients’ electronic medical records and glycemic profile parameters 1 to 2 weeks before and 1 to 2 weeks after the vaccine were extracted from the LibreView system.
Results: Seventy-two vaccines were analyzed from 44 patients with T1DM. There was no acute change of interstitial glucose measures after COVID-19 vaccination; however, there was a significant reduction in time in range and an increase in time above range after vaccination in those who were aged above 28 and had longer duration and better diabetes mellitus (DM) control. Glycated hemoglobin (HbA1c) was the only independent factor associated with the change in the glycemic profile after vaccination in multivariate regression analysis.
Conclusion: In our study population, there was no significant change in glycemic parameters after COVID-19 vaccination but those above 28 years old with longer duration of DM and lower HbA1c could have shifted upward their interstitial glucose levels. Precautions could be considered in those groups before receiving the vaccine.
Keywords: COVID-19 vaccines; Diabetes mellitus; Type 1; Glycated hemoglobin; Blood glucose
| Introduction | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which was later known as coronavirus disease 2019 (COVID-19) was identified initially in China. This pandemic disturbed the whole world’s dynamics, economy, and healthcare [1].
The fatality rate is higher among patients with multiple comorbidities including, but not limited to, diabetes, pulmonary diseases, and cardiac diseases [2]. New-onset diabetes and diabetic emergencies such as hyperosmolar hyperglycemic syndrome (HHS) and diabetic ketoacidosis (DKA) were, in some cases, first identified during COVID-19 infection [3, 4].
A nationwide COVID-19 vaccination program was started in Saudi Arabia as the Saudi Food and Drug Authority (SFDA) reviewed the data presented by vaccine manufacturers and approved the first COVID-19 vaccine in December 2020 [5]. The common side effects of available vaccinations were injection site pain, headache, fatigue, fever, and nausea or vomiting [6].
Before COVID-19, there was no research in the literature reporting specifically on the relationship between vaccines to blood glucose elevations. However, studies in the literature focused on confirming the overall safety and benefits of vaccines in patients with diabetes. Blood glucose instability was reported after influenza vaccination, which could be not only due to a reaction to the virus itself but also to the excipients in the vaccine [7].
Few studies correlated the relationship between the COVID-19 vaccine and hyperglycemia. Heald et al conducted a study on 20 patients in which eight of them received Pfizer-BioNTech while 12 of them received the Oxford/AstraZeneca vaccine. There was a significant reduction in the glucose time in range (TIR) by 14% among all participants with no difference between the type of vaccine (P-value of 0.020) [8]. They also demonstrated almost the same results on 97 consecutive adults (age ≥ 18 years) with type 1 diabetes mellitus (T1DM) and showed a decline in the time glucose was on target for the week following vaccination. However, it was a modest change, (mean 52.2±2.0%) versus pre-COVID-19 vaccination (mean 55.0±2.0%) (P = 0.030) [9].
There are many case reports published recently showing the COVID-19 vaccines induced hyperglycemia and its related complications. Acute hyperglycemia started a few days after administration of the vaccine and ranged from mild hyperglycemia to DKA and HHS required hospitalization or intensive care unit (ICU) admission regardless of the diabetic status, previous history of COVID-19 infection, and vaccine type and dose [10].
Continuous glucose monitoring (CGM) devices show the average blood glucose levels and estimate the trend direction. Nowadays, they are increasingly being used routinely in the care of people with T1DM [11]. By using flash glucose monitoring (FGM), users can retrospectively review the preceding 8 h of continuous glucose data [12]. Those devices were considered a real change in diabetes management and showed a reduction in glycated hemoglobin (HbA1c) with their usage [11, 13].
We observed in our clinical practice that some patients with DM could experience a transient instability of blood glucose levels after vaccination. Hence comes the need to study the effect of vaccination on people with diabetes mellitus (DM). The stimulation of the immune response can lead to an increase in counterregulatory hormone levels such as adrenaline, growth hormone, cortisol, and glucagon [14]. The increase in counterregulatory hormone response in people with T1DM led us to the hypothesis of a decrease in glucose TIR acutely after vaccination.
| Materials and Methods | ▴Top |
Ethical approval
Institutional Review Board of College of Medicine, King Saud University, Saudi Arabia reviewed and approved the protocol (E-22-6910). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Study design
This was a retrospective observational study of patients with T1DM who were following in the adult diabetes clinics at the University Diabetes Center (UDC), King Saud University Medical City (KSUMC), received at least one COVID-19 vaccine and were using FGM.
Subjects
We included in our analysis patients with T1DM, who received at least one COVID-19 vaccination and using FGM 1 or 2 weeks before and after the vaccine with at least more than 70% of usage. Those who received corticosteroid treatment or were affected by any concomitant condition capable of affecting glucose control in three previous months were excluded.
Materials
The LibreView reporting system provides several metrics over the selected period for each participant that are all dependent on underlying patient interstitial glucose control; these include average glucose, glucose variability (GV) and percentage of glucose results falling within given ranges: TIR 3.9 - 10 mmol/L, time above rage (TAR) ≥ 10.1 mmol/L and time below range (TBR) < 3.9 mmol/L.
Variables
The primary outcome was the percentage (%) of GV, TIR, TAR and TBR for 7 days before and after COVID-19 vaccination. Data for those metrics were also extracted for weeks -2 and +2 to evaluate the interstitial glucose stability in the period before and the speed of return to pre-vaccination control, after the main measurement period.
Variables that might have an impact on the results were also taken from the patient records. These included age, sex, HbA1c level, fasting blood sugar (FBS), type and dose of vaccine given, medication, duration with diabetes and body mass index (BMI). For continuous indicators, the participants were split into two groups across the median value of each variable. Information concerning the date of vaccination was obtained from the patient’s general practice record.
Statistical analysis
Analysis was performed using PSPP (version 1.6.2-g78a33a). Data were demonstrated by mean and standard deviation (SD) or number and percentage for numerical and categorical variables, respectively. Wilcoxon signed-rank test for the outcome measures compared results in week +1 against -1 and weeks +2 against -2.
The median of the selected indicators was then calculated for the total cohort and split into two classes for each potential factor. Wilcoxon signed-rank test was repeated for the outcome measures for each subgroup.
Multiple regression modelling was carried out with the change in the proportion of interstitial glucose results in the TIR, TAR, TBR and GV as the dependent variables. A P-value of ≤ 0.05 was considered statistically significant.
| Results | ▴Top |
Demographic and clinical characteristics
A total of 194 patients with T1DM on FGM were screened and 44 patients were included as the remaining were excluded due to insufficient FGM and vaccine data. The mean age of the participants was 28.89 ± 10.28 years. Female participants were more in our sample (73.6%). The mean duration of DM was 11.63 ± 8.68 years and mean BMI was 26.88 ± 6.4 kg/m2. The total number of COVID-19 vaccines included in the analysis was 72 and Pfizer vaccine was mostly used in our study population (86.1%). The mean HbA1c before vaccine was 9.02±1.92% and mean fasting blood glucose was 11.79 ± 5.17 mmol/L. All patients were using multiple daily insulin injection (MDI) therapy 13.9% of them were on metformin and 2.8% were on semaglutide as adjunctive treatment (Table 1).
 Click to view | Table 1. Demographic and Clinical Characteristics of the Study Population |
Outcomes
There was no significant change in the glycemic profile (TIR, TAR, TBR and GV) acutely after COVID-19 vaccine (Fig. 1). However, in subgroup analysis, we found that in patients older than 28 years, TIR reduced significantly comparing 1 week before and 1 week after vaccine (Z = -2.92, P = 0.003) and 2 weeks before and 2 weeks after vaccine (Z = -2.17, P = 0.03) with significant increase in TAR 1 week after vaccine compared to 1 week before (Z = -2.02, P = 0.043). Moreover, there was a significant reduction in TBR comparing 1 week before and 1 week after vaccine (Z = -2.75, P= 0.006) and 2 weeks before and 2 weeks after vaccine (Z = -2.14, P = 0.032) in those who were younger than 29 years old. Categorizing patients according to DM duration, the vaccine associated with a reduction in TIR 1 week after the vaccine compared to 1 week before (Z = -2.68, P = 0.007), a reduction in TIR 2 weeks after the vaccine compared to 2 weeks before (Z = -2.5, P = 0.012), an increment in TAR 1 week after vaccine compared to 1 week before (Z = -2.27, P = 0.023) and an increment in TAR 2 weeks after vaccine compared to 2 weeks before (Z = -2.12, P = 0.034) in those who had longer duration of DM (more than 10.5 years). However, for those with shorter duration of DM, TBR reduced significantly 1 week after vaccine compared to 1 week before (Z = -2.08, P = 0.037). Also, we found that patients with better control of diabetes at baseline (HbA1c ≤ 8.55% before vaccine) taking the vaccine associated with a reduction in TIR and an increase in TAR acutely. However, when categorizing the patients according to gender, BMI, vaccine type and vaccine dose, there was no acute effect of the vaccine on glycemic profile (Table 2).
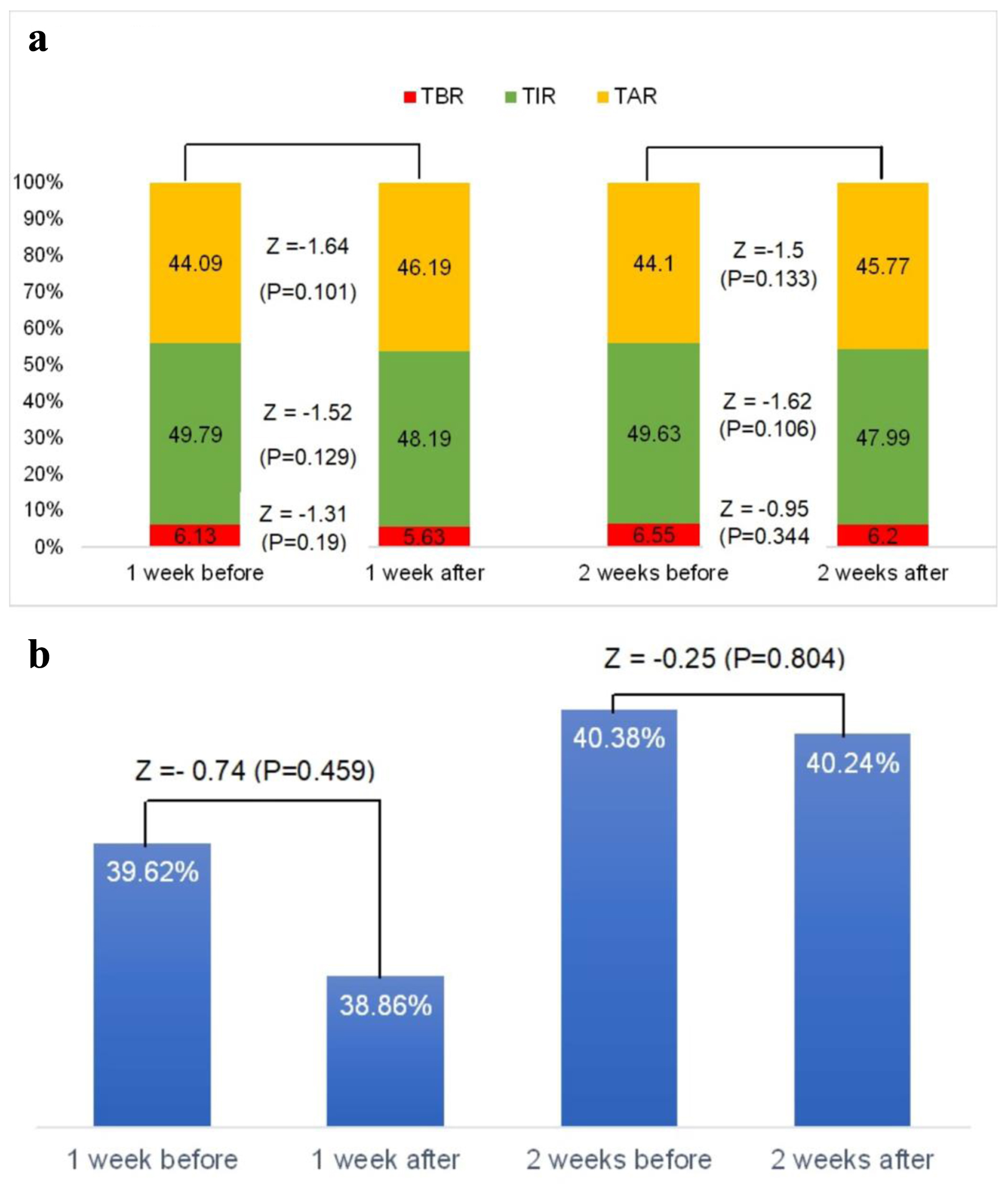 Click for large image | Figure 1. (a) Glycemic profile (time in range, time above range and time below range) changes in response to COVID-19 vaccination. (b) Glycemic variability changes in response to COVID-19 vaccination. COVID-19: coronavirus disease 2019; TAR: time above range; TBR: time below range; TIR: time in range. |
 Click to view | Table 2. Results of Statistical Subgroup Analysis (Wilcoxon Signed Rank Test) of Changes in Glycemic Parameters After COVID-19 Vaccination |
Multiple regression analysis
Multivariate linear regression analysis indicated that lower HbA1c before vaccine was independently associated with a reduction in TIR (standardized beta 0.33, P = 0.029) and an increase in TAR (standardized beta -0.4, P = 0.005) 2 weeks after vaccine. The model included the independent variables of age, BMI, duration of DM, the dose and type of vaccine, which had no significant effect on the outcomes (Table 3).
 Click to view | Table 3. Results of Multiple Regression Analysis for Variables Predicting Changes in Glycemic Parameters After COVID-19 Vaccination |
| Discussion | ▴Top |
In our study population, we did not find an acute effect of the COVID-19 vaccine on the glycemic profile using interstitial glucose monitoring parameters (TIR, TAR, TBR and GV). However, after subgroup analysis, in patients above 28 years old and those with longer duration and better control of DM, there was a significant reduction in TIR and an increase in TAR acutely after receiving the COVID-19 vaccine. In contrast, there was a reduction in TBR in those who were younger and had a shorter duration of DM.
The change in the proportion of previous interstitial glucose parameters persisted into the second week after vaccination.
There was no change in GV after the COVID-19 vaccine in those who had a reduction in TIR and TBR and an increase in TAR, suggesting that there was a shift upward in interstitial glucose levels, instead of variability changes.
PRO-VACS study and a substudy of the COVAC-DM study reported no relevant acute negative effect of COVID-19 vaccination on glycemic control in patients with T1DM which was similar to our results [15, 16]. Moreover, two studies in children and adolescents with T1DM reported no immediate effect of the vaccine on TIR [17, 18]. However, Heald et al found an incremental change in interstitial glucose levels for patients with T1DM with a reduction in TIR 1 week after vaccination in 58% of them [9]. Furthermore, a study done on the Arab population with T1DM found a temporary interstitial glucose disturbance after viral vector vaccines [19]. The population in those studies was older with longer duration and better control of DM which could explain the difference in the outcome measures compared to our study.
Furthermore, when categorized as higher or lower HbA1c (by median HbA1c), Heald et al found a significant reduction in TIR in those in the lower HbA1c group vs. no change for participants in the higher HbA1c group (P = 0.007) [9]. Similarly, Al-Ozairi et al reported a significant reduction in TIR in the group with HbA1c less than 7% compared with the higher HbA1c group [19]. This conclusion was also confirmed by our study.
Heald et al reported no significant difference in the change in glycemic profile after vaccination by age (split by median age of 44 years), and duration of diabetes (split by median duration of 17 years) which was against our results [9]. Again, this could be due to older age and longer duration of DM of his study population.
The finding that those with lower HbA1c had a significant acute change in TIR and TAR after the COVID-19 vaccine may indicate that those patients are more sensitive to the effect of the vaccine on the glycemic profile parameters as they had more percentage of interstitial glucose in the target range, so they had more to lose. Furthermore, those who were older and had a longer duration of DM had a higher percentage of TIR (above 50%) (data not shown) which also could demonstrate the acute changes in those groups’ glycemic profile.
Many published cases are reporting the induction of severe hyperglycemia crises including DKA and HHS after COVID-19 vaccination even in those who do not have a history of diabetes [10, 20]. The possible link between the vaccine and the resultant hyperglycemia is still unclear. One of the possible mechanisms is the inflammatory response to the vaccine by increasing cytokines such as interleukin (IL)-1, IL-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α which contribute to the abnormal glucose and lipid metabolism and culminate in the pancreatic endocrine system damage. Moreover, the stimulation of the immune system leads to stress response increasing the counterregulatory hormones like cortisol, growth hormone and glucagon [10, 20, 21]. It is proposed that the trigger of the inflammatory response in the mRNA vaccines is the SARS-CoV-2 spike protein and in the viral vector-based vaccine is the adenoviral vector [7]. Vaccination-induced immune response varies in degree within and between individuals affected by many factors which could be within the vaccine such as the type of adjuvant or the host like the immune response gene which could explain the difference in the results between the studies [8].
There was no evidence of any other factor than the vaccination which could affect the glycemic profile such as inter-current illness, minor operation or other events that would significantly influence interstitial glucose levels. However, we did not evaluate the post-vaccination side effects which showed in a previous study those with higher side effect scores spent significantly less TIR on days after the vaccine [16].
Heald et al studied only those who received the first dose of the vaccine and divided equally on both Pfizer and AstraZeneca vaccines and found similar outcomes in both vaccines [9]. In contrast, Al-Ozairi et al analyzed the two doses of vaccine and both viral vector and mRNA-based vaccines and concluded that only those who received the first dose of viral vector vaccines had a significant acute effect on TIR [19]. In our study, we included the three doses and three types of vaccines (Pfizer, AstraZeneca and Moderna) and there was no significant effect on glycemic profile after subgroup analysis.
HbA1c level was the only independent factor which was associated positively with TIR and negatively with TAR after the vaccine in a multivariate linear regression analysis which was confirmed by a previous study [9].
Strengths and limitations
We analyzed in our study the glycemic profile parameters from those who had more than 70% usage of the freestyle Libre over 4 weeks which could strengthen the study. Also, we included all three types and three doses of the vaccine in the analysis.
The retrospective design of the study and the few number of participants from only one center could be a limitation of our study. One of the limitations also is that we did not quantify the modification of insulin doses done after vaccination which could mask the hyperglycemia induced by the vaccine. Moreover, we did not have any inflammatory markers measurement pre- and post-vaccination to evaluate the link between the vaccine and the glycemic profile changes.
Conclusion
There was no acute effect of the COVID-19 vaccine on the glycemic profile in a group of patients with T1DM. However, there was a transient shift upward in interstitial glucose levels in those people older than 28 years old and have longer duration of DM with lesser HbA1c. This could raise the importance of educating people with T1DM about the possibility of temporary perturbation of blood glucose post-vaccination and about insulin dose adjustment and better management.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that there is no potential conflict of interest relevant to this article was reported.
Informed Consent
Since this was a retrospective data collection study, informed consent was waived by the Institutional Review Board, College of Medicine, King Saud University, Riyadh.
Author Contributions
Conceptualization: KHA and MKA. Development and design of methodology: KHA, MKA, MFA and SIA. Conducting the research and data collection: MKA, MFA, SIA, MJA, ASA and AAA. Application of statistical analysis of the study data: KHA. Writing the initial draft: MKA, MFA, SIA, MJA, ASA and AAA. Critical review and revision of the initial draft: KHA and MKA. Supervision: KHA. All the authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
BMI: body mass index; CGM: continuous glucose monitoring; COVID-19: coronavirus disease 2019; DKA: diabetic ketoacidosis; DM: diabetes mellitus; FBS: fasting blood sugar; FGM: flash glucose monitoring; GV: glycemic variability; HbA1c: glycated hemoglobin; HHS: hyperosmolar hyperglycemic syndrome; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SFDA: Saudi Food and Drug Authority; TAR: time above range; TBR: time below range; TIR: time in range; T1DM: type 1 diabetes mellitus
| References | ▴Top |
- Rawaf S, Allen LN, Stigler FL, Kringos D, Quezada Yamamoto H, van Weel C, Global Forum on Universal Health C, et al. Lessons on the COVID-19 pandemic, for and by primary care professionals worldwide. Eur J Gen Pract. 2020;26(1):129-133.
doi pubmed pmc - Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395-403.
doi pubmed pmc - Sarwani A, Al Saeed M, Taha H, Al Fardan RM. New-onset diabetes mellitus presenting as diabetic ketoacidosis in patients with COVID-19: A Case Series. Cureus. 2021;13(7):e16290.
doi pubmed pmc - Kiral E, Kirel B, Havan M, Keskin M, Karaoglan M, Yildirim A, Kangin M, et al. Increased severe cases and new-onset type 1 diabetes among children presenting with diabetic ketoacidosis during first year of COVID-19 pandemic in Turkey. Front Pediatr. 2022;10:926013.
doi pubmed pmc - Saudi Food and Drug Authority. Available from: https://www.sfda.gov.sa/en/node/73864. (updated December 10, 2020).
- Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15(2):505-508.
doi pubmed pmc - di Mauro G, Mascolo A, Longo M, Maiorino MI, Scappaticcio L, Bellastella G, Esposito K, et al. European safety analysis of mRNA and viral vector COVID-19 vaccines on glucose metabolism events. Pharmaceuticals (Basel). 2022;15(6):677.
doi pubmed pmc - Heald AH, Rea R, Horne L, Metters A, Steele T, Leivesley K, Whyte MB, et al. Analysis of continuous glucose tracking data in people with type 1 diabetes after COVID-19 vaccination reveals unexpected link between immune and metabolic response, augmented by adjunctive oral medication. Int J Clin Pract. 2021;75(12):e14714.
doi pubmed pmc - Heald AH, Stedman M, Horne L, Rea R, Whyte M, Gibson JM, Anderson SG, et al. The change in glycaemic control immediately after COVID-19 vaccination in people with type 1 diabetes. Diabet Med. 2022;39(4):e14774.
doi pubmed - Samuel SM, Varghese E, Triggle CR, Busselberg D. COVID-19 vaccines and hyperglycemia-is there a need for postvaccination surveillance? Vaccines (Basel). 2022;10(3):454.
doi pubmed pmc - Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
doi pubmed pmc - Kalra S, Gupta Y. Ambulatory glucose profile: Flash glucose monitoring. J Pak Med Assoc. 2015;65(12):1360-1362.
pubmed - Yadegarfar G, Anderson SG, Khawaja Z, Cortes G, Leivesley K, Metters A, Horne L, et al. The FreeStyle Libre flash glucose monitoring system: how it has improved glycaemic control for people with type 1 diabetes in Eastern Cheshire, UK. Cardiovasc Endocrinol Metab. 2020;9(4):171-176.
doi pubmed pmc - Mifsud S, Schembri EL, Gruppetta M. Stress-induced hyperglycaemia. Br J Hosp Med (Lond). 2018;79(11):634-639.
doi pubmed - Dicembrini I, Vitale V, Cosentino C, Cresci B, Pala L, Pieri M, Yannas D, et al. Interstitial glucose monitoring, type 1 diabetes and COVID-19 vaccine: the patient-reported outcomes and vaccine-associated changes in glucose and side effects (PRO-VACS). Acta Diabetol. 2022;59(3):435-438.
doi pubmed pmc - Aberer F, Moser O, Aziz F, Sourij C, Ziko H, Lenz J, Abbas F, et al. Impact of COVID-19 vaccination on glycemia in individuals with type 1 and type 2 diabetes: substudy of the COVAC-DM study. Diabetes Care. 2022;45(2):e24-e26.
doi pubmed - Piccini B, Pessina B, Pezzoli F, Casalini E, Toni S. COVID-19 vaccination in adolescents and young adults with type 1 diabetes: Glycemic control and side effects. Pediatr Diabetes. 2022;23(4):469-472.
doi pubmed pmc - Gouda N, Dimitriadou M, Sotiriou G, Christoforidis A. The impact of COVID-19 vaccination on glycaemic control in children and adolescents with type 1 diabetes mellitus on continuous glucose monitoring. Acta Diabetol. 2022;59(12):1609-1614.
doi pubmed pmc - Al-Ozairi E, Irshad M, Taghadom E, Varghese A, Sojan L, Alkandari J. Effect of COVID-19 vaccine on blood glucose metrics in Arabic people with type 1 diabetes. Front Endocrinol (Lausanne). 2023;14:1120384.
doi pubmed pmc - Lee HJ, Sajan A, Tomer Y. Hyperglycemic emergencies associated with COVID-19 vaccination: a case series and discussion. J Endocr Soc. 2021;5(11):bvab141.
doi pubmed pmc - Shi J, Fan J, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism. Front Endocrinol (Lausanne). 2019;10:703.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
