| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 14, Number 1, February 2024, pages 40-47
Increased Prevalence of Advanced Metabolic Dysfunction-Associated Steatotic Liver Disease Fibrosis in Type 2 Diabetics Despite Low-Risk Fibrosis-4 Index Scores
Jordan S. Woodarda, b, Gary A. Abramsa
aDepartment of Medicine, Prisma Health - Upstate Greenville, SC 29605, USA
bCorresponding Author: Jordan S. Woodard, Department of Medicine, Prisma Health - Upstate Greenville, SC 39605, USA
Manuscript submitted January 15, 2024, accepted February 27, 2024, published online February 29, 2024
Short title: FIB-4 Underestimates MASLD Fibrosis in T2DM
doi: https://doi.org/10.14740/jem935
| Abstract | ▴Top |
Background: The American Gastroenterology Association (AGA) guidelines for metabolic dysfunction-associated steatotic liver disease (MASLD) recommend screening for fibrosis in high-risk subjects with either type 2 diabetes, two or more metabolic risk factors, or steatosis on imaging. The 2021 AGA guidelines recommend calculating a fibrosis-4 index (FIB-4) score, and patients with scores above low-risk require further workup with FibroScan to assess liver stiffness measurement (LSM), a surrogate for liver fibrosis. However, FIB-4 scores have been suggested to be less accurate in patients with type 2 diabetes. The aim of our study was to identify the prevalence of significant to advanced fibrosis in subjects with a low-risk FIB-4 value with FibroScan’s LSM in type 2 diabetes.
Methods: A total of 1,153 subjects were referred to our liver center between August 2019 and September 2022; 1,114 subjects met MASLD criteria with data to calculate FIB-4 values. Subjects were categorized into age adjusted low-risk FIB-4 groups. Diagnosis of diabetes was determined by medical history.
Results: Low-risk age-adjusted FIB-4 scores were observed in 68.3% of older subjects and 73.4% of younger subjects (P = not significant (NS)). In the older group and younger cohorts, a LSM ≥ 10 kPa was noted in 21% suggesting advanced liver fibrosis. Seventy-one point six percent of older diabetic subjects had low FIB-4 values, similar to 67.2% of young diabetic subjects with low FIB4 values. Overall, 72% of subjects would not have been referred for FibroScan per AGA criteria. Despite low-risk FIB-4 scores, 257 subjects had LSM greater than or equal to 8 kPa and 148 underwent a liver biopsy. Forty-eight percent of patients with biopsies had significant fibrosis (F2-4), predominately affecting subjects with type 2 diabetes.
Conclusions: Diabetic subjects, despite having a low-risk FIB-4 tests, were four-fold more likely to demonstrate significant to advanced fibrosis, highlighting the limitations of FIB-4 in these individuals.
Keywords: MASLD; Hepatic fibrosis; FibroScan; FIB-4; Diabetes; Cirrhosis; Type 2 diabetes mellitus
| Introduction | ▴Top |
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading cause of chronic liver disease. The worldwide prevalence has increased from 5.5% to 32% over the past 30 years [1, 2]. MASLD is an umbrella term that includes a spectrum of liver injury including steatosis, metabolic dysfunction-associated steatohepatitis (MASH), fibrosis, and ultimately cirrhosis. MASLD is strongly associated with obesity, insulin resistance, metabolic syndrome, and type 2 diabetes. The increasing MASLD prevalence correlates with the current obesity epidemic and subsequent increase in metabolic disorders. Within the United States, the prevalence of MASLD is nearly two-fold greater in men and varies by ethnicity, affecting Hispanics (45%) more than White (33%) and Black (25%) individuals [2-4].
Long-term liver complications arise in the setting of moderate-to-advanced fibrosis (F2-4) [5]. Subjects with type 2 diabetes have a 65% prevalence of some fibrosis, and 38% have advanced fibrosis (F3-4) [6, 7]. Multiple metabolic risk factors increase the risk of fibrosis and cirrhosis. When compared to patients with metabolic trait (0 - 1) (defined as central obesity, triglycerides > 150 mg/dL, reduced high-density lipoprotein (HDL), < 40 mg/dL in men and < 50 mg/dL in women, hypertension, and raised fasting glucose), those with two or more metabolic traits had nearly two-fold increase in risk for progression to cirrhosis [8]. In patients with diabetes, obesity, dyslipidemia, and hypertension, the risk of progression to cirrhosis was increased by 2.6-fold [8].
The 2021 American Gastroenterology Association (AGA) guidelines recommend outpatient screening for all patients with type 2 diabetes, two or more metabolic traits, hepatic steatosis on imaging or elevated transaminases, because of the increased risk for advanced fibrosis and cirrhosis [9]. The suggested algorithm for MASLD/fibrosis screening is calculating a fibrosis-4 index (FIB-4) score using age, alanine transaminase (ALT), aspartate aminotransferase (AST), and platelets to categorize patients at higher risk for fibrosis [9]. A FIB-4 < 1.3 is considered low risk for advanced fibrosis or cirrhosis and can be managed by primary care with repeat noninvasive testing (NIT) every 2 - 3 years; however, those with FIB-4 > 2.67 are at high risk and should be referred to a hepatologist [9]. Indeterminate risk, FIB-4 between 1.3 and 2.67, necessitates additional workup with liver stiffness measurement (LSM) determined by vibration-controlled transient elastography (VCTE) to categorize patients into low-, intermediate-, or severe-risk based on LSM < 8 kPa, LSM 8 - 12 kPa, and LSM > 12 kPa, respectively [9]. Those with intermediate to high risk qualify for hepatology referral for either a liver biopsy, or magnetic resonance (MR) elastography, or surveillance every 2 - 3 years [9]. The lower end FIB-4 cutoff should be age adjusted to < 2.0 for patients aged 65 years or older [9, 10].
Recently, the 90% negative predictive value (NPV) of FIB-4 for predicting significant to advanced fibrosis has been questioned in subjects with type 2 diabetes [11-13]. The purpose of our study was to evaluate MASLD FibroScan referrals to our hepatology clinic. Our three aims were to assess their FIB-4 scores to 1) determine the prevalence of subjects that would not have been referred per 2021 AGA guidelines; 2) determine the prevalence of significant LSMs in subjects with a low FIB-4; and 3) compare LSMs, FIB-4 and liver histology in patients with type 2 diabetes and without diabetes.
| Materials and Methods | ▴Top |
Patient selection
This was a retrospective study of 1,153 consecutive adult patients, who were referred to our liver center for FibroScan between August 2019 and September 2022 with comparison of LSM values, FIB-4 scores, and liver biopsies (if performed). Inclusion in the study required a controlled attenuation parameter (CAP) score of 250+, 10 LSM values (kPa) with interquartile range (IQR)/med < 30%, and necessary lab work to calculate a FIB-4 score (age × AST/platelet count (× 109/L) × √ALT). Data collection of demographics, metabolic comorbidities including age, weight, liver transaminases, diagnoses of type 2 diabetes, hypertension, and dyslipidemia, LSM values, and liver histology was obtained on manual chart review.
The cohort was distributed into subgroups including age < 65 (group 1 “young/younger”) and ≥ 65 (group 2 “old/older”) years old and age-corrected low, intermediate, and high FIB-4 scores. We characterized low FIB-4 subjects into five LSM categories (< 8 kPa, 8 - 9.99, 10 - 13.99, 14 - 20 and 20+). Patients with and without diabetes were further characterized according to FIB-4 and LSM categories.
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Prisma Health approved this study.
Liver histology
Patients with low-risk FIB-4 scores and LSM values > 8 kPa that underwent a liver biopsy were identified. Liver biopsies were considered adequate if ≥ 1 cm and ≥ 10 portal tracts. Pathology was reported using the NASH Clinical Research system (F0: no fibrosis; F1: perisinusoidal or portal; F2: perisinusoidal and portal; F3: bridging; and F4: cirrhosis [14]). All biopsies were interpreted primarily, but not exclusively, by hepatology trained pathologists. Fibrosis staging ranged from 0 to 4 (0 to 4: F0 = absence of fibrosis; F1 = portal or perisinusoidal fibrosis; F2 = portal/ periportal and perisinusoidal; F3 = bridging fibrosis; and F4 = cirrhosis). If fibrosis staging was listed as a range, the higher stage was chosen for consistency (F2-3 was classified as F3).
Statistical analysis
Data were analyzed as both continuous and categorical variables. All continuous variables were expressed as means ± standard deviation (SD) and categorical ones as numbers or percentages. Categorial variables were analyzed using Chi-square or Fisher’s exact tests when appropriate. An independent t-test was used for normally distributed continuous variables. In all analyses, the P value < 0.05 was significant. All statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL, version 25.0 for windows).
| Results | ▴Top |
Demographics
Of the 1,153 referrals, 1,115 (97%) met MASLD criteria with complete data to calculate FIB-4 (Table 1). The average age was 53.8 ± 14 years old. Most (74.3%) were young and female (68%). Ethnic groups included non-Hispanic White (84%), 8% Black, 6% Hispanic and other (1%). Metabolic comorbidities including hypertension, dyslipidemia, and diabetes were noted in 57%, 57% and 39%, respectively. Eighty-four percent of the patients with diabetes also had hypertension, and 74% of patients with dyslipidemia were diabetic. The upper limit of normal ALT and AST (IU/L) in our system is 32 and 40, respectively. The prevalence of an elevated ALT (67.7% vs. 48.3%, P < 0.0001) and AST (34.5% vs. 26.6%, P = 0.005) were greater in men compared to women, respectively. Ninety percent of subjects had CAP scores greater than or equal to 274 dB/m (74% (300+ dB/m) and 54% (330+ dB/m)).
 Click to view | Table 1. Demographics of Entire Cohort and Age Adjusted Low-Risk Groups |
We compared younger and older age-corrected low-risk FIB-4 groups, it was demonstrated that the younger cohort had significantly (P < 0.0001) higher body mass index (BMI), CAP, ALT, and AST values. The proportion of type 2 diabetes, hypertension and dyslipidemia were significantly greater in the older cohort.
FIB-4 overview
Overall, 72% (n = 804) of the entire cohort had an age-corrected low FIB-4 score. The age-corrected low FIB-4 scores were similar in the younger group (73.3%) versus older group (68.4%, P = 0.112), as well as in the intermediate FIB-4 category. FIB-4 > 2.67 was significantly (P = 0.001) noted in the older compared to younger group (14% and 5%, respectively).
Low risk FIB-4 and VCTE results
We evaluated the low-risk FIB-4 scores according to VCTE categories of LSM. For the entire cohort, 257 (32%) patients with low-risk FIB-4 scores had LSM values greater than or equal to 8 kPa. Low, intermediate, and high age-corrected FIB-4 were compared to low, significant, and advanced LSM classification categories. FIB-4 categories were significantly (P < 0.01) differentiated in the low and advanced LSM groups in a stepwise decrease and increase fashion, respectively (Fig. 1a). In the older group, similar significant (P < 0.05) stepwise differences were noted in the low and advanced LSM categories (Fig. 1b).
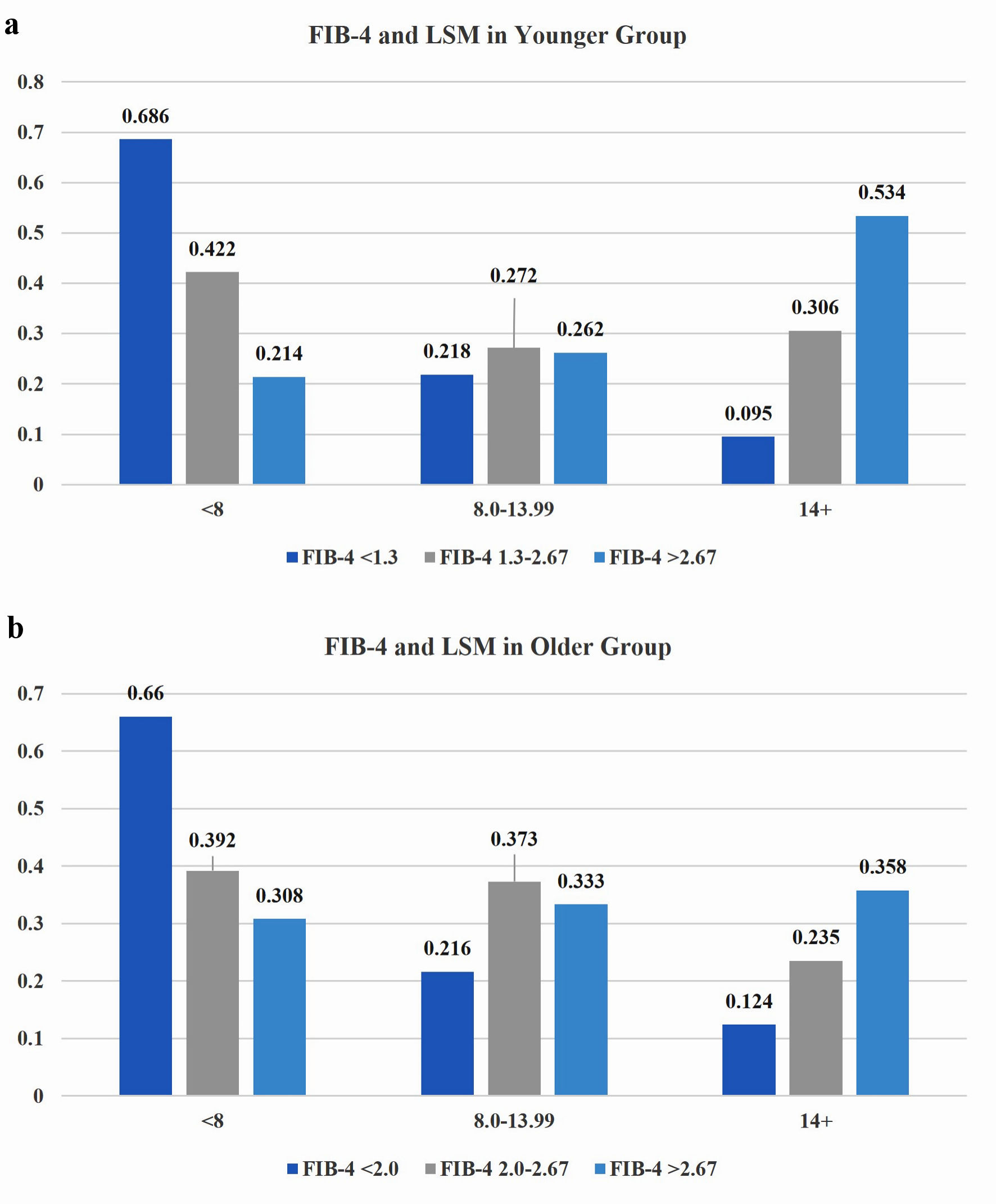 Click for large image | Figure 1. Among young adults, 31.4% (a), and among older adults, 34% (b), with a low-risk FIB-4, have significant to advanced LSM values. There is a significant stepwise decrease for FIB-4 scores for LSM < 8 kPa and increase in FIB-4 score for LSM 14+ kPa in both the young and old adult cohorts. The 8 - 13.99 kPa group have an equal distribution of FIB-4 scores in both age groups. LSM: liver stiffness measurement; FIB-4: fibrosis-4 index. |
Low risk FIB-4 and VCTE with and without diabetes
In our entire cohort, diabetic subjects showed a trend towards significantly (P = 0.08) higher LSM compared to non-diabetic subjects (Fig. 2a). However, no significant differences were noted when young or older diabetic subjects were compared with non-diabetic subjects with low FIB-4 values when categorized according to the five LSM categories (Fig. 2b, c).
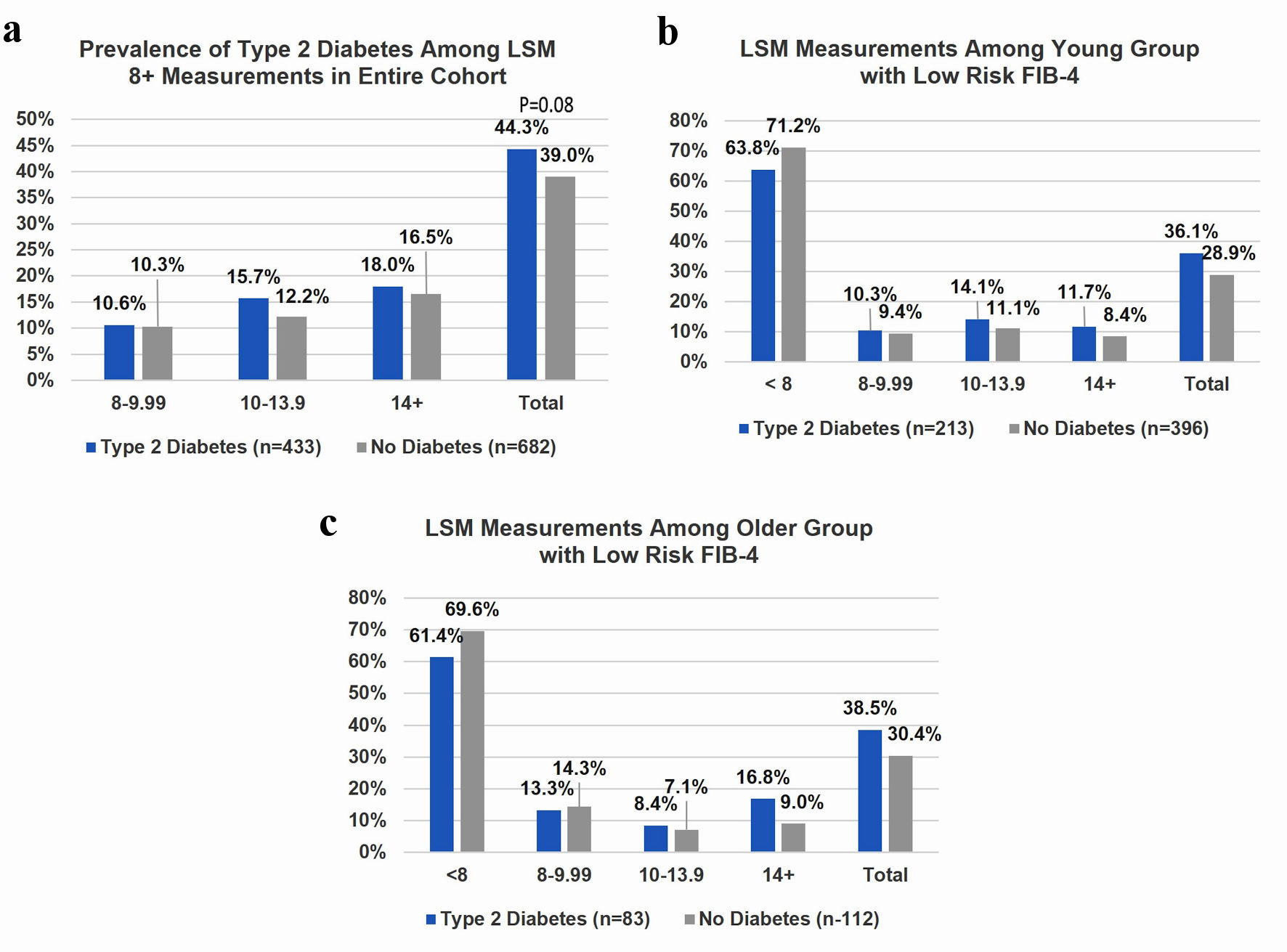 Click for large image | Figure 2. (a) In the entire cohort of subjects (n = 1,115) with and without diabetes and significant LSM (8+ kPa), no statistical differences were noted in either the total (P = 0.08) or any of the individual LSM categories. (b) Thirty-one point four percent of young adults with a low-risk FIB-4 (n = 609) have significant to advanced LSM values, and the proportion was similar to older group. There was no significant difference between LSM measurements for younger diabetic and non-diabetic patients. (c) One hundred ninety-five older patients had low risk FIB-4 scores, and 34% of older subjects with a low-risk FIB-4 have significant to advanced LSM values. Older diabetic patients were more likely to have 14+ kPa despite a low risk FIB4 score. LSM: liver stiffness measurement; FIB-4: fibrosis-4 index. |
NIT and liver histology in subjects with and without diabetes
Diabetes was present in 56% of men and 28% of women with similar proportions in younger and older groups. However, most (64%) individuals with significant fibrosis (F2-4) were diabetic women.
Totally, 257 subjects who had LSM greater than or equal to 8 kPa and 148 (48.6%) underwent a liver biopsy, and 45% (n = 67) of the biopsies were from diabetic patients (Fig. 3a). Significant fibrosis (F2-4) was identified in 72 (48.6%) individuals (Fig. 3a). Seventy-six point nine percent of subjects with advanced fibrosis and 57.6% with stage 2 fibrosis had the diagnosis of type 2 diabetes (Fig. 3b). A significantly (P = 0.0001) greater proportion of diabetic (41.8%) compared to non-diabetic (13.6%) subjects had advanced fibrosis (Fig. 3c). A higher but non-significant (P = 0.11) prevalence of stage 2 fibrosis was also noted in diabetic (28.4%) compared to non-diabetic (17.3%) subjects.
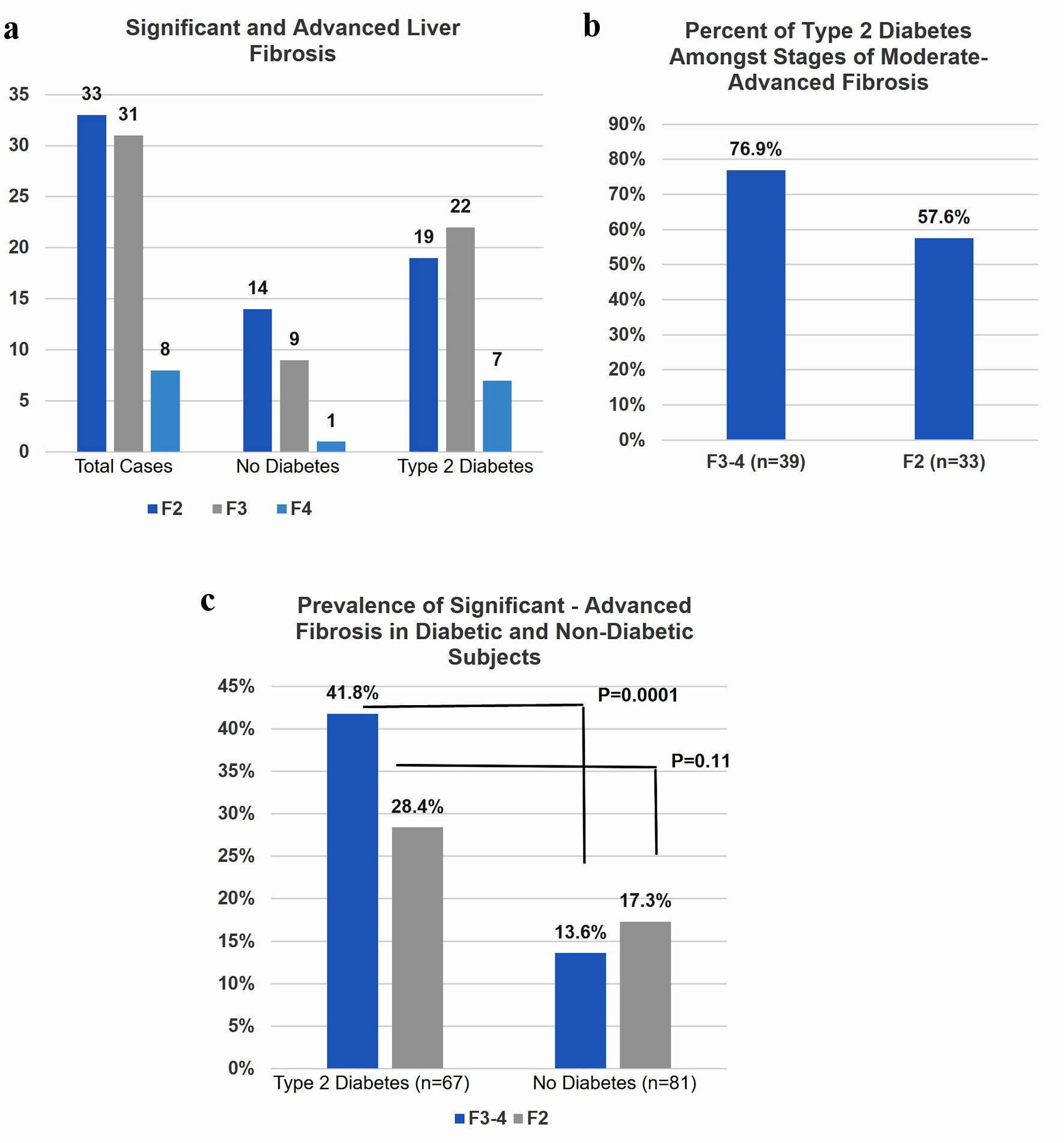 Click for large image | Figure 3. (a) Seventy-two subjects underwent liver biopsy had F2+. (b) The majority of both F2 and F3-4 subjects had type 2 diabetes. (c) Diabetic patients were significantly more likely to have F3 and F4 on biopsy despite low risk FIB4 score compared to non-diabetic patients. F2: significant fibrosis; F3: advanced fibrosis; F4: cirrhosis. |
| Discussion | ▴Top |
Based upon AGA guidelines, 30% of our subjects with significant LSM (8+ kPa) values did not meet criteria for a FibroScan referral resulting in missed opportunities for warranting close follow-up, MR elastography and/or a liver biopsy. We did not observe any significant differences in low-risk FIB-4 scores and LSM in diabetic subjects compared to those without diabetes. Liver histology did show a three-fold increase for advanced fibrosis in diabetic compared to non-diabetic subjects. Notably, 77% of individuals with advanced fibrosis and 57% with significant fibrosis had type 2 diabetes. Taken together, our data are in accordance with the recent algorithm put forth by Boursier et al [15], which directly refer MASLD diabetic subjects for a FibroScan bypassing FIB-4 evaluation.
Approximately, 72% of our referrals had a low FIB-4 score not meeting criteria for a FibroScan. A similar result (73.8%) was noted in a previous study screening 695 MASLD European subjects [16]. Recently, a higher (83%) prevalence of low-risk FIB-4 values was demonstrated in a US screening population [17]. This difference is likely due to our cohort biased towards high-risk individuals for significant to advanced fibrosis compared to screening the general population. In contrast, a lower prevalence (57%) of low FIB-4 was observed in screening 591 type 2 diabetes [6]. African Americans have been demonstrated to have a higher mean FIB-4 than non-Hispanic White individuals, and this may explain the discrepancy since their study had a greater prevalence (30%) of African Americans compared to our cohort (8%) [18].
Our FIB-4 results were greater (P = 0.04) in patients with type 2 diabetes (1.39 ± 0.9) than those without diabetes (1.27 ± 1.0), and this is consistent with other recent studies [15, 19]. FIB-4 is most useful for ruling out significant-to-advanced fibrosis. Liver stiffness cut-off values of ≥ 8.6 kPa has a 70% NPV for F2-4, and a cut-off value of ≥ 9.7 kPa has 80% NPV for F3-4 [20, 21]. We also observed similar values of 72% NPV for < 8 kPa and 80% NPV for < 10 kPa.
A recent meta-analysis by Mozes et al [22] demonstrated that LSM under 8 kPa can rule out advanced fibrosis, and values greater than 20 kPa rule in cirrhosis. Our results show 30% of individuals with low-risk FIB-4 had LSM values of 8+ kPa thereby inhibiting the ability to rule out advanced fibrosis in a significant proportion of the cohort. Our results are similar to a European/Asian study that noted a large proportion (43%) of subjects with a low FIB-4 also had LMS ≥ 8 kPa [11]. Boursier et al [15] also demonstrated a lower area under the receiver operating characteristic (ROC) curve (AUC) (0.722) for FIB-4 in detecting advanced fibrosis in diabetic subjects than non-diabetic subjects (0.819), which is consistent with our low-risk FIB-4 inaccuracy for assessing significant liver stiffness and fibrosis. In contrast, another European cohort (n = 1,799) reported a lower proportion (17.1%) with LSM values > 9.6 kPa compared to our 25% [12]. We suspect that this could be explained by a much lower criteria for MASLD (CAP ≥ 222 dB/m), and only 38% of their cohort had a CAP ≥ 290 compared to a CAP ≥ 300 dB/m in 74% of our subjects. Also, the prevalence of an elevated ALT as defined by their study (men > 30 U/L and women > 19 U/L) was significantly greater than that in our cohort (men 67% vs. 30% and women 80% vs. 21%), consistent with a greater at-risk population for higher elevated LSMs [12].
The entire cohort of diabetic individuals compared to non-diabetic ones had higher LSM values, but this was not observed when applying FIB-4 scores to these individuals. Liver stiffness median (IQR) measurements in diabetic subjects 7.3 (5.1 - 12.1) trended towards significance (P = 0.05) compared to non-diabetic subjects 6.7 (5.1 - 10.5). In contrast, a retrospective European study (n = 1,051) demonstrated a significantly higher median kPa in diabetics (10.4) compared to non-diabetic patients (6.9) [15]. The higher LSM values can be explained by their cohort having significantly more men, patients with type 2 diabetes, and a greater proportion of biopsy proven significant F2-4 (66%) and advanced F3/4 (39%) fibrosis, compared to F2-4 (49%) and F3/4 (26%) noted in our study [15].
The positive predictive value (PPV) for F3-4 fibrosis ranges from 45% to 75% and for F2-4 fibrosis 53-90%, using various FibroScan kilopascal cut-offs [13]. Approximately 50% (n = 148) of our subjects with LSM 8+ kPa underwent liver biopsy and the PPV was 49% for F2-4 fibrosis. Similarly, several prior studies that focused on solely diabetic patients have identified VCTE as a successful initial screening tool for fibrosis in diabetes as 13-18% of patients had elevated VCTE measurements, and about 50% of patients undergoing biopsy revealed advanced fibrosis [12, 23].
Overall, our diabetic subjects were 3- and 1.6-fold more likely than non-diabetic subjects to demonstrate advanced and significant fibrosis, respectively. Although FIB-4 adequately eliminates most individuals with significant to advanced fibrosis, diabetic individuals are significantly over-represented. As suggested by several authors, diabetic MASLD subjects are best served with FibroScan as a noninvasive, safe, and relatively inexpensive screening tool primarily to exclude advanced liver fibrosis [15, 24-26]. Additional serology such as the enhanced liver fibrosis (ELF) test or FibroMeter VCTE can be an additional noninvasive step after VCTE to minimize biopsies [15, 27]. Although noninvasive tests are primarily used to screen for advanced fibrosis, significant (F2) fibrosis is important to identify in diabetic subjects due to a recent MASH Clinical Research Network study that demonstrated a greater proportion of diabetic (26%) than non-diabetic subjects (14%) will progress from F0-2 to F3-4 over a shorter timeframe, further supporting VCTE referrals for diabetic individuals [19]. Finally, our data are in line with the 2023 AGA Clinical Practice Update in MASLD subjects with pre-diabetes/type 2 diabetes, or two metabolic risk factors stating that either FibroScan or ELF test should be the initial screening test for liver fibrosis, in lieu of FIB-4 testing [28].
Strengths and limitations
The overall sample size is large and represents referrals from primary care and specialists to a tertiary integrated hospital system; however, our cohort may not be representative of the general population. Our liver center has years of experience performing FibroScan, and subjects were identified from a prospectively established MASLD database. The data were retrospectively analyzed, and disadvantages include propensity for inconsistencies, lack of controlled conditions, and measurements under different conditions. Liver biopsy sampling variability could limit staging fibrosis. Clinical indications for obtaining a liver biopsy were from subjects interested in participating in a clinical trial or standard of care. Biopsies were interpreted by several pathologists and although this limits consistency, it is representative of pathologic interpretations in the general community.
Conclusions
Primary care providers should be aware of the increased risk of liver fibrosis in MASLD patients with diabetes. Ongoing community education of the 2023 AGA MASLD guidelines is necessary to mitigate unnecessary referrals, as well as correctly utilize the noninvasive tests for diabetic individuals. FibroScan cost and availability can be limiting factors for patients therefore, increasing point of care FibroScan access within primary and specialty offices (endocrine, gastrointestinal (GI)) can provide convenient point of care service and is relatively inexpensive.
Acknowledgments
The authors would like to extend special thanks to our office research coordinators Alicia Jones, Donna West and Dede Coffi, Prisma Health Gastroenterology and Liver Center, Greenville, SC, for assistance with conducting FibroScan studies.
Financial Disclosure
None to declare.
Conflict of Interest
J.S.W. has no financial disclosures. G.A.A. has no financial disclosures. No funding was received for this study.
Informed Consent
Informed consent was waived by the IRB.
Author Contributions
J.S.W. planned the study, reviewed charts and collected data, wrote initial manuscript draft. G.A.A. conceived the study, completed statistical analysis, reviewed data, and reviewed and edited the final manuscript. Both authors approve the final version of the manuscript. J.S.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524-530.e521.
doi pubmed - Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851-861.
doi pubmed - Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387-1395.
doi pubmed - Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5-10.
doi pubmed - Tamaki N, Higuchi M, Kurosaki M, Loomba R, Izumi N, MRCH Liver Study Group. Risk difference of liver-related and cardiovascular events by liver fibrosis status in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022;20(5):1171-1173.e1172.
doi pubmed pmc - Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, Portillo Romero J, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399-406.
doi pubmed pmc - Castera L, Laouenan C, Vallet-Pichard A, Vidal-Trecan T, Manchon P, Paradis V, Roulot D, et al. High prevalence of NASH and advanced fibrosis in type 2 diabetes: a prospective study of 330 outpatients undergoing liver biopsies for elevated ALT, using a low threshold. Diabetes Care. 2023;46(7):1354-1362.
doi pubmed - Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, Asch SM, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):808-819.
doi pubmed - Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161(5):1657-1669.
doi pubmed pmc - McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740-751.
doi pubmed pmc - Graupera I, Thiele M, Serra-Burriel M, Caballeria L, Roulot D, Wong GL, Fabrellas N, et al. Low accuracy of FIB-4 and NAFLD fibrosis scores for screening for liver fibrosis in the population. Clin Gastroenterol Hepatol. 2022;20(11):2567-2576.e2566.
doi pubmed - Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359-1368.
doi pubmed - Zhang X, Wong GL, Wong VW. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(2):128-141.
doi pubmed pmc - Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321.
doi pubmed - Boursier J, Canivet CM, Costentin C, Lannes A, Delamarre A, Sturm N, Le Bail B, et al. Impact of type 2 diabetes on the accuracy of noninvasive tests of liver fibrosis with resulting clinical implications. Clin Gastroenterol Hepatol. 2023;21(5):1243-1251.e1212.
doi pubmed - Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, Suri D, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371-378.
doi pubmed - Udompap P, Therneau TM, Canning RE, Benson JT, Allen AM. Performance of American Gastroenterological Association Clinical Care Pathway for the risk stratification of patients with nonalcoholic fatty liver disease in the US population. Hepatology. 2023;77(3):931-941.
doi pubmed - Kim RG, Chu JN, Vittinghoff E, Deng J, Reaso JN, Grenert JP, Khalili M. Racial/ethnic differences in fibrosis prevalence and progression in biopsy-proven steatosis: A focus on the Asian American population. Hepatol Commun. 2022;6(11):3024-3035.
doi pubmed pmc - Huang DQ, Wilson LA, Behling C, Kleiner DE, Kowdley KV, Dasarathy S, Amangurbanova M, et al. Fibrosis progression rate in biopsy-proven nonalcoholic fatty liver disease among people with diabetes versus people without diabetes: a multicenter study. Gastroenterology. 2023;165(2):463-472.e465.
doi pubmed pmc - Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156-163.e152.
doi pubmed pmc - Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717-1730.
doi pubmed - Mozes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, Fournier C, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71(5):1006-1019.
doi pubmed pmc - Roulot D, Roudot-Thoraval F, G NK, Kouacou N, Costes JL, Elourimi G, Le Clesiau H, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int. 2017;37(12):1897-1906.
doi pubmed - Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419-430.
doi pubmed - Castera L. Non-invasive tests for liver fibrosis in NAFLD: Creating pathways between primary healthcare and liver clinics. Liver Int. 2020;40(Suppl 1):77-81.
doi pubmed - Pennisi G, Enea M, Falco V, Aithal GP, Palaniyappan N, Yilmaz Y, Boursier J, et al. Noninvasive assessment of liver disease severity in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes. Hepatology. 2023;78(1):195-211.
doi pubmed - Canivet CM, Costentin C, Irvine KM, Delamarre A, Lannes A, Sturm N, Oberti F, et al. Validation of the new 2021 EASL algorithm for the noninvasive diagnosis of advanced fibrosis in NAFLD. Hepatology. 2023;77(3):920-930.
doi pubmed - Wattacheril JJ, Abdelmalek MF, Lim JK, Sanyal AJ. AGA clinical practice update on the role of noninvasive biomarkers in the evaluation and management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2023;165(4):1080-1088.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
