| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 13, Number 3, August 2023, pages 126-133
Successful Heart Rate Management of Atrial Fibrillation Due to Thyroid Storm Using Intravenous Amiodarone
Mimori Abea, Yuichiro Matsuob, e, Masao Horiuchic, Koichi Kitamurad, Toshihiko Suzukid
aDepartment of General Pediatrics, Tokyo Metropolitan Children’s Medical Center, Tokyo 183-8561, Japan
bDepartment of Clinical Epidemiology and Health Economics, School of Public Health, The University of Tokyo, Tokyo 113-8655, Japan
cDepartment of Internal Medicine, Tokyo-Bay Urayasu Ichikawa Medical Center, Chiba 279-0001, Japan
dDepartment of Nephrology, Endocrinology, and Diabetes, Tokyo-Bay Urayasu Ichikawa Medical Center, Chiba 279-0001, Japan
eCorresponding Author: Yuichiro Matsuo, Department of Clinical Epidemiology and Health Economics, School of Public Health, The University of Tokyo, Tokyo 113-8655, Japan
Manuscript submitted June 24, 2023, accepted August 2, 2023, published online August 25, 2023
Short title: Intravenous Amiodarone for Thyroid Storm
doi: https://doi.org/10.14740/jem887
| Abstract | ▴Top |
Intravenous amiodarone is commonly used for heart rate control in patients with atrial fibrillation who are at risk of hemodynamic instability; however, its administration in patients with severe thyroid dysfunction is often avoided because of its known thyroid toxicity. Furthermore, the existing literature focusing on this topic is limited. Here, we present a case of thyroid storm, atrial fibrillation, and reduced left ventricular systolic function, whose rapid heart rate was successfully controlled using intravenous amiodarone in conjunction with antithyroid drugs. An 83-year-old man presented to the emergency department with worsening dyspnea for over 1 week and was diagnosed with impending thyroid storm and heart failure with reduced ejection fraction. Treatment with thiamazole, potassium iodine, hydrocortisone, and intravenous landiolol, a short-acting beta-blocker, was initiated. Although the patient initially had a sinus rhythm, he developed atrial fibrillation with a rapid ventricular response associated with low blood pressure. An increased dose of landiolol further lowered blood pressure but failed to control the heart rate. Therefore, intravenous amiodarone was initiated, leading to a reduction in heart rate, without causing further hemodynamic instability, and an eventual return to sinus rhythm. There was no recurrence of atrial fibrillation, and the intravenous amiodarone was discontinued 48 h after administration. The final diagnosis was thyroid storm caused by painless thyroiditis. He was transferred to a long-term care hospital on day 69. Theoretical evidence suggests that amiodarone used in combination with antithyroid drugs may mitigate the potential thyrotoxicity, rendering it a favorable choice for patients with thyroid storm and atrial fibrillation. Hence, intravenous amiodarone may be considered for heart rate control in patients with atrial fibrillation if the initial therapy for thyroid storm with beta-blockers fails. However, further research and clinical studies are required to validate the safety and efficacy of this approach.
Keywords: Thyroid storm; Atrial fibrillation; Heart failure; Amiodarone
| Introduction | ▴Top |
Atrial fibrillation is a common complication of hyperthyroidism, affecting approximately 10% of hospitalized patients [1]. The prevalence of atrial fibrillation increases with severe hyperthyroidism, with approximately 30% of patients presenting with atrial fibrillation during thyroid storm [2, 3]. Beta-blockers are the preferred drugs for controlling heart rate in atrial fibrillation associated with hyperthyroidism, including thyroid storm [4]. They reduce excessive adrenergic activation and alleviate signs and symptoms induced by thyroid storm, rendering them a key treatment for thyroid storm itself [4]. Therefore, the use of beta-blockers to control the heart rate in patients with atrial fibrillation complicated by thyroid storm is a physiologically reasonable approach.
However, in patients with thyroid storm, development of decompensated heart failure, including those with reduced left ventricular systolic function, is also common [3, 5]. In such cases, beta-blockers should be cautiously administered owing to the risk of cardiac collapse [6, 7]. In situations unrelated with thyroid storm, when patients with atrial fibrillation present with decompensated heart failure or reduced left ventricular systolic function, intravenous amiodarone is a drug of choice for acute heart rate control [8], because of its effectiveness [9] and its relatively mild negative inotropic effect [10].
However, amiodarone is associated with thyroid toxicities [11], including amiodarone induced thyroid storm [5]. Even though some authors have suggested that the thyroid toxicity of amiodarone should not preclude its use in patients with thyrotoxic atrial fibrillation, the new use of amiodarone in patients with thyroid storm is often avoided because of concerns about exacerbating the thyrotoxic state [12, 13].
However, the existing literature focusing on the use of intravenous amiodarone for acute heart rate control in patients with atrial fibrillation and thyroid storm is limited [14, 15]. Therefore, we present a case of thyroid storm associated with atrial fibrillation and reduced left ventricular systolic function that was successfully treated with intravenous amiodarone in conjunction with antithyroid drugs.
| Case Report | ▴Top |
Investigations
An 83-year-old man presented to the emergency department with a 1-week history of worsening dyspnea. He also experienced fatigue for the past 2 months and unintentional weight loss of 4 kg in 1 month. The patient had no relevant medical history or current medications.
Upon presentation, the patient was alert, and his vital signs were as follows: body temperature of 37.1 °C, blood pressure of 121/74 mm Hg, heart rate of 135 beats/min in sinus rhythm, respiratory rate of 30/min, and 98% oxygen saturation on ambient air. The neck examination by palpation revealed no goiter or tenderness. The lung sounds were bilaterally diminished, and a systolic murmur of 2/6 was present at the apex. The laboratory test results are listed in Table 1. The significant findings were hyperthyroidism and increased serum brain natriuretic peptide levels. Chest radiography revealed pulmonary congestion and bilateral pleural effusion (Fig. 1a) and electrocardiogram showed sinus tachycardia (Fig. 2a). Echocardiography revealed a dilated left ventricle with a reduced left ventricular ejection fraction of 20% and moderate mitral regurgitation (Fig. 3a). Thyroid ultrasonography revealed a normoechoic, normal-sized thyroid with increased blood flow within the thyroid gland; no thyroid nodules were present.
 Click to view | Table 1. Laboratory Test Results on the Day of Admission |
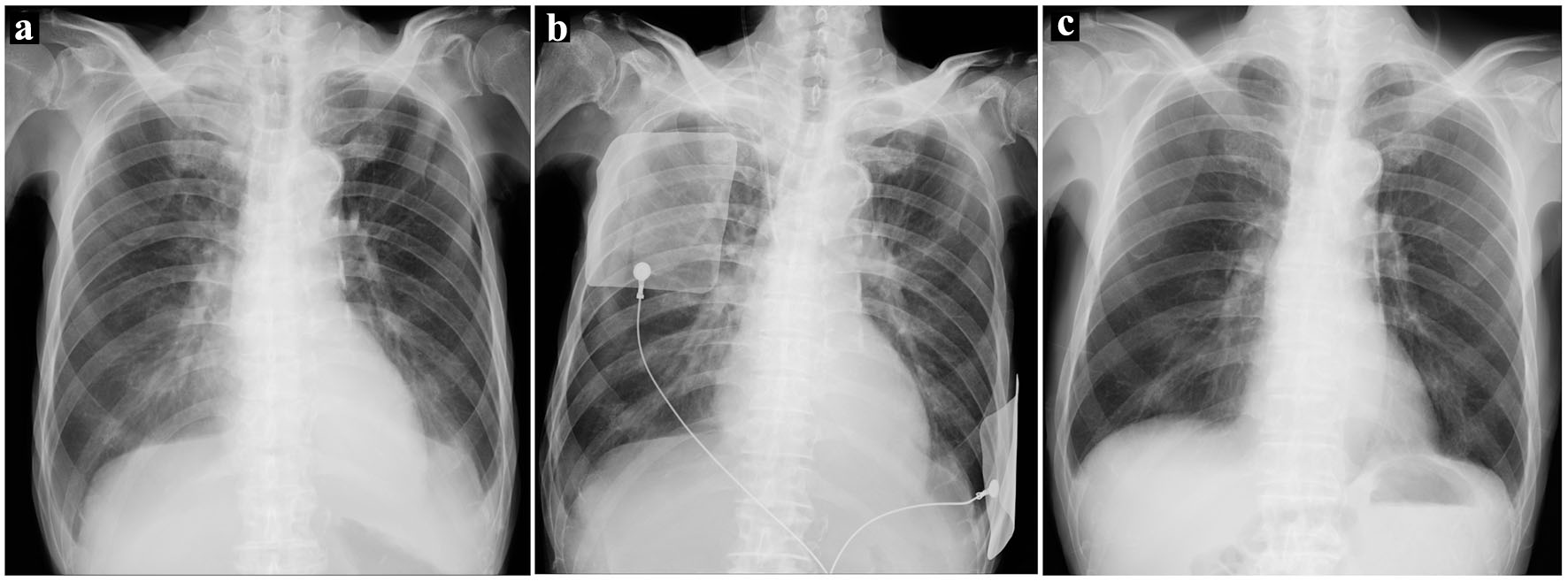 Click for large image | Figure 1. (a) Chest radiography on admission showing pulmonary congestion and bilateral pleural effusion. (b) Chest radiography when the patient developed atrial fibrillation. Pulmonary congestion was more prominent than on admission. (c) Chest radiography before discharge. Pulmonary congestion was resolved, and pleural effusion was absent. |
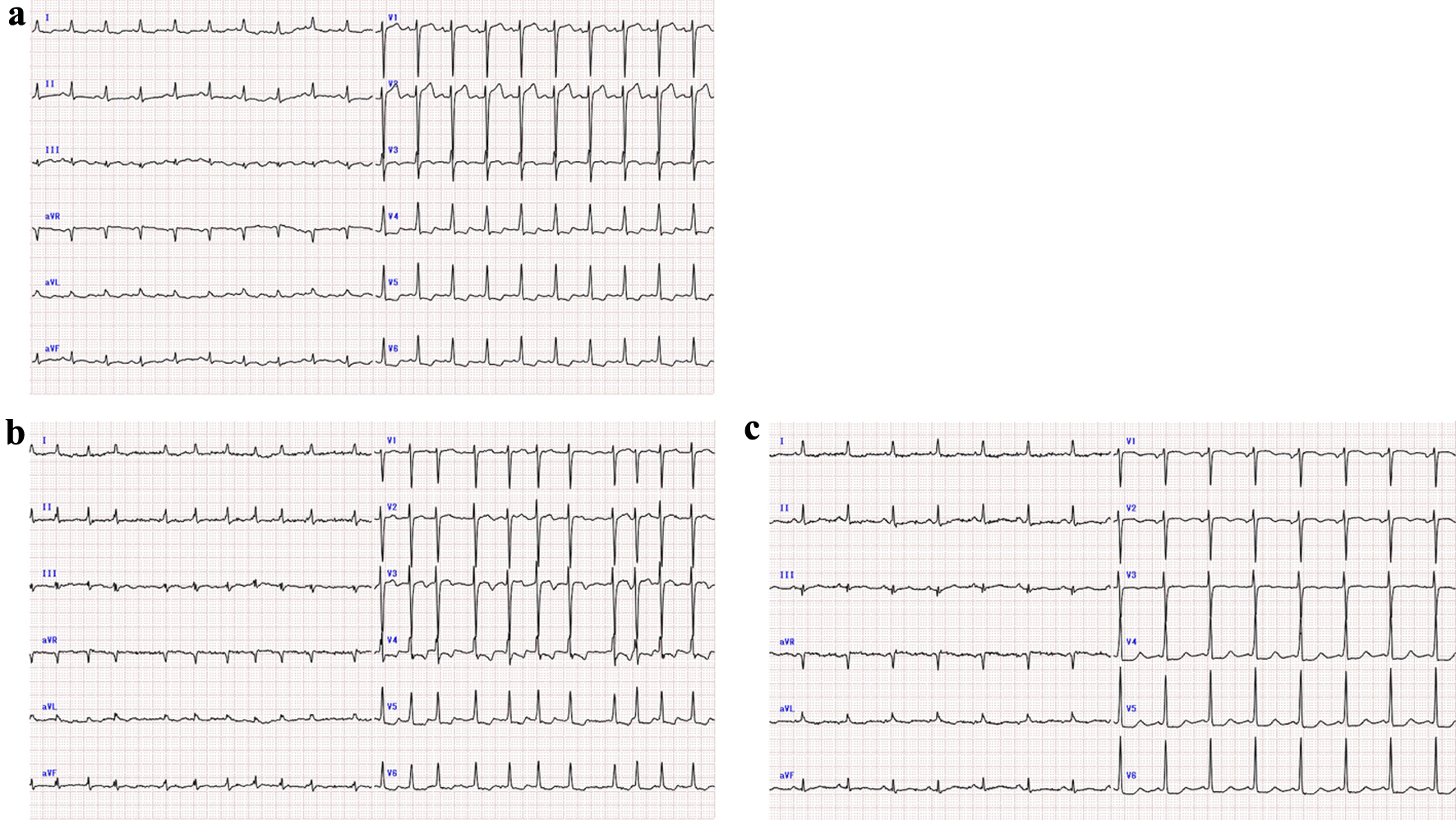 Click for large image | Figure 2. (a) Electrocardiogram on admission (heart rate 119 beats per minute, sinus rhythm). (b) Electrocardiogram when the patient developed atrial fibrillation (heart rate 130 beats per minute). (c) Electrocardiogram after conversion to sinus rhythm (heart rate 91 beats per minute). |
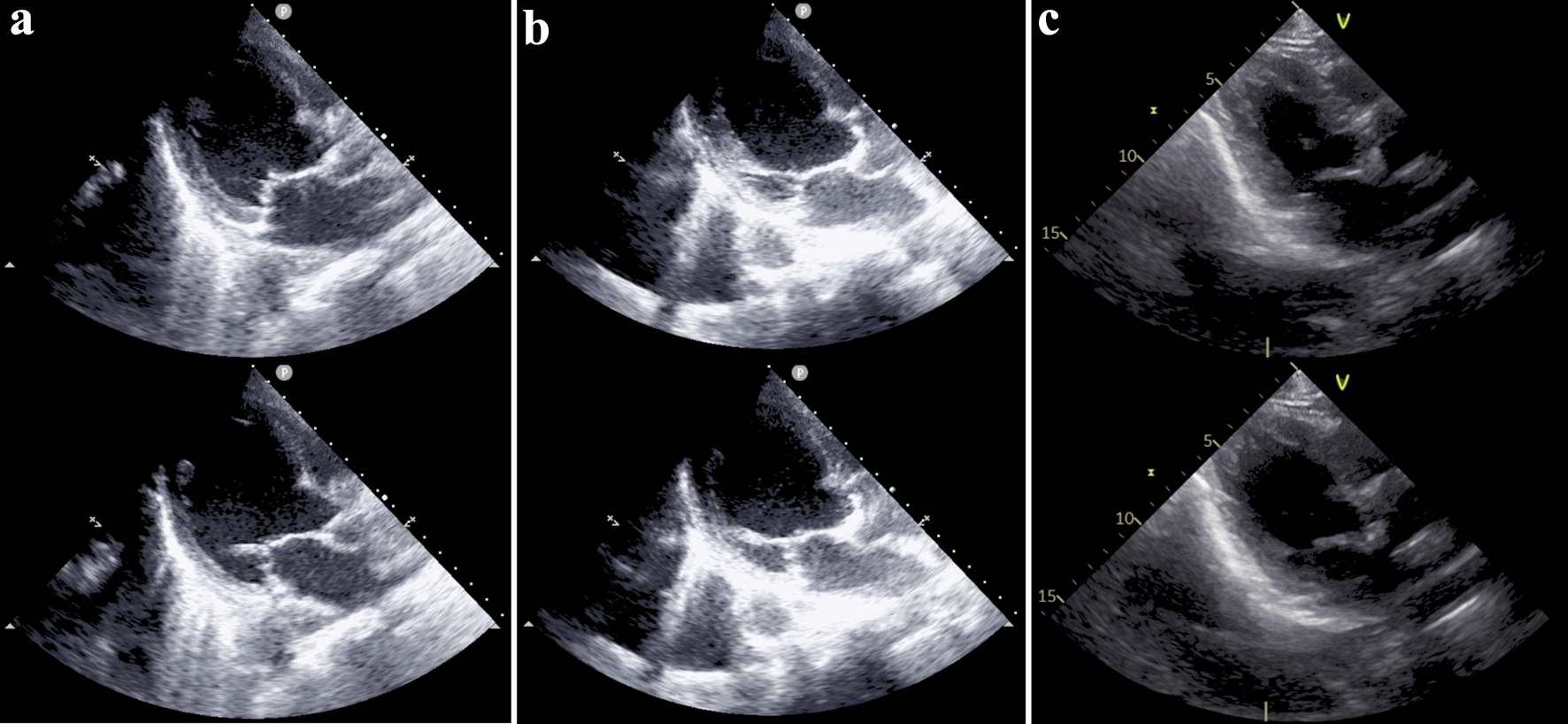 Click for large image | Figure 3. (a) Bedside echocardiogram performed on admission showing a dilated left ventricle and reduced left ventricular systolic function. Ejection fraction was visually estimated to be 20%. (b) Bedside echocardiogram performed when the patient developed atrial fibrillation. The reduced left ventricular systolic function remained unchanged. (c) Echocardiogram performed after recovery from the acute phase. Dilation and systolic function of the left ventricle were improved, and the ejection fraction calculated by Simpson method was 43%. In each column, the upper image represents the end-systolic phase, and the lower image represents the end-diastolic phase. |
Diagnosis
A diagnosis of thyrotoxicosis complicated by heart failure was made; however, the underlying thyroid disease was unclear at this point. The reduced left ejection fraction was assumed to result from tachycardia-induced cardiomyopathy. The patient scored 35 points on the Burch-Wartofsky Point Scale [16], which was suggestive of an impending thyroid storm. In accordance with the diagnostic criteria for thyroid storm established by the Japan Thyroid Association [4], two symptom criteria (tachycardia, congestive heart failure) were fulfilled, suggesting a suspected thyroid storm.
Treatment
Treatment was initiated in accordance with thyroid storm management [4] with a combination of oral thiamazole at a dose of 10 mg three times daily, oral potassium iodine at a dose of 50 mg twice daily, intravenous hydrocortisone at a dose of 100 mg three times daily, and intravenous landiolol, a short-acting beta-blocker. Thiamazole was chosen as the antithyroid drug, as both thiamazole and propylthiouracil are recommended as first-line drugs in the guidelines for the management of thyroid storm by the Japan Thyroid Association and Japan Endocrine Society [4]. Landiolol was initiated at a dose of 2 µg/kg/min and increased to 4 µg/kg/min based on the heart rate. The heart rate was controlled at approximately 110 beats/min with landiolol, and blood pressure was 110/70 mm Hg.
On the night of admission (16 h after hospitalization), the patient developed atrial fibrillation. His heart rate increased to 130 - 160 beats/min (Fig. 2b), and blood pressure decreased to 80/65 mm Hg. Chest radiography showed more prominent pulmonary congestion than on admission (Fig. 1b). Bedside echocardiography was conducted, revealing unchanged reduced left ventricular systolic function and moderate mitral regurgitation (Fig. 3b). Due to hemodynamic instability, one attempt of electrical cardioversion was made with 150 J, but failed to convert to sinus rhythm. Increasing the dosage of landiolol resulted in a further drop in blood pressure, requiring the administration of norepinephrine; therefore, landiolol administration was withheld. Instead, amiodarone and digoxin were intravenously administered. Amiodarone was introduced with an initial dose of 125 mg, followed by continuous infusion at a rate of 49.5 mg/h, while digoxin was administered at a dose of 0.25 mg. Shortly after the initiation of amiodarone, the tachycardia improved and returned to sinus rhythm 1 h after amiodarone initiation (Fig. 2c), resulting in blood pressure recovery and norepinephrine discontinuation (Fig. 4). Intravenous amiodarone was discontinued after 48 h without switching to oral amiodarone. No recurrence of atrial fibrillation was observed.
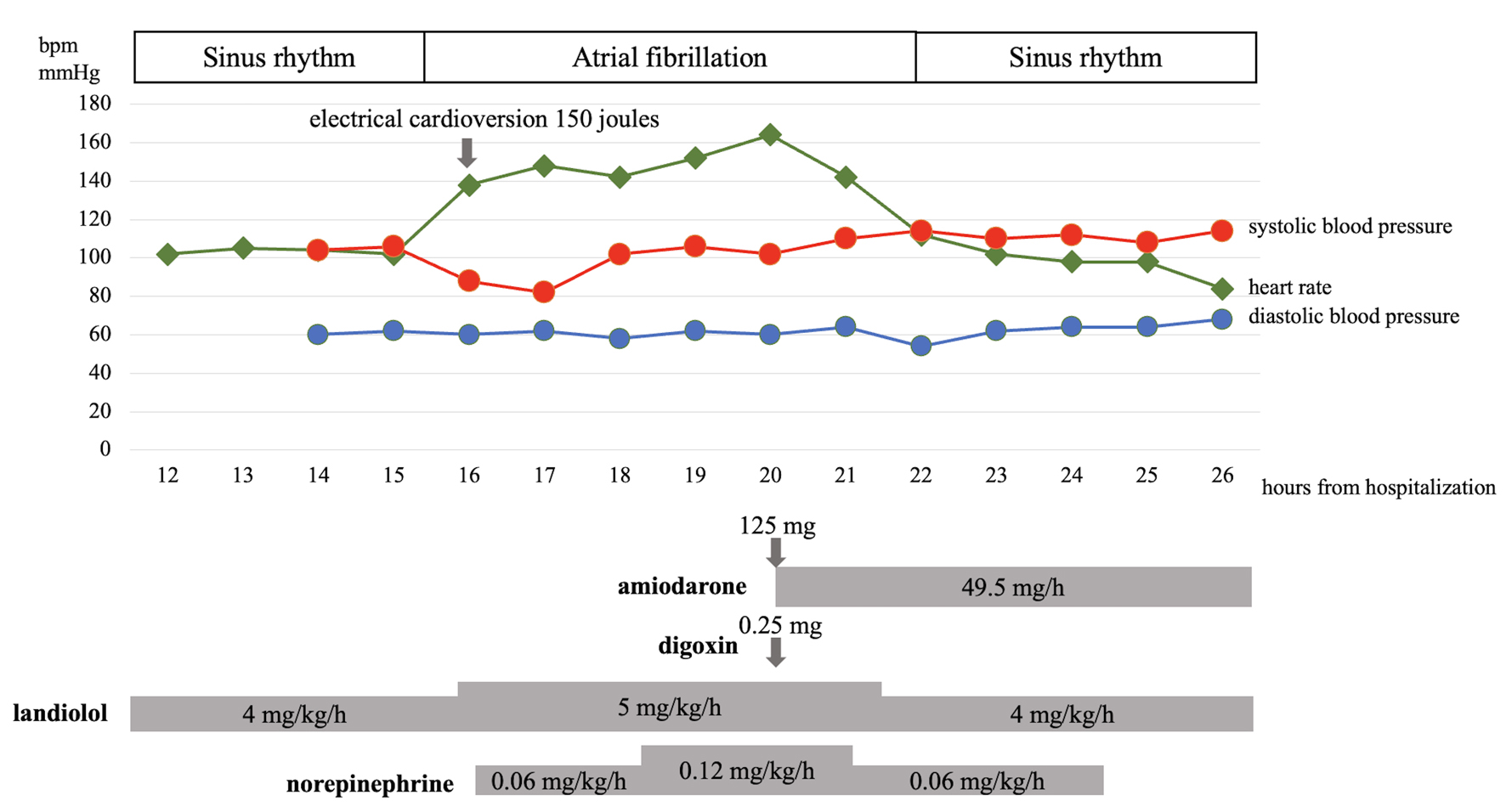 Click for large image | Figure 4. The patient’s heart rate, blood pressure, doses of amiodarone, digoxin, landiolol, and norepinephrine, and timing of electrical cardioversion during the first night of admission. bpm: beats per minute. |
Follow-up and outcomes
Hydrocortisone and potassium iodine were tapered and discontinued, and landiolol was switched to low-dose oral bisoprolol. The temporary use of diuretics improved the patient’s pulmonary congestion (Fig. 1c). Rapid improvement in hyperthyroidism and the subsequent development of a hypothyroid state, which required temporary administration of levothyroxine, were observed (Fig. 5).
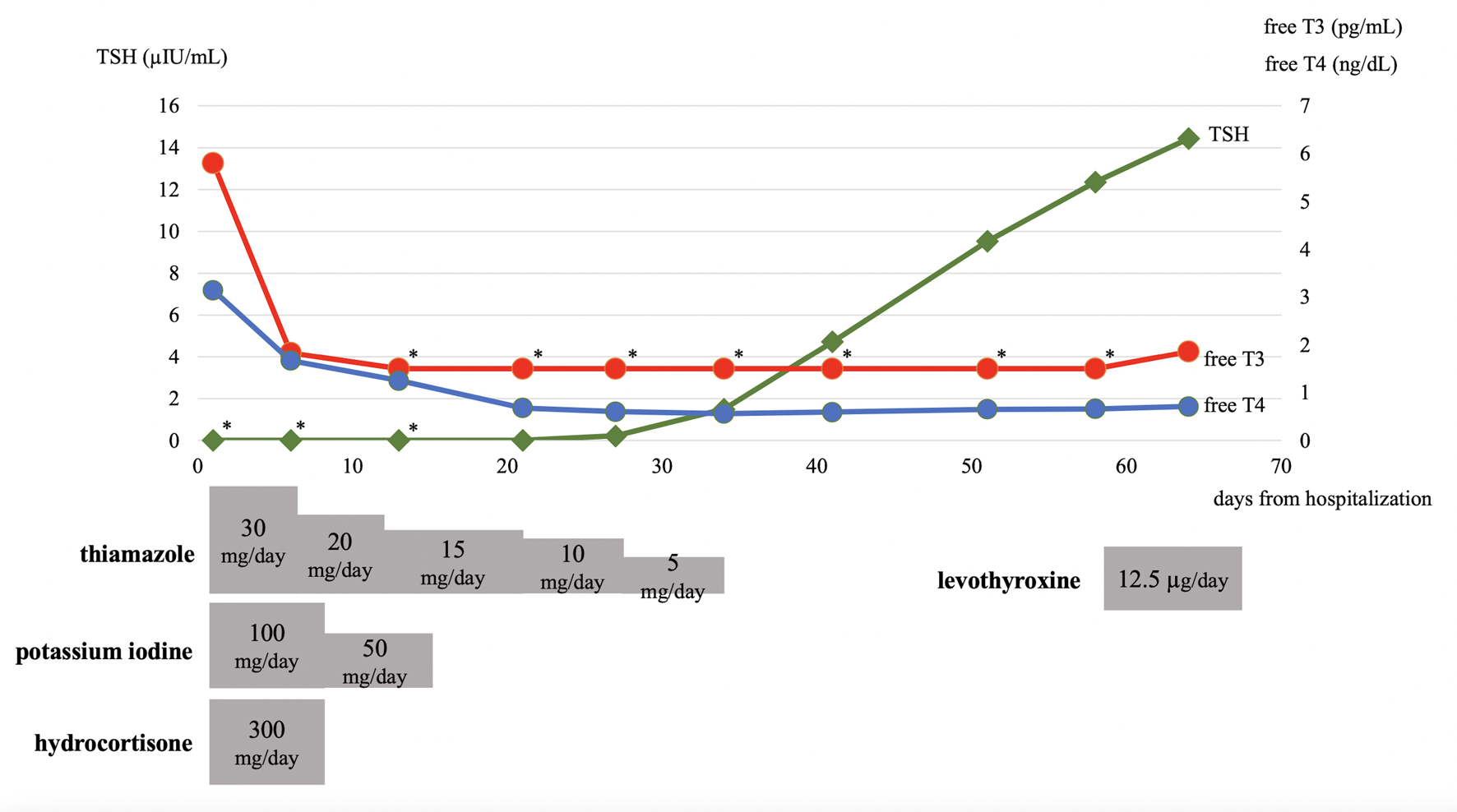 Click for large image | Figure 5. Trends of thyroid function tests and dosages of thiamazole, potassium iodine, hydrocortisone, and levothyroxine during hospitalization are depicted. Asterisks in the thyroid function test graph represent values below the detection limits (0.008 µIU/mL for TSH and 1.50 pg/mL for free T3). free T3: free triiodothyronine; fT4: free thyroxine; TSH: thyroid-stimulating hormone. |
Considering the absence of antithyroid antibodies and the rapid improvement of hyperthyroidism, the etiology of thyrotoxicosis was attributed to destructive thyroiditis rather than excessive thyroid hormone synthesis. The patient did not exhibit typical features of subacute thyroiditis, such as pain or hypoechogenic regions observed on thyroid ultrasonography. Hence, the final diagnosis was established as thyroid storm induced by painless thyroiditis.
The patient recovered from his acute phase but experienced a decline in physical function. Due to the deterioration in swallowing function, he required nasogastric tube feeding from day 8 to day 37. In addition, he developed delirium, which necessitated the occasional use of antipsychotic drugs. These conditions gradually improved, and he was transferred to a long-term care hospital on day 69. At the time of discharge, he regained his ability of orally intaking meals, and was able to ambulate with the use of walking aids. Following discharge, his thyroid function normalized, and levothyroxine was discontinued. The patient remained euthyroid for 18 months after discharge. Concurrently, there was improvement in his left ventricular systolic function, with an ejection fraction of 43% (Fig. 3c). The mildly reduced left ventricular function suggested the presence of structural heart disease, such as coronary artery disease. However, further investigations to determine the underlying cause of heart disease, such as coronary artery imaging tests or cardiac stress tests, were not performed due to the patient’s advanced age, impaired physical function, and the absence of cardiac-specific symptoms like shortness of breath or chest pain.
| Discussion | ▴Top |
Here, we reported on a case of thyroid storm complicated by tachycardiac atrial fibrillation and subsequent reduced left ventricular systolic function. Despite attempts to manage the patient’s atrial fibrillation with a beta-blocker alone, effective control was not achieved owing to hemodynamic instability. Heart rate control and hemodynamic stability were successfully achieved following the administration of intravenous amiodarone and digoxin. We posit that the reduction in heart rate and subsequent return to sinus rhythm were primarily attributed to the rapid onset and action of intravenous amiodarone [17], while digoxin, in contrast, has a relatively slower onset and peak of action [18].
Concerns regarding the use of amiodarone in patients with thyroid storm arise from its potential thyrotoxic effects, which are complex and multifactorial. Amiodarone thyrotoxicity involves both the effect of iodine overload (200 mg of amiodarone contains 75 mg of iodine) and the intrinsic effects of amiodarone and its metabolites [11], which may result in both hyperthyroidism and hypothyroidism.
Iodine is a substrate for thyroid synthesis, and its administration may lead to excessive thyroid hormone synthesis, known as Jod-Basedow syndrome, especially in patients with low regular iodine intake [19]. On the other hand, an excessive iodine load leads to transient suppression of thyroid hormone synthesis, known as the Wolff-Chaikoff effect [20]. Patients with autoimmune thyroid disease such as Hashimoto’s disease and Graves’ hyperthyroidism frequently experience continuous suppression of thyroid hormone synthesis and iodine-induced hypothyroidism [21] as a result of “failing to escape” from the Wolff-Chaikoff effect.
The intrinsic effects of amiodarone or its metabolites include anti-thyroid effects through the inhibition of thyroxine (T4) to triiodothyronine (T3) activation [22] and the blockade of T3 binding to its receptors [23], as well as destructive thyroiditis through the direct toxic effect on thyroid follicular cells [24], resulting in thyrotoxicosis.
Several small studies have investigated the efficacy of oral amiodarone for the acute treatment of thyrotoxicosis [25, 26]. One of these studies showed that the use of oral amiodarone in conjunction with antithyroid drugs for 4 weeks resulted in a more rapid reduction in serum thyroid hormone levels and improvement in clinical symptoms than antithyroid drugs alone, without causing major side effects [26]. On the other hand, an equivalent clinical effect may not be achieved with short-term use of intravenous amiodarone. Desethylamiodarone, an amiodarone metabolite, has a stronger effect on blocking T3 from binding to its receptors than amiodarone itself [27]. However, approximately a week of amiodarone administration is required to achieve a plateau for the desethylamiodarone plasma concentration [28]. This might imply that short-term intravenous amiodarone use may be less effective in reducing the thyrotoxic effect compared with oral treatment administered for a longer duration. Nevertheless, short-term use of intravenous amiodarone has also shown to decrease peripheral conversion of T4 to T3 [28], suggesting that it may still have the potential to ameliorate the symptoms caused by thyrotoxicosis when used in conjunction with antithyroid drugs.
However, considering the potential for amiodarone to cause hyperthyroidism, the possibility of exacerbating the thyrotoxic state in thyroid storm should also be taken into account. Long-term oral administration of amiodarone is clearly associated with an elevated risk of amiodarone-induced thyrotoxicosis (AIT) [29, 30]. According to a report, the median onset time of AIT from initiation of amiodarone therapy was 3.5 months for type 1 AIT (AIT due to excessive iodine-induced hormone synthesis), and 30 months for type 2 AIT (destructive thyroiditis due to the direct thyrotoxic effect of amiodarone) [31]. While there have been limited case reports suggesting AIT following short-term intravenous use [32, 33], the causal relationship between short-term intravenous amiodarone use and the onset or exacerbation of thyrotoxicosis has not been thoroughly investigated.
Few reports have focused on the use of intravenous amiodarone for the management of atrial fibrillation during thyroid storm. We searched the MEDLINE database on July 21, 2023, using the keywords ((thyrotoxicosis) OR (thyroid storm)) AND (amiodarone), and identified three case reports that utilized intravenous amiodarone to control tachycardic supraventricular arrythmia, including atrial fibrillation, in patients with thyroid storm [14, 15, 34]. We provide a comprehensive summary of the clinical features, treatment, and outcomes from these case reports, along with the information of our case (Supplementary Material 1, www.jofem.org)
In one report, a patient with an impending thyroid storm and atrial fibrillation resulting from factitious thyrotoxicosis (thyrotoxicosis due to excessive intake of thyroid hormone medication) was effectively managed with intravenous amiodarone in combination with propranolol [14]. In another report, the authors unintentionally used intravenous amiodarone, followed by oral amiodarone, to control multifocal atrial tachycardia complicated with cardiogenic shock in a patient with thyroid storm without realizing that the patient was in thyrotoxic state [15]. They retrospectively confirmed the thyrotoxic state due to Graves’ disease by measuring the thyroid function from day 1 to day 7 after the acute phase. It is noteworthy heart rate control and conversion to sinus rhythm were achieved, along with improvement in the patient’s thyrotoxic state in the acute phase, without administering antithyroid drugs. This improvement was possibly due to the antithyroid effect of amiodarone and its high iodine content. However, as the authors acknowledged, it would have been preferable to administer antithyroid drugs prior to (or simultaneously with) amiodarone administration to prevent excessive thyroid hormone synthesis by iodine overload, if they were aware of the thyrotoxic state. In contrast, in another report, intravenous amiodarone was not immediately effective in controlling the rapid heart rate of atrial fibrillation [34]. Importantly, all three reports did not observe any apparent exacerbation of the thyrotoxic state with the use of intravenous amiodarone. Notably, clinical guidelines retain the option of continuing amiodarone in cases of AIT when alternative approaches for managing life-threatening arrhythmias are unavailable [35].
Based on the above discussion, we suggest that intravenous amiodarone may be considered a treatment choice for the management of patients with atrial fibrillation and thyroid storm; however, there are several caveats. First, the efficacy and safety of intravenous amiodarone in patients with thyroid storm have not been studied in clinical trials or large observational studies. Therefore, beta-blockers should be the first-line drugs [4], and intravenous amiodarone should only be used if beta-blockers are contraindicated or if initial therapy with beta-blockers fails to control the heart rate. Second, antithyroid drugs should be administered before or simultaneously with intravenous amiodarone to prevent excessive thyroid production due to iodine overload. This suggestion is consistent with the guidelines for managing thyroid storms, which recommend administering iodine at least 1 h after [35] or simultaneously [4] with antithyroid drugs. Third, to avoid the thyroid toxicity associated with the long-term use of amiodarone, it would be safer to limit the use of intravenous amiodarone in the acute phase.
In our case of thyroid storm complicated by atrial fibrillation and reduced left ventricular systolic function, short-term intravenous amiodarone treatment was effective. However, further research and clinical studies are required to validate the safety and efficacy of this approach.
| Supplementary Material | ▴Top |
Suppl 1. Summary of the Reported Cases Utilizing Intravenous Amiodarone for the Control of Tachycardic Supraventricular Arrythmia During Thyroid Storm.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Owing to the patient’s health condition and inability to provide consent, written informed consent was obtained from the patient’s family for the publication of this case report, including any accompanying images or other identifiable information.
Author Contributions
MA drafted the original manuscript. YM and the other authors contributed to draft revision. All authors have reviewed and approved the final version of the manuscript and agreed to be accountable for any part of the work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
bpm: beats per minute; free T3: free triiodothyronine; fT4: free thyroxine; TSH: thyroid-stimulating hormone
| References | ▴Top |
- Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004;164(15):1675-1678.
doi pubmed - Waqar Z, Avula S, Shah J, Ali SS. Cardiovascular events in patients with thyroid storm. J Endocr Soc. 2021;5(6):bvab040.
doi pubmed pmc - Akamizu T. Thyroid Storm: A Japanese Perspective. Thyroid. 2018;28(1):32-40.
doi pubmed pmc - Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Kanamoto N, et al. 2016 Guidelines for the management of thyroid storm from the Japan Thyroid Association and Japan Endocrine Society (First edition). Endocr J. 2016;63(12):1025-1064.
doi pubmed - Bourcier S, Coutrot M, Kimmoun A, Sonneville R, de Montmollin E, Persichini R, Schnell D, et al. Thyroid storm in the ICU: a retrospective multicenter study. Crit Care Med. 2020;48(1):83-90.
doi pubmed - Ngo AS, Lung Tan DC. Thyrotoxic heart disease. Resuscitation. 2006;70(2):287-290.
doi pubmed - Bokhari SFH, Sattar H, Abid S, Vohra RR, Sajid S. Cardiovascular collapse secondary to beta-blocker administration in a setting of coexisting thyroid storm and atrial fibrillation: a case report. Cureus. 2022;14(9):e29321.
doi pubmed pmc - Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498.
doi pubmed - Delle Karth G, Geppert A, Neunteufl T, Priglinger U, Haumer M, Gschwandtner M, Siostrzonek P, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med. 2001;29(6):1149-1153.
doi pubmed - van Erven L, Schalij MJ. Amiodarone: an effective antiarrhythmic drug with unusual side effects. Heart. 2010;96(19):1593-1600.
doi pubmed - Trohman RG, Sharma PS, McAninch EA, Bianco AC. Amiodarone and thyroid physiology, pathophysiology, diagnosis and management. Trends Cardiovasc Med. 2019;29(5):285-295.
doi pubmed pmc - Parmar MS. Thyrotoxic atrial fibrillation. MedGenMed. 2005;7(1):74.
pubmed pmc - Obi MF, Namireddy V, Garg Y, Sharma M. Benefit and preference of propranolol over metoprolol in thyrotoxicosis-induced atrial fibrillation: a case report and review of literature. Cureus. 2023;15(1):e34474.
doi pubmed pmc - Aulia D, Ardiany D. The role of amiodarone in post-operative hypothyroidism patient with factitious thyrotoxicosis and atrial fibrillation: A case report. Int J Surg Case Rep. 2023;106:108252.
doi pubmed pmc - Yamamoto H, Monno S, Ohta-Ogo K, Ishibashi-Ueda H, Hashimoto T. Delayed diagnosis of dilated thyrotoxic cardiomyopathy with coexistent multifocal atrial tachycardia: a case report. BMC Cardiovasc Disord. 2021;21(1):124.
doi pubmed pmc - Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993;22(2):263-277.
pubmed - Desai AD, Chun S, Sung RJ. The role of intravenous amiodarone in the management of cardiac arrhythmias. Ann Intern Med. 1997;127(4):294-303.
doi pubmed - Ehle M, Patel C, Giugliano RP. Digoxin: clinical highlights: a review of digoxin and its use in contemporary medicine. Crit Pathw Cardiol. 2011;10(2):93-98.
doi pubmed - Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83-100.
doi pubmed - Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174(2):555-564.
pubmed - Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-Induced hypothyroidism. Thyroid. 2001;11(5):501-510.
doi pubmed - Rao RH, McCready VR, Spathis GS. Iodine kinetic studies during amiodarone treatment. J Clin Endocrinol Metab. 1986;62(3):563-568.
doi pubmed - Franklyn JA, Davis JR, Gammage MD, Littler WA, Ramsden DB, Sheppard MC. Amiodarone and thyroid hormone action. Clin Endocrinol (Oxf). 1985;22(3):257-264.
doi pubmed - Roti E, Minelli R, Gardini E, Bianconi L, Braverman LE. Thyrotoxicosis followed by hypothyroidism in patients treated with amiodarone. A possible consequence of a destructive process in the thyroid. Arch Intern Med. 1993;153(7):886-892.
pubmed - Sheldon J. Effects of amiodarone in thyrotoxicosis. Br Med J (Clin Res Ed). 1983;286(6361):267-268.
doi pubmed pmc - Rajatanavin R, Chailurkit LO, Kongsuksai A, Teeravaninthorn U, Himathongkam T. The effect of amiodarone on the control of hyperthyroidism by propylthiouracil. Clin Endocrinol (Oxf). 1990;33(2):193-203.
doi pubmed - Latham KR, Sellitti DF, Goldstein RE. Interaction of amiodarone and desethylamiodarone with solubilized nuclear thyroid hormone receptors. J Am Coll Cardiol. 1987;9(4):872-876.
doi pubmed - Iervasi G, Clerico A, Bonini R, Manfredi C, Berti S, Ravani M, Palmieri C, et al. Acute effects of amiodarone administration on thyroid function in patients with cardiac arrhythmia. J Clin Endocrinol Metab. 1997;82(1):275-280.
doi pubmed - Keidar S, Grenadier E, Palant A. Amiodarone-induced thyrotoxicosis: four cases and a review of the literature. Postgrad Med J. 1980;56(655):356-358.
doi pubmed pmc - Ruzieh M, Moroi MK, Aboujamous NM, Ghahramani M, Naccarelli GV, Mandrola J, Foy AJ. Meta-analysis comparing the relative risk of adverse events for amiodarone versus placebo. Am J Cardiol. 2019;124(12):1889-1893.
doi pubmed - Tomisti L, Rossi G, Bartalena L, Martino E, Bogazzi F. The onset time of amiodarone-induced thyrotoxicosis (AIT) depends on AIT type. Eur J Endocrinol. 2014;171(3):363-368.
doi pubmed - Ozcan EE, Dogdus M, Yilancioglu RY, Adiyaman SC, Turan OE. Invasive heart rate control as a salvage therapy in amiodarone-induced thyroid storm. Medeni Med J. 2022;37(1):119-122.
doi pubmed pmc - Katoh D, Yoshino H, Ikehara K, Kumashiro N, Uchino H, Tsuboi K, Hirose T. Successful treatment of amiodarone-induced thyrotoxicosis type 1 in combination with methimazole and potassium iodide in a patient with Hashimoto's thyroiditis. Intern Med. 2020;59(3):383-388.
doi pubmed pmc - Miller A, Silver KD. Thyroid storm with multiorgan failure treated with plasmapheresis. Case Rep Endocrinol. 2019;2019:2475843.
doi pubmed pmc - Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
