| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 13, Number 3, August 2023, pages 114-120
Study of Plasma Corticosterone Upon Chronic Stress Induction and Its Effect on Follicular Development in Rattus norvegicus
Nitin Kalsi Rajashekaraa, Bindu Jayashankaraswamya, Raghu Nataraja, b
aDivision of Molecular Biology, School of Life Sciences-Mysuru, JSS Academy of Higher Education and Research, Mysuru, Karnataka 570015, India
bCorresponding Author: Raghu Nataraj, Division of Molecular Biology, School of Life Sciences-Mysuru, JSS Academy of Higher Education and Research, Mysuru, Karnataka 570015, India
Manuscript submitted April 20, 2023, accepted July 17, 2023, published online August 25, 2023
Short title: Corticosterone’s Influence on Folliculogenesis
doi: https://doi.org/10.14740/jem880
| Abstract | ▴Top |
Background: Sex hormones controlling the ovarian function are centrally controlled by the hypothalamus, and the downstream control are carried through the pituitary secretion via the hypothalamus-pituitary-gonadal (HPG) axis. In rodents, corticosterone under stress conditions is known to disrupt the hormones of HPG axis at multiple stages and exhibits pathological impact on ovaries. The current study aimed to investigate the role of chronic stress effect on the regulation of corticosterone and its counter effectiveness in dysregulating testosterone, estradiol levels, along with the interference in ovarian follicular development and polycystic ovary syndrome (PCOS) induction in Rattus norvegicus.
Methods: The study identified the rat models into three groups, i.e., control, stress and stress with ketoconazole treated. The high dosage of ketoconazole exhibits the corticosterone suppression effect. Control animals were left undisturbed and maintained under standard conditions. Stress group animals were exposed to different stress induction parameters such as restraining, dark shift, forced swimming, acute heat, and social isolation. Another stress group of animals were treated with 50 mg/kg body weight of ketoconazole prior to the stress exposure which was conducted for 30 days. At the end of stress observations, animals were euthanized, ovaries collected, stored in neutral buffer formalin for further histopathology studies and the plasma was collected and stored under -20 °C for further hormonal investigations.
Results: The study demonstrates a significant increase in number of days per estrous cycle among the stress group animals by vaginal cytology analysis. Histopathology study of ovaries revealed healthy follicular development in control group of animals and cystic follicular growth among the stress group animals. Animals treated with 50 mg/kg body weight ketoconazole exhibited improper follicular growth. All the study observations were correlated with the hormonal estimations across the animal groups against Rotterdam criteria and the results inferred.
Conclusions: Our observation is that damaged impaired folliculogenesis which has led to ovarian failure may be imputed to change in hormonal secretion pattern.
Keywords: Stress; Ketoconazole; Corticosterone; Testosterone; Estradiol; Polycystic ovary syndrome
| Introduction | ▴Top |
polycystic ovary syndrome (PCOS) is one of the major endocrine system-related syndromes that is encountered in the reproductive age group females, with the symptoms like, hyperandrogenism, oligo/anovulation and cystic ovaries with a high risk of type-2 diabetes, dysregulated follicle-stimulating hormone (FSH)-luteinizing hormone (LH) ratio, modulated testosterone to estrogen ratio, etc. [1, 2]. Corticosterone is a stress responsive hormone which plays a huge role in normal physiological activities of an organism. Being a stress responsive element, it helps damaged tissues to overcome the stress effects by availing them of glucose [1, 3].
Pathologically, elevation in corticosterone has shown to lead multiple dysfunctions in the body, including the disruption in the hormonal profile of hypothalamus-pituitary-gonadal (HPG) axis, insulin metabolism, growth and development, neurobehavioral response, disruption of immunity, disrupting reproductive health, etc. [1, 3-8]. Corticosterone under stress conditions is known to disrupt the hormones of HPG axis at multiple stages and exhibiting its pathological impact on the ovaries [1, 5, 8-11].
Elevated cortisol levels in humans during stress have been shown to interfere with the follicular development, ovulation, steroidogenesis and fertility in human [1, 9, 10]. Study on the effect of chronic stress in causing the variation in estrous cyclicity, testosterone levels, its impact on the follicular development in association with the reactive oxygen species (ROS) and other free radicles has been conducted on rat models [8]. The current study aimed to investigate the role of chronic stress’ effect in the regulation of corticosterone and its consequences on dysregulating testosterone, estradiol levels and interfering in follicular development and hence causing PCOS induction in rat models assessed as per Rotterdam criteria.
| Materials and Methods | ▴Top |
Animal ethics
Current study has been reviewed, and the ethical clearance for the current study was granted from the Institutional Animal Ethical Committee (IAEC), JSS AHER, Mysuru, Karnataka, India (JSSAHER/CPT/IAEC/084/2021). The study was conducted in compliance with the ethical guidelines from the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), under the Ministry of Fisheries, Animal Husbandry and Dairying Department of Animal Husbandry and Dairying, Government of India.
Animal grouping
A total of 15 rats (Rattus norvegicus) of age group 8 - 12 weeks, weighing 180 g to 200 g were obtained from the animal facility of JSS AHER, Mysuru. Animals were maintained under the standard conditions as per the CPCSEA guidelines, with 12 h dark - 12 h light and fed with ad libitum of food pellets and reverse osmosis water. For the study, all animals were grouped as control, stress and stress with corticosterone inhibitor with five rats in each group.
Study design
For a total of 30 days at 6 days each week, animals in the corticosteroid inhibitor dosed group and the stress group underwent varying degrees of stress. First, the animals were restrained for 45 min in a wire mesh restrainer (Fig. 1a), then the animals were subjected to 2 h of darkness (Fig. 1b), immediately after which the animals were made to swim for 15 min (Fig. 1c), and finally, the animals were subjected to a brief exposure to heat (Fig. 1d). The rats were randomly exposed to social isolation twice a week as part of these routine stress regimes (Fig. 1e). These stress factors may interfere with folliculogenesis and the production of PCOS [12]. Stress with corticosterone inhibitor group animals were fed with 50 mg/kg body weight of ketoconazole (purchased from JSS pharmacy) suspended in 0.5% carboxymethyl cellulose (CMC) before exposing to stress parameters, where the control group animals were maintained under the standard conditions without any stress exposure. The dosage of ketoconazole was standardized according to the standard procedures [13].
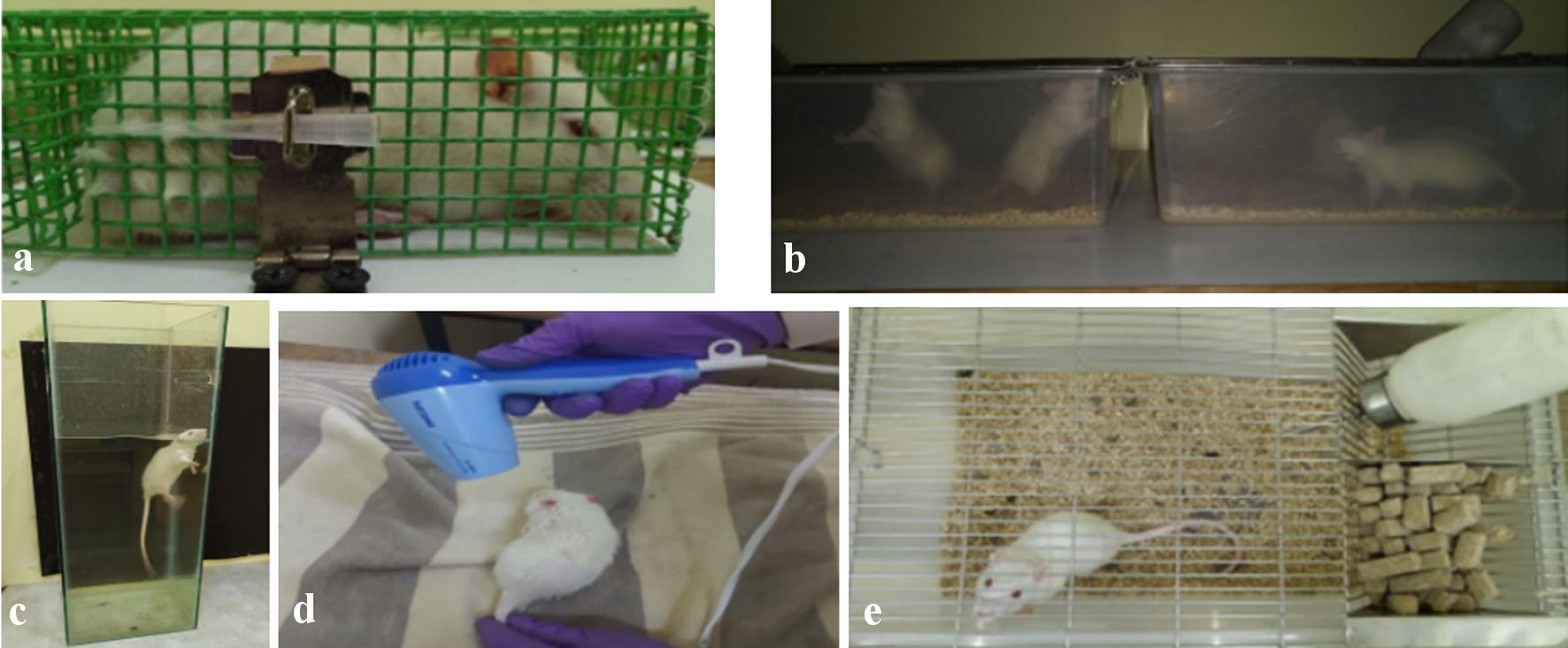 Click for large image | Figure 1. (a) Animal under restraining. (b) Animals under dark condition. (c) Animal under forced swimming. (d) Anima exposing to flash heat. (e) Animal under social isolation. |
Ovarian histology
Ovaries were collected and stored in neutral buffer formalin and processed for paraffin sectioning. Five-µm thick sections were taken using Leica RM2125RT microtome followed by hematoxylin and eosin (H&E) staining and observed under Olympus BX53 microscope. The presentations of the histology sections have been discussed (Figs. 2-5).
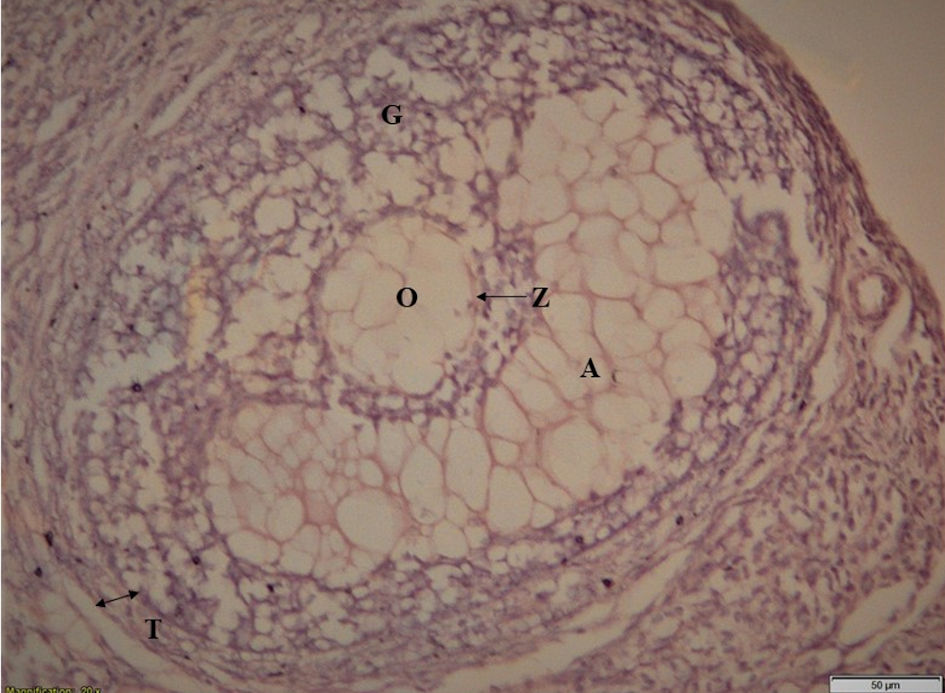 Click for large image | Figure 2. Matured healthy follicle. T: theca layer; G: granulosa layer; O: ovule; Z: zona pellucida; A: antrum. |
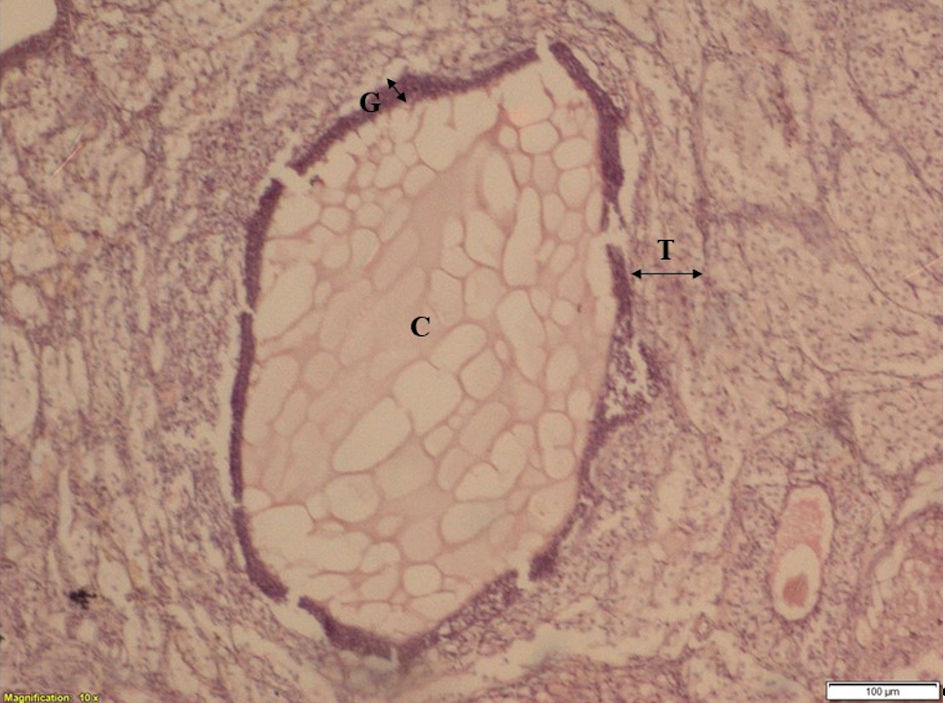 Click for large image | Figure 3. The ovarian section of stress group animal showing cystic follicle (after 30 days of stress exposure). C: cyst; T: theca layer; G: granulosa layer. |
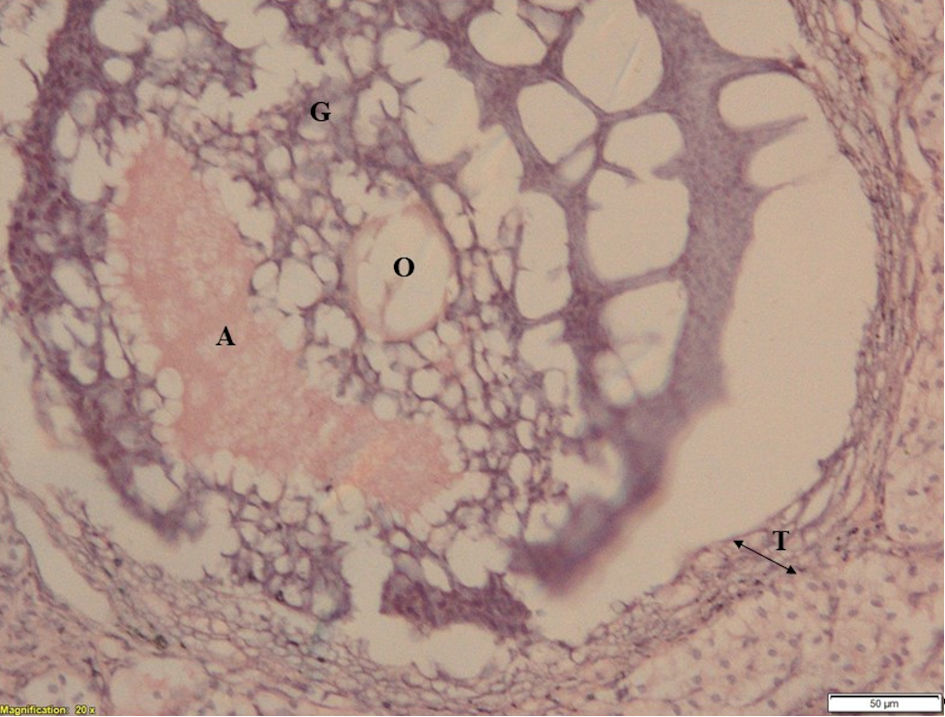 Click for large image | Figure 4. Ovarian section of ketoconazole-treated animal showing pseudo healthy follicle. (after 30 days of 50 mg/kg body weight ketoconazole with stress exposure). T: theca layer; G: granulosa layer; O: ovule; A: antrum. |
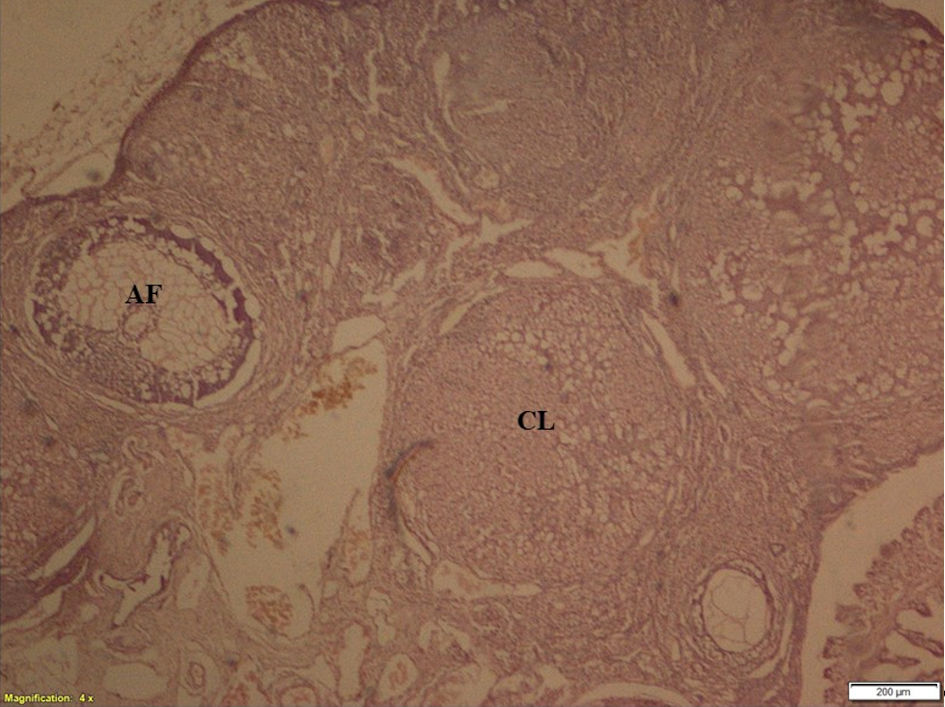 Click for large image | Figure 5. Ovarian section showing corpus luteum and antral follicle. CL: corpus luteum; AF: antral follicle. |
Hormonal assay
Plasma corticosterone was estimated with KinesisDx Corticosterone enzyme-linked immunoassay (ELISA) kit. Testosterone and estradiol were estimated using Calbiotech ELISA kit and the results established.
Estrous cycle analysis
Variations in estrous cycle across all the three groups were studied by the vaginal smear preparation according to standard protocol [12], and the results presented (Fig. 6).
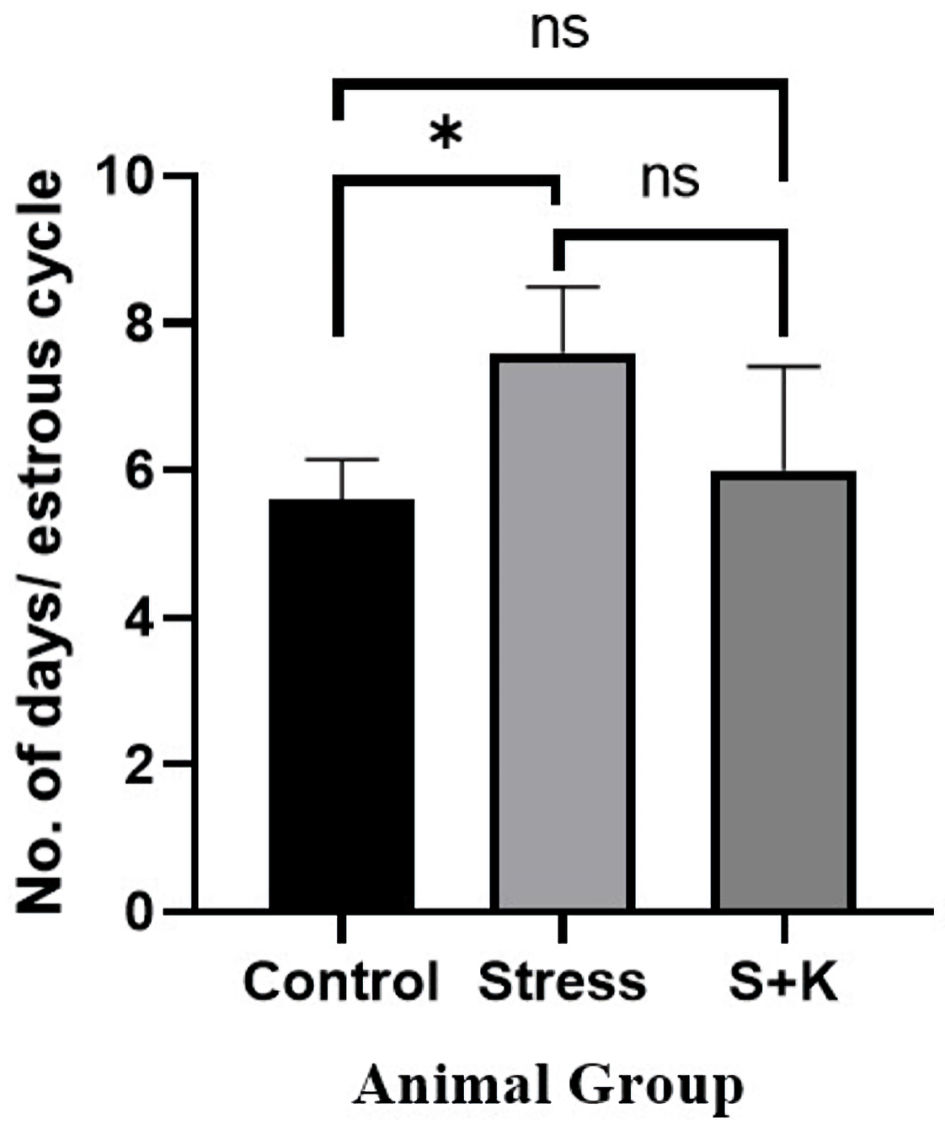 Click for large image | Figure 6. Number of days per estrous cycle of control, stress, and ketoconazole-induced group of animals. *P ≤ 0.05. S+K: animal group with 50 mg/kg body weight ketoconazole prior to stress; ns: not significant. |
Statistical analysis
The significance between the hormonal values and average number of days per estrous cycle among the control, stress, and stress with corticosterone inhibitor group animals were established using one-way analysis of variance (ANOVA) and judged as significant only if P < 0.05.
| Results | ▴Top |
Effect of stress on ovarian histology
Ovarian section of control group animals has shown a healthy follicle with proper theca layer, and granulosa layer with a developing ovule in it (Fig. 2); whereas, the ovarian sections of stress group animals have shown a cystic follicular development with thickened theca layer, reduced granulosa cells and the absence of ovule in it (Fig. 3), which suggests the follicular atresia caused by the elevated testosterone through the autophagic reaction. Follicles of ketoconazole-induced animals have exhibited a pseudo development, i.e., the theca membrane shows a thickening in its size with some degradation pattern in granulosa membrane, and the developed ovule has shown the degradation patterns in its membrane (Fig. 4).
Effect of stress on plasma corticosterone level
As corticosterone is a primary stress hormone in rodents, its level was expected to be high in the stress group of animals. The one-way ANOVA revealed a significant P value of 0.0418 at P < 0.05, as predicted; however, the Tukey’s multiple comparison test between the animal groups has shown no significant difference for the adjusted P value at P < 0.05, i.e., the Tukey’s multiple comparison test has given the adjusted P value of 0.0743, 0.9776, and 0.0551 between control to stress, control to ketoconazole-induced group and stress to ketoconazole-induced group of animals, respectively, which are not significant at P < 0.05 (Fig. 7).
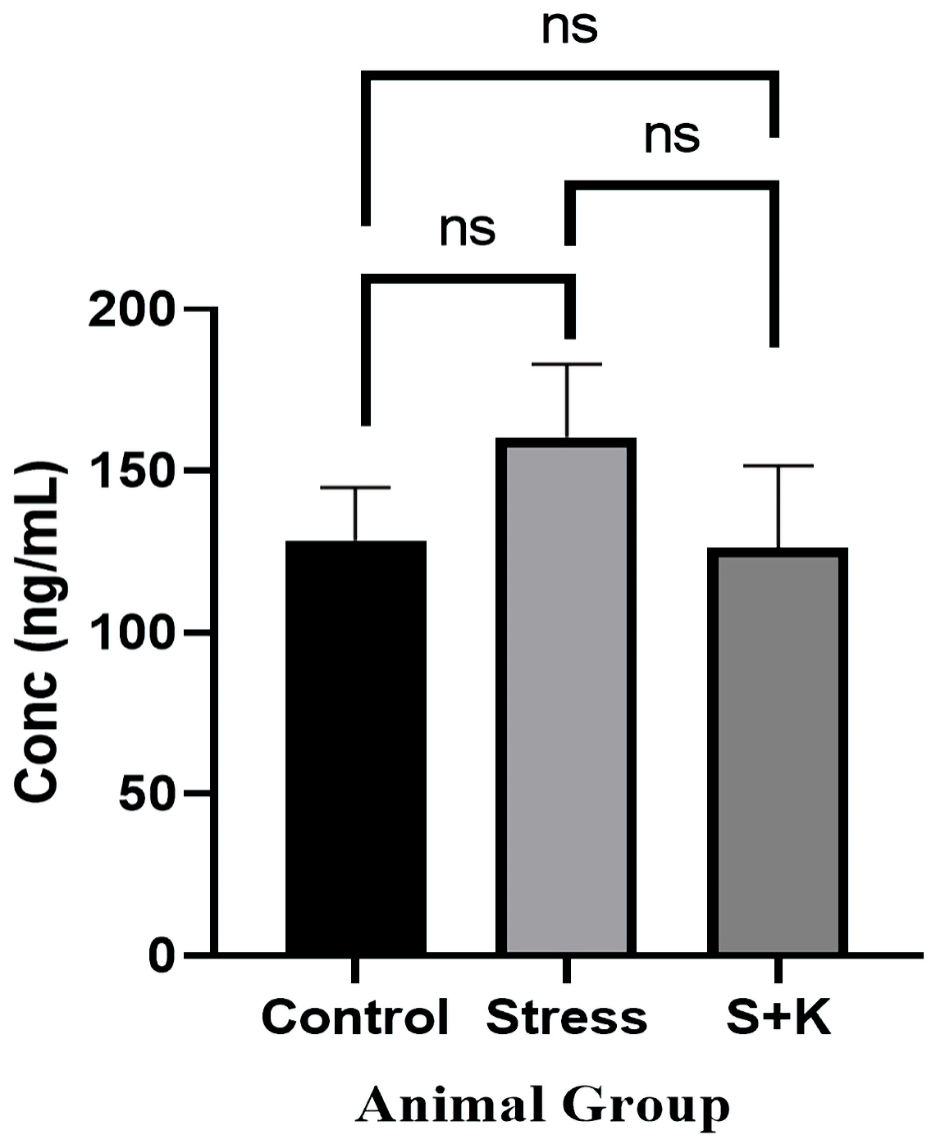 Click for large image | Figure 7. Plasma corticosterone level of control, stress and ketoconazole-induced group of animals. S+K: animal group with 50 mg/kg body weight ketoconazole prior to stress; ns: not significant. |
Effect of stress on plasma testosterone level
A P value of 0.0380, which is significant at P < 0.05, was found in the one-way ANOVA for the plasma testosterone level between the control, stress, and ketoconazole-induced group of animals. Tukey’s multiple comparison tests revealed an adjusted P value of 0.0449 for the control and stress group animals, which is significant at P < 0.05. However, the same test between the control to the ketoconazole-induced group and the stress to ketoconazole-induced group revealed the adjusted P values of 0.0798 and 0.9201 for respective groups, which are not significant at P < 0.05 (Fig. 8).
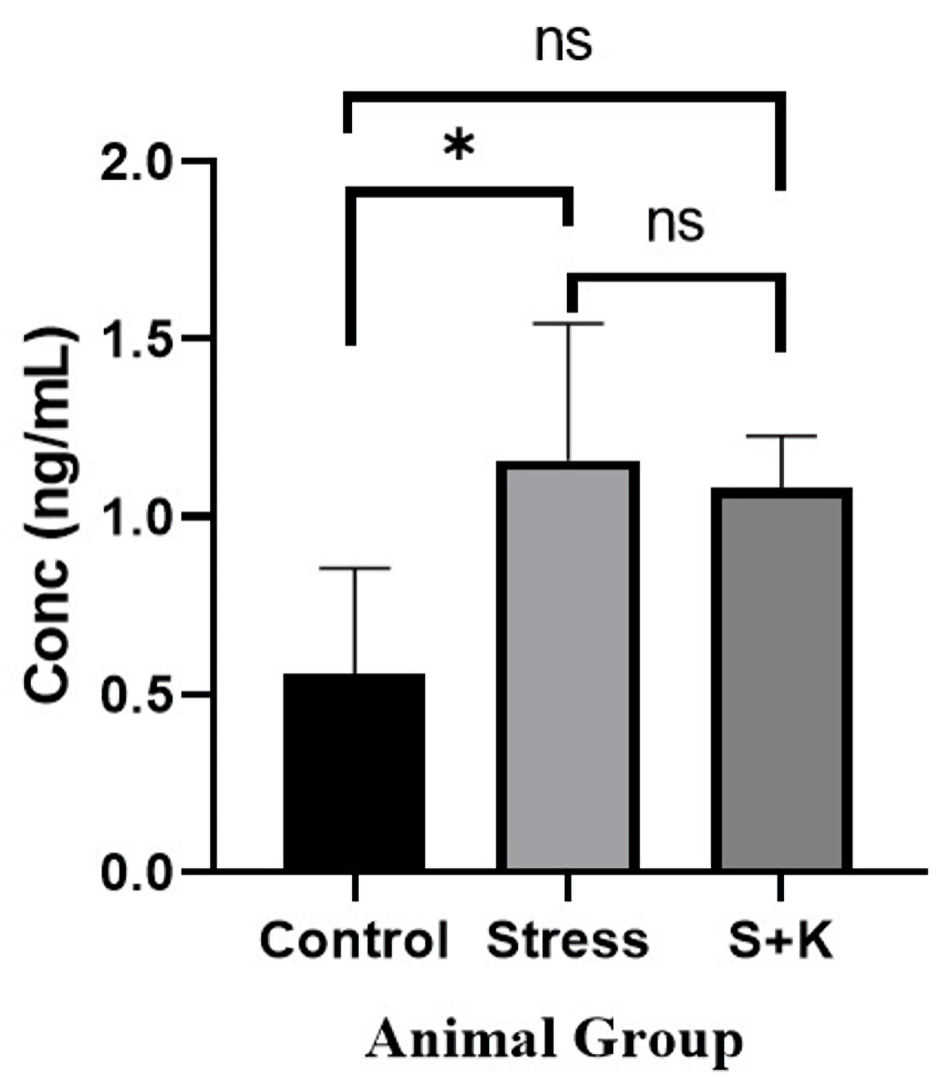 Click for large image | Figure 8. Plasma testosterone level of control, stress and ketoconazole-induced group of animals. *P ≤ 0.05. S+K: animal group with 50 mg/kg body weight ketoconazole prior to stress; ns: not significant. |
Effect of stress on plasma estradiol levels
A P value of 0.0067, which is significant at P < 0.05, was found in the one-way ANOVA for the plasma estradiol level between the control, stress, and ketoconazole-induced group of animals. The adjusted P value from Tukey’s multiple comparison test between the control and stress group animals was 0.8424, which is insignificant at P < 0.05, but the adjusted P values from the tests comparing the control group to the group that received ketoconazole and the stress group to the group that received ketoconazole were 0.0182 and 0.0084, respectively, which are significant at P < 0.05 (Fig. 9).
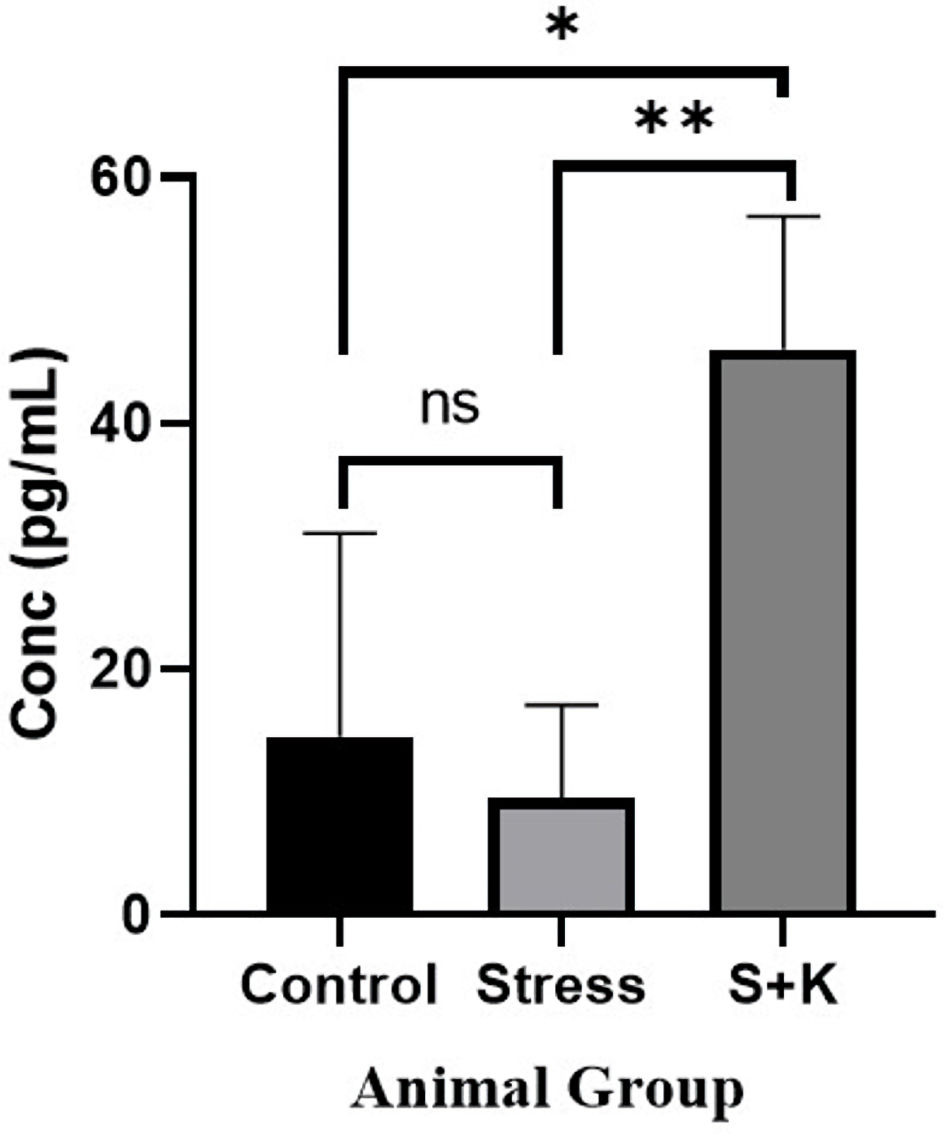 Click for large image | Figure 9. Plasma estradiol level of control, stress and ketoconazole-induced group of animals. *P ≤ 0.05. **P ≤ 0.01. S+K: animal group with 50 mg/kg body weight ketoconazole prior to stress; ns: not significant. |
Effect of stress on estrous cycle
The one-way ANOVA revealed a P value of 0.0375, which is significant at P < 0.05, for the average number of days per estrous cycle between the control, stress, and ketoconazole-induced group of animals. Tukey’s multiple comparison test between the control group and the stress group of animals revealed an adjusted P value of 0.0397, which is significant at P < 0.05. In contrast, the same test between the control group and the stress group of animals given ketoconazole revealed adjusted P values of 0.8225 and 0.0962, respectively, which are not significant at P < 0.05 (Fig. 6).
| Discussion | ▴Top |
Under stress regime, corticosterone, a predominant glucocorticoid in Rattus norvegicus is known to be produced by the activation of hypothalamus-pituitary-adrenal (HPA) axis thereby elevating adrenocorticotropic hormone (ACTH) in the circulation. The higher concentration of ACTH trigger cytochrome P450 family 17 subfamily A member 1 (CYP17A1) of zona fasciculata/reticularis to produce more adrenal androgen into the circulation [14]. Similarly, elevated corticosterone levels in the animals exposed to the stress regime of another study conducted elsewhere has proven the possible HPA-axis upregulation in the stress group animals [8].
One-way ANNOVA test results for our study demonstrates a significant variation for the plasma corticosterone levels across the animal groups with a P value of 0.0418, however, there was no statistical significance have been observed between the individual groups. Though the rise in the plasma corticosterone in the stress group animals is statistically insignificant, it has shown a mathematically significant rise in numbers, i.e., the plasma corticosterone in control group animals have shown a mean value of 128.5 ng/mL, whereas the stress group animals have shown 160.4 ng/mL of plasma corticosterone and similarly the corticosterone inhibitor group of animals have shown 126 ng/mL of plasma corticosterone level. This obtained null-hypothesis in the case of plasma corticosterone could be because of the small population size that has chosen for the study or any unknown parameters beyond the current study. The study has shown a comparatively less raised plasma corticosterone values compared to the previously reported studies [8], with corticosterone level showing a significant deviation at P < 0.05 from the one-way ANNOVA. This lower corticosterone response may be attributed to the change in response of HPA axis to ACTH [15]. To study the effect of stress-induced corticosterone elevation on the follicular development and PCOS induction, corticosterone inhibitor, ketoconazole was administered to suppress the 17α-hydroxylase activity, 11β-hydroxylase activity and thereby reducing the production of the primary stress hormone [16-18]. The inclusion of ketoconazole in the study proved to be effective as the stressed animals dosed with 50 mg/kg body weight ketoconazole showed similar corticosterone values in the plasma of control animals (Fig. 7).
The results of testosterone values showed a significant elevation in stress group animals in comparison to control group (Fig. 8). This rise could be attributed to the higher ACTH in circulation that stimulates the CYP17A1 of adrenal and theca cells of the ovaries [14], which in turn elevates the 3β-hydroxysteroid dehydrogenase (3β-HSD) [8] that in turn elevates the production of testosterone. The study deviates from the early reports of testosterone values, which showed no change in elevation even after animals were exposed to 4 - 8 weeks of stress regime. But, in the same study, rats with 12 weeks of stress exposure have exhibited 1.56 ± 0.12 ng/mL of testosterone which exhibits a significant elevation compared with the control. In addition to the rats, a similar study using the same dosage of ketoconazole (50 mg/kg body weight) in male rabbits also revealed a considerable drop in testosterone, measuring 0.80 ± 0.24 ng/mL, which is significantly lower when compared to the control [19]. This effect is because of the potential inhibiting effect of ketoconazole on 17, 20-desmolase, which is an enzyme that helps in the production of androgen [16]. The hyperandrogenism in females is one of the key symptoms that has commonly be observed in PCOS as it has been mentioned in many studies [1, 2, 8, 9, 20]. The exact cause for the hyperandrogenism that has been observed in the ketoconazole-induced rats (Fig. 8) is not clear till date.
A physiological concentration of androgen is a key hormone in the development of pre-antral follicle to early antral follicle by helping in expression of FSH receptors on granulosa cells [9], but the hyper expression has the pathogenic effect known as atresia. This atretic effect is thought to be because of higher concentration of androgen or higher ratio of androgen to estrogen which causes the fragmentation of oocyte [9, 16]. Results in the current study have shown that control group of animals have exhibited a 14.5 ± 1.70 pg/mL of plasma estradiol which is physiologically a normal amount. But, in the case of stress-induced animals, the estradiol levels were decreased and has shown a 9.5 ± 1.37 pg/mL of plasma estradiol, which is much lower than that of control group animals (Fig. 9). Though the decrease value is still statistically insignificant, the imbalance imparted by the varied ratio of testosterone to estradiol would still play a key role in follicular atresia. A study has shown that the reason for the decreased level of estradiol in the stress group animals may be due to the non-conversion of testosterone into estradiol by the granulosa cells [21].
The study has also observed a significant variation in the estrous cycle in stress group animals compared to control group, i.e., stress group animals have taken 7.6 ± 0.4 days to complete one estrous cycle, whereas, the control animals have taken 5.6 ± 0.24 days to complete one estrous cycle, and the ketoconazole-induced animals have taken 6 ± 0.63 days to complete one estrous cycle, which is not significant when compared to control group (Fig. 6).
The results obtained from histology study have revealed a healthy follicular structure in the ovarian section of control group animals with proper theca layer, granulosa layer with a developing ovule (Fig. 2). Meanwhile, the ovarian sections of stress group animals have shown evidence of cystic follicular development with thickened theca layer, degenerated granulosa cells with an absence of ovule (Fig. 3), suggesting the follicular atresia caused by the elevated testosterone through the autophagic reaction [21]. Other studies conducted elsewhere have also shown the morphological changes that occurred in the follicles of the stress-exposed animals with thickened theca layer, degenerated granulosa and the ovule [8, 22]. In the present study, follicles of animals treated with ketoconazole showed a pseudo granulosa membrane development with a thickened degraded theca membrane and the development of ovule exhibiting degradation patterns on its membrane (Fig. 4). This phenomenon is attributed to the compensatory effect of estrogen which can minimize the autophagy effect in follicles [21].
In the current investigation, only the minimum number of animals that was required to demonstrate the results’ statistical significance has been taken into consideration, but, the small population size that has been used in this study may have its limitation in: 1) potential false negative findings; and 2) generalization of the results obtained to a larger population, as the individual animal has its own physiological and behavioral differences. Hence a more extensive study is recommended to correlate the obtained results to a larger population size.
Conclusions
The current study suggests that the chronic stress-induced elevation in corticosterone levels may have its potential involvement in interrupting follicular development. Our conclusion is that damaged impaired folliculogenesis which has led to ovarian failure may be imputed to change in hormonal secretion pattern. However, the study has further identified novel lacunas which need to be addressed with respect to the elevation in testosterone and estradiol in the corticosterone inhibitor group of animals in future studies.
Acknowledgments
The authors would like to thank JSS Academy of Higher Education & Research, Mysuru for their financial support and IAEC, JSS AHER, Mysuru for providing the Central Animal Facility to carry out the work.
Financial Disclosure
The current research work was supported by JSS Academy of Higher Education & Research, Mysuru, under University Research Grant (JSSAHER/REG/RES/URG/54/2011-12/10419, dated February 24, 2022) and JSS AHER Fellowship (JSSAHER/REG/RES/JSSURF/29910/2010-11, dated November 15, 2021).
Conflict of Interest
The authors hereby declare no conflict of interest related to this publication.
Informed Consent
Not applicable.
Author Contributions
Raghu Nataraj and Nitin Kalsi Rajashekara: substantial contribution to the conception, design of the work; acquisition, analysis and interpretation of the data for the work; drafting the work, revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Bindu Jayashankaraswamy: data acquisition and analysis of the data, revising the draft critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The data presented in this study are available on request from the corresponding author.
| References | ▴Top |
- Nitin KR, Nataraj R. Hormones of HPG-axis and their active role during chronic stress and PCOS Induction: a review. International Journal of Basic and Applied Sciences. 2022;11(1):1-8.
- Iervolino M, Lepore E, Forte G, Lagana AS, Buzzaccarini G, Unfer V. Natural molecules in the management of polycystic ovary syndrome (PCOS): an analytical review. Nutrients. 2021;13(5):1677-1688.
doi pubmed pmc - Mishima Y, Shinoda Y, Sadakata T, Kojima M, Wakana S, Furuichi T. Lack of stress responses to long-term effects of corticosterone in Caps2 knockout mice. Sci Rep. 2015;5:8932.
doi pubmed pmc - Assad S, Khan HH, Ghazanfar H, Khan ZH, Mansoor S, Rahman MA, Khan GH, et al. Role of sex hormone levels and psychological stress in the pathogenesis of autoimmune diseases. Cureus. 2017;9(6):e1315.
doi pubmed pmc - Austin SH, Harris RM, Booth AM, Lang AS, Farrar VS, Krause JS, Hallman TA, et al. Isolating the role of corticosterone in the hypothalamic-pituitary-gonadal transcriptomic stress response. Front Endocrinol (Lausanne). 2021;12:632060.
doi pubmed pmc - Kinlein SA, Phillips DJ, Keller CR, Karatsoreos IN. Role of corticosterone in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2019;102:248-255.
doi pubmed pmc - H NS, H NY. Duration dependent effect of chronic stress on primary and secondary lymphoid organs and their reversibility in rats. Immunobiology. 2019;224(1):133-141.
doi pubmed - Divyashree S, Yajurvedi HN. Long-term chronic stress exposure induces PCO phenotype in rat. Reproduction. 2016;152(6):765-774.
doi pubmed - Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35(2):109-125.
pubmed pmc - Andersen CY. Possible new mechanism of cortisol action in female reproductive organs: physiological implications of the free hormone hypothesis. J Endocrinol. 2002;173(2):211-217.
doi pubmed - Mohan V, Mehreen TS, Ranjani H, Kamalesh R, Ram U, Anjana RM. Prevalence of polycystic ovarian syndrome among adolescents and young women in India. Journal of Diabetology. 2021;12(3):319-325.
doi - Nitin KR, Bindu J, Nataraj R. A study on the effect of randomized chronic stress on estrous cycle and ovarian cyst in Rattus norvegicus. International Journal of Emerging Technologies and Innovative Research. 2022;9(8):860-867.
- Burrin JM, Yeo TH, Ashby MJ, Bloom SR. Effect of ketoconazole on adrenocorticotrophic hormone secretion in vitro and in vivo. J Endocrinol. 1986;108(1):37-41.
doi pubmed - Bertagna X. Effects of chronic ACTH excess on human adrenal cortex. Front Endocrinol (Lausanne). 2017;8:43.
doi pubmed pmc - Rich EL, Romero LM. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1628-1636.
doi pubmed - Shirley M. Ketoconazole in Cushing’s syndrome: a profile of its use. Drugs & Therapy Perspectives. 2021;37(2):55-64.
doi - Nagaoka M, Fukami T, Kisui F, Yamada T, Sakai Y, Tashiro K, Ogiso T, et al. Arylacetamide deacetylase knockout mice are sensitive to ketoconazole-induced hepatotoxicity and adrenal insufficiency. Biochem Pharmacol. 2022;195:114842.
doi pubmed - Attoui N, Guedri K, Makhloufi K. Effect of ketoconazole on early maternal separation stress model in rats: a neurobehavioral and biochemical approach. Adv Anim Vet Sci. 2019;7(9):761-769.
doi - Mohamed A, Hassan A, Amer M, Abdel-Aziz ES. The effects of oral ketoconazole and griseofulvin on the fertility of male rabbits. Mansoura Veterinary Medical Journal. 2020;21(2):32-38.
doi - Wei Y, Li W, Meng X, Zhang L, Shen M, Liu H. Corticosterone injection impairs follicular development, ovulation and steroidogenesis capacity in mice ovary. Animals (Basel). 2019;9(12):1047-1056.
doi pubmed pmc - Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706-2733.
doi pubmed pmc - Chukwuebuka NB, Emeka OA, Irukefe OS, Iju WJ, Temitope OU, Nneamaka EC, Peter AC. Stress-induced morphological changes of ovarian histology in female Wistar rats. Biomedical & Pharmacology Journal. 2020;13(4):1625-1643.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
