| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 12, Number 4-5, October 2022, pages 134-139
The Difference in Glucagon Response to Breakfast Between Non-Obese Patients With Long-Duration Type 1 and Type 2 Diabetes
aHamasaki Clinic, 2-21-4 Nishida, Kagoshima 890-0046, Japan
bDepartment of Diabetes, Kanmachi Imakiire Hospital, Kagoshima, Japan
cEmail:
Manuscript submitted August 15, 2022, accepted September 22, 2022, published online October 9, 2022
Short title: Glucagon Response to Breakfast in Diabetes
doi: https://doi.org/10.14740/jem834
| Abstract | ▴Top |
Background: A balanced action of insulin and glucagon is essential for treating diabetes. This study aimed to assess the change in blood glucagon levels from fasting to a postprandial state and to compare them between patients with type 1 diabetes (T1DM) and type 2 diabetes (T2DM).
Methods: This study enrolled patients with T1DM (n = 13) who had undetectable serum C-peptide levels (< 0.02 ng/mL) and patients with T2DM (n = 13) whose age, gender, and body mass index were matched to cases (1:1) as controls. Plasma glucose, serum C-peptide, and plasma glucagon in fasting and 2 h after consuming a standard breakfast for diabetic patients were measured and compared between groups.
Results: There were no significant differences in plasma glucose and hemoglobin A1c levels between patients with T1DM and T2DM. However, fasting plasma glucagon levels were significantly lower in patients with T1DM than those in patients with T2DM (19.2 ± 13.0 pg/mL vs. 31.6 ± 18.3 pg/mL, P = 0.029). Furthermore, the glucagon’s response to a standardized meal for diabetic patients differed between patients with T1DM and T2DM.
Conclusions: The significant difference in glucagon response to the meal may be caused by the abnormal postprandial secretion of glucagon in patients with T1DM. The nutrient ratio of the meal may also influence glucagon secretion.
Keywords: Glucagon; C-peptide; Type 1 diabetes; Type 2 diabetes; Standardized meal; Postprandial state
| Introduction | ▴Top |
Recent research on the pathophysiological role of glucagon in glycemic control has drawn the attention of diabetologists to the fact that a balanced action of insulin and glucagon is required for the treatment of diabetes [1, 2]. In patients with type 1 diabetes mellitus (T1DM), glucagon secretion is decreased, increasing glycemic variability and the risk of insulin-induced hypoglycemia. Nevertheless, glucagon secretion in these patients is not depleted when compared to insulin secretion [3]. Glucagon secretion, which is stimulated by a decrease in blood glucose level, is regulated reciprocally to insulin secretion and decreases progressively in patients with T1DM [3]. However, the glucagon response to insulin-induced hypoglycemia is not depleted in patients with T1DM [4]. On the other hand, glucagon secretion is significantly excessive in type 2 diabetes mellitus (T2DM) [5]. Glucagon secretion from the pancreatic α-cells is regulated by insulin secreted from β-cells adjacent to the α-cells [6], and the balance between insulin and glucagon levels is vital for glucose homeostasis. However, the difference in glucagon levels between patients with T1DM and T2DM in both fasting and postprandial states has not been thoroughly investigated. Recently, a specific double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) for measuring blood glucagon levels has been developed [7], which may shed light on the role of glucagon in glucose metabolism regulation.
This study aimed to determine the change in blood glucagon levels from fasting to a postprandial state and to compare blood glucagon levels between patients with T1DM and T2DM.
| Materials and Methods | ▴Top |
Study design and subjects
This is a comparative study of patients with diabetes admitted to Imakiire General Hospital. Between April 2017 and December 2019, subjects who had their plasma glucagon levels measured during hospitalization were included. Patients under the age of 20 years or those who had been treated with glucagon-like peptide 1 analogues were excluded. Patients with cancers were also excluded. Patients with T1DM who had their diagnosis confirmed by a diabetologist and had undetectable serum C-peptide levels (< 0.02 ng/mL) were enrolled in this study. Additionally, patients with T2DM whose age, gender, and body mass index (BMI) were matched to cases (1:1) were enrolled and served as controls.
The study protocol was approved by the Medical Ethics Committee of the Imakiire General Hospital (reference no. 244) and adhered to the Helsinki Declaration. All study subjects were informed about the research and how the opt-out method would preserve their personal information and confidentiality.
Study procedure
Height and weight were measured using a rigid stadiometer and calibrated scales, respectively. BMI was calculated as follows: weight in kilograms/(height in meters)2. Waist circumference was measured at the level of the umbilicus in a standing position at the end of exhalation. All subjects consumed a standardized breakfast designed specifically for diabetic patients (calorie intake: 25 - 30 kcal/kg × ideal body weight; nutrient ratio: 55% carbohydrates, 18% protein, and 27% fat) during hospitalization. The author examined the change in blood glucagon levels from fasting to a postprandial state after patients’ medical condition was improved and patients could complete meals.
Blood measurements
The meal test was performed 9.9 ± 10.3 days on average after admission. Fasting plasma glucose (PG0), 2-h postprandial glucose (PG2h), fasting serum C-peptide (CPR0) (E-test TOSOH II; Tosoh, Tokyo, Japan), 2-h postprandial C-peptide (CPR2h), fasting plasma glucagon (G0) (glucagon ELISA kit; Cosmic, Tokyo, Japan) [7], 2-h postprandial plasma glucagon (G2h), and hemoglobin A1c (HbA1c) levels were measured. In addition, plasma glucagon levels were divided by plasma glucose levels at fasting (G0/PG0) and a 2-h postprandial state (G2h/PG2h). Glucagon response to hypoglycemia and glucagon’s counter-regulatory effect that balances insulin action are essential to maintain euglycemia in a postprandial state as well as a fasting state [3, 8]. Circulating glucagon levels were correlated with fasting glucose levels in subjects with impaired glucose tolerance [9]. Therefore, measuring the glucagon to glucose ratio is significant to investigate the glucagon response to meals. The estimated glomerular filtration rate (eGFR) was also calculated to compare renal function between patients with T1DM and T2DM [10].
Statistical analysis
Statistical analyses were performed using the SPSS software, version 25 (IBM Co., Ltd., Chicago, IL). Quantitative variables are presented by the mean and standard deviation, while categorical variables are presented by numbers. The Mann-Whitney U test was used to compare clinical data between groups. Fisher’s exact test was used to compare medication between groups. Wilcoxon’s signed-rank test was used to assess changes in PG, G, and G/PG levels from fasting to a 2-h postprandial state. P values < 0.05, as determined by the two-sided test, were considered statistically significant.
| Results | ▴Top |
A total of 357 patients (323 with T2DM and 34 with T1DM) were screened and 13 patients with T1DM and 13 patients with T2DM whose age, gender, and BMI matched were included in this study. The characteristics of study subjects are summarized in Table 1.
 Click to view | Table 1. Characteristics of Patients With Type 1 Diabetes and Type 2 Diabetes |
In patients with T1DM, causes of hospitalization were uncontrolled diabetes (76.9%), orthopedic diseases such as a fracture (15.4%), and inguinal hernia (7.7%). On the other hand, in patients with T2DM, causes of hospitalizations were uncontrolled diabetes (30.8%), respiratory diseases such as pneumonia and pneumothorax (30.8%), orthopedic diseases such as a fracture (15.4%), intestinal obstruction (7.7%), benign prostatic hyperplasia (7.7%), and rhabdomyolysis (7.7%).
There were no significant differences in PG0, PG2h, HbA1c levels, or eGFR between patients with T1DM and those with T2DM. However, G0 and G0/PG0 levels were significantly lower in patients with T1DM than in patients with T2DM. On the other hand, G2h and G2h/PG2h levels did not differ significantly between groups. Figure 1 depicts the changes in mean PG and G levels from fasting to the 2-h postprandial state in patients with T1DM and T2DM. In patients with T1DM, G (P = 0.49) and G/PG (P = 0.89) levels did not change. However, in patients with T2DM, G/PG levels decreased significantly from 0.25 ± 0.19 to 0.13 ± 0.07 (P = 0.008), but G levels alone did not change 2 h after breakfast (P = 0.95).
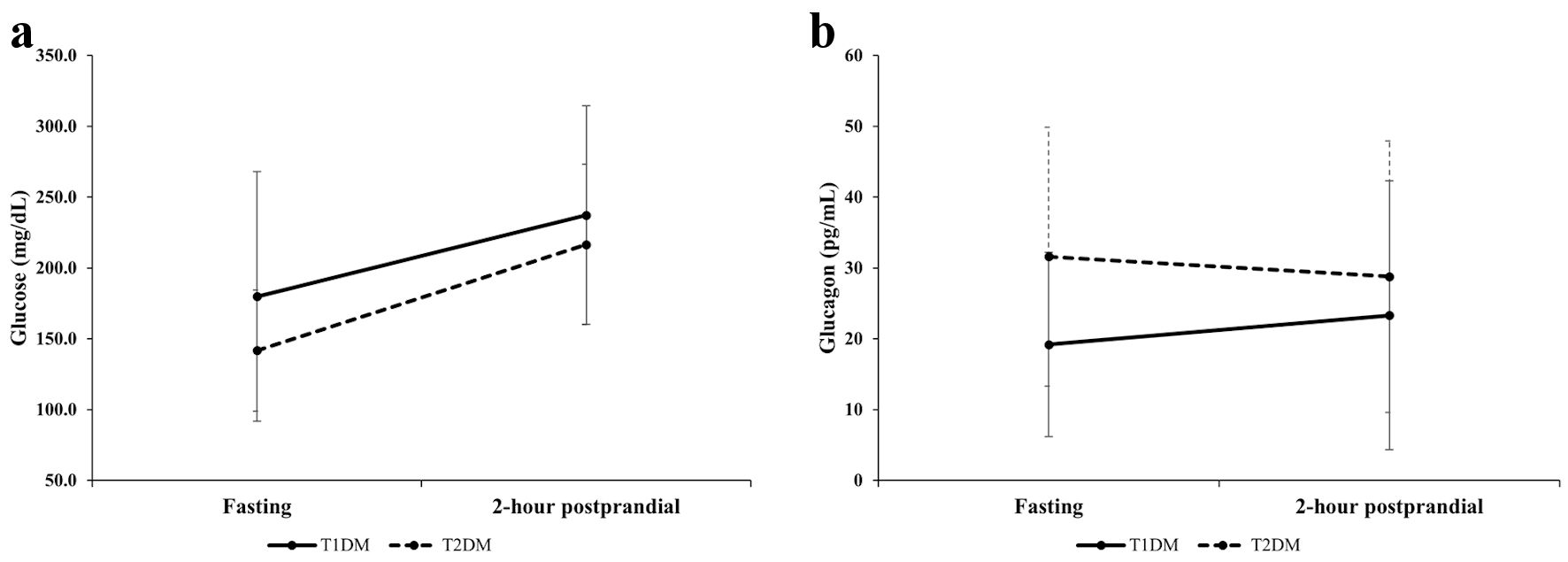 Click for large image | Figure 1. Comparison of changes in glucose (a) and glucagon (b) levels between patients with T1DM and those with T2DM from fasting to 2-h postprandial state. T1DM: type 1 diabetes; T2DM: type 2 diabetes. |
| Discussion | ▴Top |
The present study demonstrated that fasting plasma glucagon levels were lower in patients with T1DM than in those with T2DM. However, the glucagon’s response to a standardized meal for diabetic patients differed between T1DM and T2DM.
There have been contradictory results regarding the difference in fasting glucagon levels between patients with T1DM and T2DM. Kawamori et al [11] reported that fasting plasma glucagon levels in patients with T1DM were 28.1 ± 17.7 pg/mL, comparable with those in patients with T2DM. On the other hand, Kodama et al [12] reported that fasting glucagon levels of fulminant T1DM patients were lower than those of T2DM patients. This inconsistency may be due to the difference in residual β-cell function between study subjects. The present study included T1DM patients with undetectable serum C-peptide levels (< 0.02 ng/mL) as with fulminant T1DM. Fasting glucagon secretion may significantly reduce if the insulin secretion is completely depleted.
Previous studies have reported that patients with T1DM excessively secrete glucagon after a mixed-meal stimulation as opposed to individuals without diabetes [13]. In healthy individuals, blood glucagon levels reach a peak 30 - 60 min after consuming a mixed-meal (700 kcal; 100 g of carbohydrate, 26 g of protein, and 22 g of fat), return to near baseline after 120 min, then increase again; however, the range of fluctuation is small compared to patients with diabetes [14]. The excessive secretion of glucagon after a mixed-meal stimulation in patients with T1DM relates to low paracrine insulin secretion, which could cause postprandial hyperglycemia due to hyperglucagonemia in patients with T1DM [13].
Furthermore, the macronutrient ratio (carbohydrate, protein, and fat) may be a key to the glucagon response to meals in patients with T1DM. Consumption of amino acids (proteins) stimulates pancreatic α-cells to secrete glucagon [15]. A randomized controlled trial showed that a high-protein breakfast (35% proteins and 45% carbohydrates) resulted in 34% higher glucagon secretion than a high-carbohydrate breakfast (15% proteins and 65% carbohydrates) in patients with T2DM. However, glucagon secretion remained unchanged 2 h after a high-protein diet or decreased after a high-carbohydrate diet [16]. Contrariwise, a recent randomized study in patients with T1DM found that both high-protein, high-fat and low-protein, low-fat meals increased glucagon secretion [17]. Glucagon level is abnormally elevated in patients with T1DM at 30, 60, and 90 min after oral glucose administration and returns to near baseline after 2 h [18]. These findings imply that proteins have a longer impact on postprandial glucagon secretion than carbohydrates in patients with T1DM, which may improve glycemic control by minimizing the risk of persistent hypoglycemia. Indeed, patients with T1DM who followed a high-protein, low-carbohydrate diet experienced less hypoglycemia and improved glycemic control [19]. In the present study, the nutrient ratio of breakfast was 55% carbohydrates, 18% protein, and 27% fat, indicating a low-protein, high-carbohydrate diet. The Japanese Clinical Practice Guideline for Diabetes 2019 recommends that carbohydrates and proteins account for 50-60% and up to 20% of the diet therapy for diabetes, respectively. However, it should also be flexible based on patient criteria such as age, physical activity, and preferences [20]. In the present study, the nutrient ratio of breakfast was set up based on this recommendation. A recent meta-analysis showed that both low (< 40%) and high (> 70%) carbohydrate intake was associated with an increased risk of cardiovascular disease and all-cause mortality, whereas moderate (50-55%) carbohydrate intake minimized mortality [21]. In contrast, low-carbohydrate diets may be useful in improving obesity, blood pressure, and serum lipid profile in obese individuals as well as low-fat diets [22, 23]. Horikawa et al [24] reported that the proportion of carbohydrate intake was not associated with the incidence of diabetic complications in Japanese patients with T2DM. The long-term effect of low-carbohydrate diets on health remains controversial because not only the nutrient ratio but also the quality of macronutrients impacts the long-term health [25]. In patients with T1DM, the effect of low-carbohydrate diets on glycemic control is also controversial [26]. Few studies examined the long-term effect of low carbohydrate or high-protein diets on the risk of cardiovascular disease or mortality in patients with T1DM [27]. However, the short-term high-protein, low-carbohydrate diet may be effective for improving glycemic control in both patients with T2DM [28] and T1DM [19]. Given the significant difference in glucagon response to a standardized diet for diabetes between T1DM and T2DM, the diet therapy should be modified based on diabetes type. In patients with T1DM, a high-protein, low-carbohydrate diet, for example, may suppress postprandial hyperglycemia and prevent prolonged hypoglycemia caused by insulin injections or exercise [29, 30]. However, excessive glucagon secretion promotes hyperglycemia, therefore balancing insulin and glucagon is critical for treating diabetes.
Strengths and limitations
This study has several strengths and limitations. First, the differences in patients’ characteristics between T1DM and T2DM were adjusted by matching both groups for age, gender, and BMI. Recently, Ito et al [31] compared glucagon response to a mixed meal tolerance test between patients with T1DM and T2DM; however, there were significant differences in patient characteristics between groups. Such differences can affect the blood glucagon levels; thus, it is vital to match basic characteristics such as age, gender ratio, and BMI when comparing glucagon response to a meal between patients with T1DM and T2DM. Second, this study included patients with T1DM who had a completely depleted endogenous insulin secretion. Glucagon secretion is regulated reciprocally to insulin secretion. Also, the decline in insulin secretion in patients with T1DM is variable, and there is little evidence of changes in insulin secretion in patients with T1DM over time. Therefore, it is important to match endogenous insulin secretion levels of study subjects to investigate the glucagon response in patients with T1DM. Third, glucagon levels were measured using a high-accuracy dual-antibody sandwich ELISA. However, this study is limited by the small sample size and insufficient glucagon measures. Ideally, changes in glucagon levels should be investigated in more detail and longer duration (i.e., 30, 60, 90, and 180 min). Second, although subjects treated with glucagon-like peptide 1 analogues were excluded, medications such as dipeptidyl peptidase-4 inhibitors [32] might have affected blood glucagon levels. However, no statistical difference in the proportion of patients with dipeptidyl peptidase-4 inhibitors was observed in this study (P = 0.096). Finally, the author did not investigate the glucagon response to meals with varying nutrient ratios (e.g., low- or high-carbohydrate, high-protein) in this cohort; thus, whether the glucagon response to such specific meals differs between patients with T1DM and T2DM is unknown. Therefore, further studies are warranted to elucidate the role of glucagon in glycemic control and the difference in glucagon response to meals between patients with T1DM and T2DM.
Conclusions
The difference in glucagon’s response to a standardized meal between patients with T1DM and T2DM observed in this study may provide a clue to optimal diet therapy depending on the types of diabetes. There is still a lot to understand about the physiology of glucagon secretion in response to meals; however, further research on the glucagon response to meals with various nutrient ratios will shed light on the relationship between the balanced action of insulin and glucagon and diet, which ensures the establishment of personalized treatment of diabetes in the future.
Acknowledgments
None to declare.
Financial Disclosure
The author has no relevant financial disclosure.
Conflict of Interest
The author has no conflict of interest to declare.
Informed Consent
The opt-out method of obtaining informed consent was adopted because this study was an observational study.
Author Contributions
Hamasaki H solely conceived the idea of the study, conducted the study, analyzed and interpreted the data, drafted the manuscript, and revised it critically.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; BMI: body mass index; PG0: fasting plasma glucose; PG2h: 2-h postprandial plasma glucose; CPR0: fasting C-peptide; CPR2h: 2-h postprandial C-peptide; G0: fasting plasma glucagon; G2h: 2-h postprandial plasma glucagon; HbA1c: hemoglobin A1c; eGFR: estimated glomerular filtration rate
| References | ▴Top |
- Hamasaki H, Morimitsu S. Association of glucagon with obesity, glycemic control and renal function in adults with type 2 diabetes mellitus. Can J Diabetes. 2021;45(3):249-254.
doi pubmed - Harp JB, Yancopoulos GD, Gromada J. Glucagon orchestrates stress-induced hyperglycaemia. Diabetes Obes Metab. 2016;18(7):648-653.
doi pubmed - Gromada J, Chabosseau P, Rutter GA. The alpha-cell in diabetes mellitus. Nat Rev Endocrinol. 2018;14(12):694-704.
doi pubmed - Flatt AJS, Greenbaum CJ, Shaw JAM, Rickels MR. Pancreatic islet reserve in type 1 diabetes. Ann N Y Acad Sci. 2021;1495(1):40-54.
doi pubmed - Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28(3):253-283.
doi pubmed - Svendsen B, Larsen O, Gabe MBN, Christiansen CB, Rosenkilde MM, Drucker DJ, Holst JJ. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 2018;25(5):1127-1134.e1122.
doi pubmed - Sloan JH, Siegel RW, Ivanova-Cox YT, Watson DE, Deeg MA, Konrad RJ. A novel high-sensitivity electrochemiluminescence (ECL) sandwich immunoassay for the specific quantitative measurement of plasma glucagon. Clin Biochem. 2012;45(18):1640-1644.
doi pubmed - Capozzi ME, Wait JB, Koech J, Gordon AN, Coch RW, Svendsen B, Finan B, et al. Glucagon lowers glycemia when β-cells are active. JCI Insight. 2019;4(16):e129954.
doi pubmed - Ortega FJ, Moreno-Navarrete JM, Sabater M, Ricart W, Fruhbeck G, Fernandez-Real JM. Circulating glucagon is associated with inflammatory mediators in metabolically compromised subjects. Eur J Endocrinol. 2011;165(4):639-645.
doi pubmed - Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992.
doi pubmed - Kawamori D, Katakami N, Takahara M, Miyashita K, Sakamoto F, Yasuda T, Matsuoka TA, et al. Dysregulated plasma glucagon levels in Japanese young adult type 1 diabetes patients. J Diabetes Investig. 2019;10(1):62-66.
doi pubmed - Komada H, Hirota Y, Sakaguchi K, Okuno Y, Ogawa W, Seino S. Impaired glucagon secretion in patients with fulminant type 1 diabetes mellitus. Endocrine. 2019;63(3):476-479.
doi pubmed - Bisgaard Bengtsen M, Moller N. Mini-review: Glucagon responses in type 1 diabetes - a matter of complexity. Physiol Rep. 2021;9(16):e15009.
doi pubmed - McGlone ER, Malallah K, Cuenco J, Wewer Albrechtsen NJ, Holst JJ, Vincent RP, Ling C, et al. Differential effects of bile acids on the postprandial secretion of gut hormones: a randomized crossover study. Am J Physiol Endocrinol Metab. 2021;320(4):E671-E679.
doi pubmed - Holst JJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis. Diabetes. 2017;66(2):235-240.
doi pubmed - Park YM, Heden TD, Liu Y, Nyhoff LM, Thyfault JP, Leidy HJ, Kanaley JA. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J Nutr. 2015;145(3):452-458.
doi pubmed - Garcia A, Moscardo V, Ramos-Prol A, Diaz J, Boronat M, Bondia J, Rossetti P. Effect of meal composition and alcohol consumption on postprandial glucose concentration in subjects with type 1 diabetes: a randomized crossover trial. BMJ Open Diabetes Res Care. 2021;9(1):e002399.
doi pubmed - Kramer CK, Borgono CA, Van Nostrand P, Retnakaran R, Zinman B. Glucagon response to oral glucose challenge in type 1 diabetes: lack of impact of euglycemia. Diabetes Care. 2014;37(4):1076-1082.
doi pubmed - Dimosthenopoulos C, Liatis S, Kourpas E, Athanasopoulou E, Driva S, Makrilakis K, Kokkinos A. The beneficial short-term effects of a high-protein/low-carbohydrate diet on glycaemic control assessed by continuous glucose monitoring in patients with type 1 diabetes. Diabetes Obes Metab. 2021;23(8):1765-1774.
doi pubmed - Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11(4):1020-1076.
doi pubmed - Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health. 2018;3(9):e419-e428.
doi - Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147-157.
doi pubmed - Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, Desai M, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667-679.
doi pubmed - Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Matsunaga S, Hanyu O, et al. Is the proportion of carbohydrate intake associated with the incidence of diabetes complications? An analysis of the Japan diabetes complications study. Nutrients. 2017;9(2):113.
doi pubmed - Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523.
doi pubmed - Turton JL, Raab R, Rooney KB. Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS One. 2018;13(3):e0194987.
doi pubmed - Seckold R, Fisher E, de Bock M, King BR, Smart CE. The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: a review of clinical outcomes. Diabet Med. 2019;36(3):326-334.
doi pubmed - Skytte MJ, Samkani A, Petersen AD, Thomsen MN, Astrup A, Chabanova E, Frystyk J, et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62(11):2066-2078.
doi pubmed - Schmidt S, Christensen MB, Serifovski N, Damm-Frydenberg C, Jensen JB, Floyel T, Storling J, et al. Low versus high carbohydrate diet in type 1 diabetes: A 12-week randomized open-label crossover study. Diabetes Obes Metab. 2019;21(7):1680-1688.
doi pubmed - Ranjan A, Schmidt S, Damm-Frydenberg C, Holst JJ, Madsbad S, Norgaard K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: A randomized open-label crossover trial. Diabetes Obes Metab. 2017;19(10):1479-1484.
doi pubmed - Ito A, Horie I, Miwa M, Sako A, Niri T, Nakashima Y, Shigeno R, et al. Impact of glucagon response on early postprandial glucose excursions irrespective of residual beta-cell function in type 1 diabetes: A cross-sectional study using a mixed meal tolerance test. J Diabetes Investig. 2021;12(8):1367-1376.
doi pubmed - Ahren B. DPP-4 inhibitors. Best Pract Res Clin Endocrinol Metab. 2007;21(4):517-533.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
