| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Short Communication
Volume 14, Number 4, August 2024, pages 207-212
Effect of Mitochondrial Transfer via Centrifugation on Insulin Resistance in C2C12 Cells
Kyung-Soo Kima, d, Yeon Kyung Choib, Mi Jin Kimb, Chang-Koo Yunb, Jung Wook Hwangb, Kyunghoon Minc, Sang Youn Junga, Soo-Kyung Kima, Yong-Wook Choa, Yong-Soo Choib, d
aDepartment of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
bDepartment of Biotechnology, CHA University, Seongnam, Korea
cDepartment of Rehabilitation Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
dCorresponding Author: Kyung-Soo Kim, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Bundang-gu, Seongnam-si, Gyeonggi-do, Korea; Yong-Soo Choi, Department of Biotechnology, CHA University, Bundang-gu, Seongnam-si, Gyeonggi-do, Korea
Manuscript submitted May 27, 2024, accepted July 18, 2024, published online July 30, 2024
Short title: Effect of Mitochondrial Transfer on IR
doi: https://doi.org/10.14740/jem994
| Abstract | ▴Top |
Background: Insulin resistance is associated with mitochondrial dysfunction. Whether mitochondrial transfer using the centrifugation method has a beneficial effect on insulin resistance in muscles has not been reported yet. This study aimed to investigate the effect of mitochondrial transfer via centrifugation on insulin resistance in C2C12 cells.
Methods: Insulin resistance was induced in C2C12 cells using palmitate. Healthy mitochondria from C2C12 cells were transferred into insulin-resistant C2C12 cells using the centrifugation method. Glucose uptake was evaluated to confirm the effect of mitochondrial transfer on insulin resistance. Whether mitochondrial dysfunction was improved was assessed based on changes of mitochondrial contents and function.
Results: Healthy mitochondria were transferred into C2C12 cells using the centrifugation method. Mitochondrial transfer improved 2-deoxy glucose uptake in palmitate-treated C2C12 cells. It enhanced mitochondrial function by restoring adenosine triphosphate synthesis. Mitochondrial transfer increased the relative level of mitochondrial DNA copy number.
Conclusions: Mitochondrial transfer via centrifugation improved insulin resistance in C2C12 cells.
Keywords: Insulin resistance; Mitochondria; Mitochondrial replacement therapy; Type 2 diabetes mellitus
| Introduction | ▴Top |
Mitochondrial dysfunction is associated with insulin resistance [1]. Some studies have shown that improving mitochondrial dysfunction is associated with beneficial effects on type 2 diabetes mellitus [2, 3]. However, an improvement in mitochondrial dysfunction by targeted therapies would be limited since mitochondrial dysfunction is caused by complex mechanisms [4]. Mitochondrial transplantation might be one of the promising options for improving mitochondrial dysfunction [5, 6].
There are several ways to transfer mitochondria, including direct injection, co-incubation, magnetomitotransfer, cell-penetrating peptide, and biocompatible polymer [6]. Recently, a centrifugation-based method has been reported as a simple and rapid way to improve the efficacy of mitochondrial transfer [7]. Mitochondrial transfer using the centrifugation method has demonstrated that exogenous mitochondria can be successfully delivered into damaged tenocytes to improve tendinopathy [8]. However, whether mitochondrial transfer using the centrifugation method has a beneficial effect on insulin resistance in muscle cells has not been reported yet. Thus, the aim of this study was to investigate the effect of mitochondrial transfer on insulin resistance in C2C12 cells.
| Materials and Methods | ▴Top |
Cell culture
This study was approved by CHA University Institutional Review Board (201803-BR-015-04) and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. C2C12 cells derived from mouse skeletal muscles (CRL-1772, passage #6) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) high glucose supplemented with 10% fetal bovine serum (FBS; Gibco, Waltham, MA, USA), 1% penicillin/streptomycin (P/S; Hyclone) at 37 °C under a humidified atmosphere containing 5% CO2. For differentiation of myoblasts to myotubes, the media was replaced with differentiation media containing DMEM supplemented with 2% horse serum (Gibco) and 1% P/S and continued to incubate for 4 days.
Induction of insulin resistance
To induce insulin resistance, the differentiated C2C12 myotubes were treated with palmitate (Sigma, Darmstadt, Germany). Palmitate was dissolved in 95 °C heated ethanol to a concentration of 100 mM. After filtration, 100 mM palmitate was diluted 1:100 with DMEM containing 2% bovine serum albumin (BSA; Bio Basic, Markham, Canada) to yield a final palmitate concentration of 1 mM. The palmitate-BSA mixture in DMEM was incubated at 37 °C for 2 h to conjugate with BSA. And then, C2C12 cells were incubated with 1 mM palmitate for 24 h as an in vitro model for insulin resistance.
Preparation of mitochondria
Using differential centrifugation as we previously described, mitochondria were isolated from allogeneic C2C12 cells [7]. Briefly, 2 × 107 C2C12 cells were suspended in SHE buffer ((0.25 M sucrose, 20 mM hydroxyethyl piperazine ethane sulfonic acid (HEPES) (pH 7.4), 2 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA), and 0.1% defatted BSA) and lysed using a 1 mL syringe. To remove cell debris, centrifugation was performed at 1,500 × g for 5 min at 4 °C. The supernatant was collected to another tube and centrifuged at 20,000 × g for 10 min at 4 °C to remove the cytosol fraction. And then, the mitochondrial pellet was suspended in 1.8 mL SHE buffer and centrifuged at 20,000 × g for 10 min at 4 °C. The pellet was suspended in 1 mL SHE buffer without BSA (respiration buffer) and centrifuged at 20,000 × g for 10 min at 4 °C. Finally, the pellet was resuspended in 60 µL phosphate-buffered saline (PBS) after removing the supernatant and kept on ice until measurements were performed. Isolated mitochondria were quantified by determining the protein concentration using a bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). All the experiments were performed with freshly isolated mitochondria.
Mitochondrial transfer into C2C12 cells
Isolated mitochondria were transferred into the palmitate-induced insulin resistant C2C12 myotubes by centrifugation as we previously described [7]. For transfer of mitochondria, cells were suspended in 100 µL of PBS and kept on ice. Mitochondria doses (1, 10 µg) refer to the weight of donor cell mitochondria per 1 × 105 C2C12 cells. The prepared mitochondria suspension (in 10 µL of PBS) was added to the tube of C2C12 cells suspended in PBS. The mixture was centrifuged at 1,500 × g for 5 min at 4 °C and washed twice with PBS. And then, C2C12 cells were re-seeded in a six-well plate for further experiments.
2-deoxy D-glucose uptake assay
After treatment with palmitate, cells were stimulated with 100 nM insulin (Eli Lilly Nederland, Indianapolis, IN, USA) for 30 min. The palmitate-untreated group was used as a negative group for comparing insulin-stimulated glucose uptake. Also, as a positive control, insulin-sensitizing agent pioglitazone (50 µM) was used. Accumulated 2-deoxy glucose-6-phosphate in C2C12 cells treated with mitochondrial transfer or pioglitazone was measured using a glucose uptake assay kit (Abcam, Cambridge, UK).
Measurement of adenosine triphosphate (ATP) contents and ATP synthesis
Intracellular ATP levels were measured using a CellTiter-Glo 2.0 assay kit (Promega, Madison, WI, USA). To measure ATP, insulin resistance-induced cells by palmitate were seeded in opaque-walled 96-well plates after mitochondrial transfer. After 24 h, cells were washed with PBS and added 50 µL of CellTiter-Glo luminescence test solution and incubated for 30 min at room temperature. Also, to determine ATP synthesis, cells were incubated with adenosine diphosphate for 45 min before adding the ATP reagent. Luminescence signals were determined using a luminescence microplate reader.
Real-time polymerase chain reaction
Mitochondrial-encoded gene (COX2) and nuclear-encoded gene (Rsp18) expression levels were measured using reverse transcription polymerase chain reaction (PCR; Applied Biosystems, Foster City, CA, USA). The ratio of COX2 versus Rsp18 was used for calculating the relative level of mitochondrial DNA (mtDNA) copy number.
Statistical analysis
All statistical analyses were performed using SigmaPlot 14.0 Software (Systat Software, San Jose, CA, USA). Significant differences of investigated parameters between groups were evaluated using Student’s t-test or one-way analysis of variance test followed by Bonferroni post hoc test. Statistical significance was defined at P < 0.05.
| Results | ▴Top |
Effect of mitochondrial transfer on glucose uptake
To evaluate the effect of mitochondrial transfer on insulin resistance induced by palmitate, 2-deoxy glucose (2-DG) uptake was measured (Fig. 1). The decrease of 2-DG uptake by treatment of palmitate was confirmed compared to palmitate-nontreated group. And 2-DG uptake was improved by mitochondrial transfer compared to the palmitate-treated group. Additionally, significant improvement was also found in the pioglitazone-treated group.
 Click for large image | Figure 1. Effect of mitochondrial transfer on glucose uptake in C2C12 cells. Results are expressed as fold change of mean ± standard deviation (n ≥ 3). *P < 0.05 vs. insulin-stimulated group. #P < 0.05 vs. insulin and palmitate treated group. MT: mitochondria; Pio: pioglitazone. |
Effect of mitochondrial transfer on mitochondrial function and contents
The intracellular ATP content and synthesis activity were decreased in the insulin-resistant muscle cells by treatment of palmitate (Fig. 2a and Supplementary Material 1, www. jofem.org). Although ATP content was not changed (Supplementary Material 1, www. jofem.org), ATP synthesis was increased by mitochondrial transfer at 10 µg (Fig. 2a). Furthermore, mitochondrial transfer increased the relative level of mtDNA copy number compared to palmitate-treated groups (Fig. 2b).
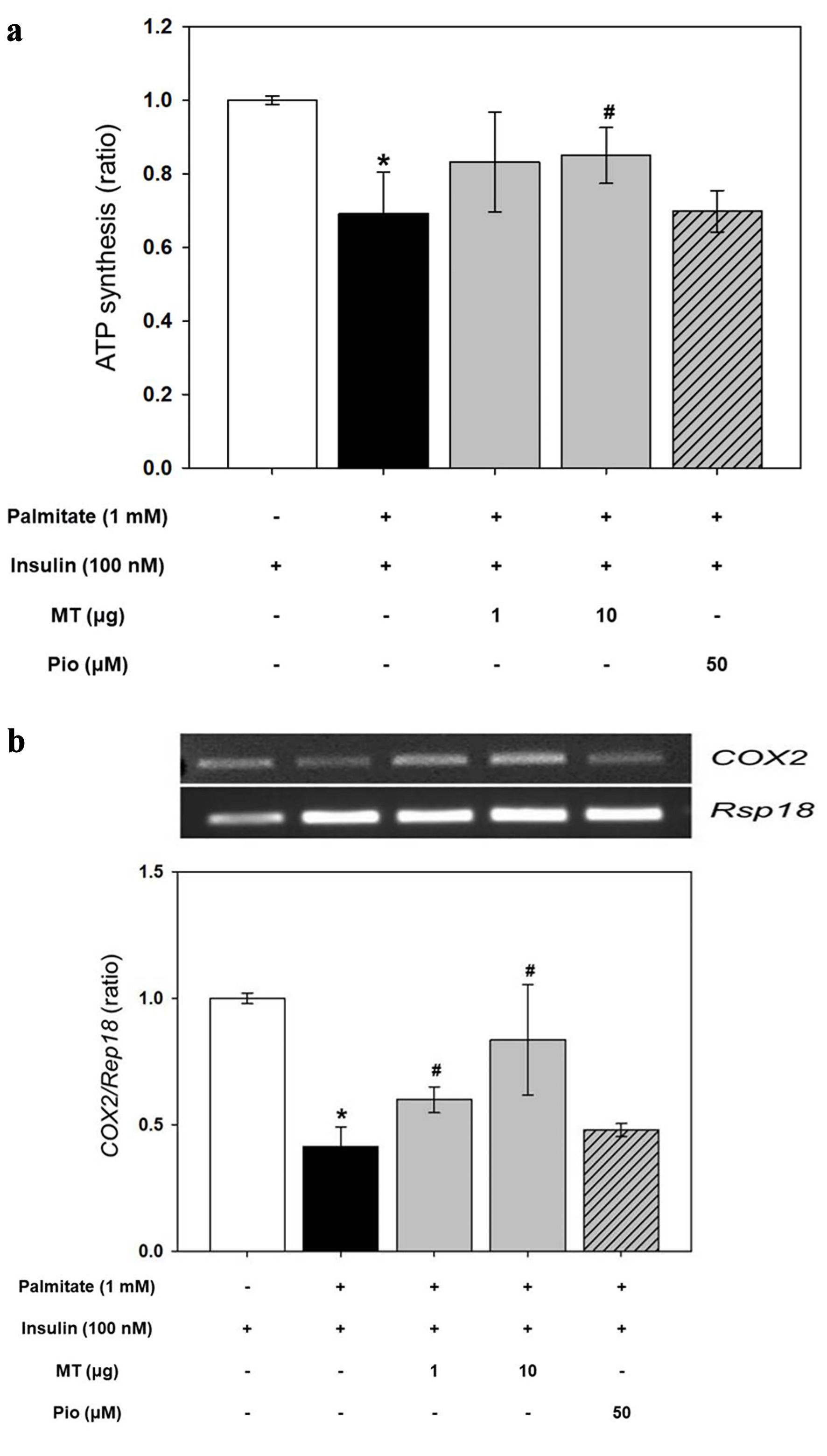 Click for large image | Figure 2. Effects of mitochondrial transfer on mitochondrial function and content. (a) Adenosine triphosphate (ATP) synthesis. (b) Relative level of mitochondrial DNA (mtDNA) copy number. Results are expressed as fold change of mean ± standard deviation (n ≥ 3). *P < 0.05 vs. insulin-stimulated group. #P < 0.05 vs. insulin and palmitate treated group. MT: mitochondria; Pio: pioglitazone. |
| Discussion | ▴Top |
This study demonstrated that mitochondrial transfer to C2C12 cells treated with palmitate using centrifugation as a mitochondria intracellular delivery method effectively ameliorates insulin resistance. This is the first study to evaluate the efficacy of mitochondrial transfer using the centrifugation method in mitigating insulin resistance in muscle cells.
Many studies have described a complex bidirectional association between insulin resistance and mitochondrial function. Insulin can stimulate mitochondrial activity that can reduce hepatic lipid accumulation and enhance insulin sensitivity and glucose homeostasis [9]. In addition, mutations of mitochondrial DNA can inhibit mitochondrial β-oxidation and glucose transporter type 4 (GLUT4) translocation, which can increase fatty acid accumulation and induce insulin resistance [10].
Several modes including direct injection, co-incubation, magnetomitotransfer, cell-penetrating peptide, biocompatible polymer, photothermal nanoblade, and fluidic force microscope have been established to transfer isolated healthy mitochondria to recipient cells [6]. The centrifugation method used in this study is a simple and quick way to deliver exogenous mitochondria into culture cells without further co-incubation. It has a high transfer efficiency and reproducibility [7]. This strategy is not accompanied by membrane disruption. It has no technical requirements like microinjection, photothermal nanoblade, or fluidic force microscope. We have previously reported that exogenous mitochondria can be successfully transferred by centrifugation with protective effects on damaged tenocytes [8]. In this study, although we were unable to directly trace the transferred mitochondria, we could assume that mitochondrial transfer into the cells would have been successful.
Mitochondrial function is closely linked to ATP content and synthesis. Previously, we found that mitochondrial transfer normalized ATP production in human umbilical cord-derived mesenchymal stem cells [7]. In the present study, mitochondrial transfer to insulin-resistant muscle cells led to an enhancement in ATP synthesis, which may have improved insulin resistance assessed by 2-DG uptake. However, although ATP synthesis was not increased by mitochondrial transfer at a dose of 1 µg, insulin resistance was improved. This might be because insulin resistance was associated with various mechanisms including GLUT4 translocation, insulin signaling pathway, oxidative stress, and mitochondrial dynamics. Mitochondrial transfer may have a beneficial effect on these mechanisms as well, thereby improving insulin resistance. Further research is required to evaluate exactly how mitochondrial transfer may improve insulin resistance. In addition, mitochondrial transfer did not result in an increase in the total intracellular ATP level. This might be because insulin-resistant cells might have more complex defects of ATP production than other cells. Another possibility was that ATP generation is a complex process and is associated with various factors, so mitochondrial transfer might not restore it completely.
There have been many studies that have tried to treat diabetes by restoring the function of damaged mitochondria [11, 12]. Potential mitochondria-targeting agents such as NAD+ booster [13] and oxidative phosphorylation modulators [14] might improve insulin resistance through modulation of mitochondrial function. However, it has been difficult to completely restore dysfunctional mitochondria. Mitochondrial transfer is an attractive approach for treating various diseases because dysfunctional mitochondria in defective cells could be replaced with healthy mitochondria. It has been reported to be useful for treating myocardial ischemia-reperfusion injury, acute respiratory distress syndrome, Parkinson’s disease, spinal cord injury, liver injury, and acute kidney injury [15-20]. Treatment with mitochondria isolated from human HepG2 cells can reduce reactive oxygen species (ROS) and increase energy supply in an in vitro Parkinson’s disease model [17]. In addition, exogenous mitochondria from HepG2 cells can decrease ROS, increase energy supply, and reduce hepatotoxicity in in vivo and in vitro models of acetaminophen-induced liver injury [19]. Nevertheless, little is known about the association between mitochondrial transfer and diabetes. One study has reported that mitochondria from human adipose mesenchymal stromal cells can be successfully transferred into human islet β cells to enhance insulin secretion [21]. As far as we know, this is the first study to show that insulin resistance in muscle cells can be improved by mitochondrial transfer.
Unfortunately, this study was not conducted in human muscle cells. However, the C2C12 cell line has been used extensively to understand the molecular mechanisms underlying the progression of diabetes and obesity [22]. The use of C2C12 myoblast cell line has been meaningful in pharmaceutical and biomedical research due to their expression of GLUT-4 and other features that are representative to human skeletal muscle cells [23]. Furthermore, in the preclinical stage of pharmaceutical development, the C2C12 myoblast cell line has enabled the establishment of the potential safety profile and effective dosage range prior to testing of therapeutic agents in vivo by using animal models [23]. We believe that the results of this study conducted on the C2C12 cell line are likely to be reproduced in humans. Further studies are needed to evaluate whether the results of this study are replicated in human muscular metabolism.
In conclusion, mitochondrial transfer via centrifugation improved insulin resistance in muscle cells. Further study is warranted to elucidate effects of mitochondrial transfer on insulin resistance in animals or humans.
| Supplementary Material | ▴Top |
Suppl 1. Effects of mitochondrial transfer on adenosine triphosphate (ATP) contents. Results are expressed as fold change of mean ± standard deviation (n ≥ 3). *P < 0.05 vs. insulin-stimulated group. MT: mitochondria; Pio: pioglitazone.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the Korean Endocrine Society of Hyangseol Young Investigator Award 2022, the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. NRF-2018R1C1B5042633), and the Bio Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2018M3A9B5023052).
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
This study does not contain any studies with human subjects performed by any of the authors.
Author Contributions
Study concept and design: KSK and YSC; acquisition of data: KSK, YKC, MJK, JWH, and YSC; analysis and interpretation of data: KSK, YKC, MJK, CKY, JWH, KM, SYJ, SKK, YWC, and YSC; drafting of the manuscript: KSK, YKC, MJK, and YSC; critical revision of the manuscript: KSK, YKC, MJK, CKY, JWH, KM, SYJ, SKK, YWC, and YSC; statistical analysis: KSK, YKC, MJK, and YSC; obtained funding: KSK and YSC; administrative, technical, or material support: KSK and YSC; and study supervision: KSK and YSC.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4(1):R1-R15.
doi pubmed pmc - Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572-579.
doi pubmed pmc - Vial G, Chauvin MA, Bendridi N, Durand A, Meugnier E, Madec AM, Bernoud-Hubac N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes. 2015;64(6):2254-2264.
doi pubmed - Montgomery MK. Mitochondrial dysfunction and diabetes: is mitochondrial transfer a friend or foe? Biology (Basel). 2019;8(2):33.
doi pubmed pmc - Chen Y, Yang F, Chu Y, Yun Z, Yan Y, Jin J. Mitochondrial transplantation: opportunities and challenges in the treatment of obesity, diabetes, and nonalcoholic fatty liver disease. J Transl Med. 2022;20(1):483.
doi pubmed pmc - Zhang TG, Miao CY. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm Sin B. 2023;13(3):1028-1035.
doi pubmed pmc - Kim MJ, Hwang JW, Yun CK, Lee Y, Choi YS. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018;8(1):3330.
doi pubmed pmc - Lee JM, Hwang JW, Kim MJ, Jung SY, Kim KS, Ahn EH, Min K, et al. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants (Basel). 2021;10(5):696.
doi pubmed pmc - Szendroedi J, Kaul K, Kloock L, Strassburger K, Schmid AI, Chmelik M, Kacerovsky M, et al. Lower fasting muscle mitochondrial activity relates to hepatic steatosis in humans. Diabetes Care. 2014;37(2):468-474.
doi pubmed - Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286(5440):774-779.
doi pubmed - Krako Jakovljevic N, Pavlovic K, Jotic A, Lalic K, Stoiljkovic M, Lukic L, Milicic T, et al. Targeting mitochondria in diabetes. Int J Mol Sci. 2021;22(12):6642.
doi pubmed pmc - Singh A, Faccenda D, Campanella M. Pharmacological advances in mitochondrial therapy. EBioMedicine. 2021;65:103244.
doi pubmed pmc - van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193-1201.
doi pubmed pmc - Vial G, Lamarche F, Cottet-Rousselle C, Hallakou-Bozec S, Borel AL, Fontaine E. The mechanism by which imeglimin inhibits gluconeogenesis in rat liver cells. Endocrinol Diabetes Metab. 2021;4(2):e00211.
doi pubmed pmc - Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, Ericsson M, et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One. 2016;11(8):e0160889.
doi pubmed pmc - Sun CK, Lee FY, Kao YH, Chiang HJ, Sung PH, Tsai TH, Lin YC, et al. Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J Pineal Res. 2015;58(2):137-150.
doi pubmed - Shi X, Zhao M, Fu C, Fu A. Intravenous administration of mitochondria for treating experimental Parkinson's disease. Mitochondrion. 2017;34:91-100.
doi pubmed - Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, Sullivan PG, et al. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma. 2018;35(15):1800-1818.
doi pubmed pmc - Shi X, Bai H, Zhao M, Li X, Sun X, Jiang H, Fu A. Treatment of acetaminophen-induced liver injury with exogenous mitochondria in mice. Transl Res. 2018;196:31-41.
doi pubmed - Doulamis IP, Guariento A, Duignan T, Kido T, Orfany A, Saeed MY, Weixler VH, et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am J Physiol Renal Physiol. 2020;319(3):F403-F413.
doi pubmed pmc - Rackham CL, Hubber EL, Czajka A, Malik AN, King AJF, Jones PM. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells. 2020;38(4):574-584.
doi pubmed pmc - Yudhani RD, Sari Y, Nugrahaningsih DAA, Sholikhah EN, Rochmanti M, Purba AKR, Khotimah H, et al. In vitro insulin resistance model: a recent update. J Obes. 2023;2023:1964732.
doi pubmed pmc - Wong CY, Al-Salami H, Dass CR. C2C12 cell model: its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. J Pharm Pharmacol. 2020;72(12):1667-1693.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
