| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 14, Number 5, October 2024, pages 240-249
Exploring Cystatin C as an Early Indicator of End-Stage Diabetic Nephropathy in Patients With Type 2 Diabetes
Hojjat Zadabbasa, Morteza Golbashirzadehb, Atousa Moradzadegana, c
aDepartment of Experimental Sciences, Dezful Branch, Islamic Azad University, Dezful, Iran
bSchool of Nursing, Iranshahr University of Medical Sciences, Chabahar, Iran
cCorresponding Author: Atousa Moradzadegan, Department of Experimental Sciences, Dezful Branch, Islamic Azad University, Dezful, Iran
Manuscript submitted May 6, 2024, accepted August 7, 2024, published online October 31, 2024
Short title: Cystatin C Indicates Diabetic Nephropathy
doi: https://doi.org/10.14740/jem991
| Abstract | ▴Top |
Background: Diabetes, a prevalent metabolic disorder worldwide, significantly impacts various bodily organs. Type 2 diabetes, in particular, leads to chronic complications affecting the brain, eyes, cardiovascular system, and kidneys. Diabetic nephropathy, characterized by altered glomerular filtration rate (GFR) and increased urinary albumin excretion, represents a progressive kidney dysfunction. This study aimed to explore the association between serum cystatin C levels and diabetic nephropathy as a predictive marker.
Methods: In this investigation, a total of 179 patients were categorized into four groups: a diabetic nephropathy group, a nephropathy group, a diabetes group, and a healthy control group. After obtaining informed consent and recording demographic indicators and clinical symptoms, a 5 mL blood sample was collected from each participant to assess the cystatin C levels using the enzyme-linked immunosorbent assay method.
Results: The study findings revealed no significant differences in demographic indicators among the groups (P > 0.05). Additionally, the average serum cystatin C level in the diabetes group did not significantly differ from that in the control group (P > 0.05). However, a notable increase was observed in the mean serum cystatin C levels between the healthy group and both the diabetic nephropathy and nephropathy groups (P = 0.001).
Conclusion: The study findings indicate that serum cystatin C levels serve as a potential warning and predictive factor for nephropathy in diabetic patients.
Keywords: Diabetes; Nephropathy; Cystatin C; Metabolic disorder
| Introduction | ▴Top |
Diabetes mellitus is a metabolic disorder marked by elevated blood glucose levels due to inadequate insulin production or impaired cellular response to insulin [1]. Certainly, diabetes mellitus is indeed a prevalent chronic condition globally. It ranks among the top five causes of mortality worldwide [2, 3].
In diabetes, there is a disruption in carbohydrate metabolism, leading to impaired utilization of sugars by cells as an energy source. This primarily results from either insufficient insulin production or dysfunction in insulin’s action [4, 5]. Diabetes, being a chronic condition with gradual progression, indeed places a significant burden on affected families. The associated complications further exacerbate the impact, affecting both the financial aspect and overall quality of life [6].
Chronic kidney disease (CKD) arises when a condition impairs kidney function, leading to gradual kidney damage over several months or years. This condition is associated with various chronic disorders, including diabetic nephropathy (kidney disease resulting from poorly controlled diabetes), retinopathy affecting vision, neuropathy affecting the peripheral nervous system, atherosclerosis in large blood vessels and elevated blood pressure. The underlying reason of these complications is an extreme rise in blood glucose levels. Additionally, it is worth noting that glomerular diseases can also impact kidney function. These diseases affect the glomeruli, which are the involved system of blood vessels accountable for filtering waste and surplus fluids in the kidneys [7, 8].
Hyperglycemia, a common feature of diabetes, has been associated with inflammation. Research indicates that elevated blood glucose levels can trigger inflammatory responses throughout the body [9]. In addition, tissue fibrosis in some organs due to long-term diabetes has been reported [10, 11]. Additionally, chronic hyperglycemia may lead to tissue fibrosis in various organs. Among these, the kidney system is predominantly susceptible to efficient and tissue disorders prejudiced by diabetes-related inflammation [12]. Disturbance in this system can be one of the main causes in the progression of the disease and its irreversible complications leading to death [10, 13].
Persistent inflammation can lead to tissue damage in sensitive organs, including the heart, blood vessels, and kidneys. Additionally, it increases the likelihood of fibrosis development [14]. CKDs can progress to end-stage renal disease (ESRD) and cardiovascular complications, imposing a significant societal and economic burden. Regardless of the underlying cause, resident fibroblasts play crucial roles in CKD development. These fibroblasts transform into myofibroblasts upon injury, expressing alpha smooth muscle actin (αSMA) and producing excessive extracellular matrix (ECM) proteins, ultimately leading to renal fibrosis. Additionally, these resident fibroblasts contribute to inflammation during kidney injury by activating nuclear factor-κB (NF-κB) signaling and producing pro-inflammatory cytokines [15]. Therefore, early diagnosis and solving this problem has always been one of the important issues for maintaining the health of people with diabetes [16]. In the present day, the increased incidence of metabolic diseases and associated complications has underscored the significance of identifying predictive factors, capturing the attention of researchers [17]. Also, many biomarkers play an important role in the diagnosis and prognosis of diabetic nephropathy [18].
Multiple studies have demonstrated the close association between proteinuria, glomerular filtration rate (GFR), creatinine clearance, blood pressure, and glomerular lesions with the development of diabetic nephropathy [19, 20]. In addition, serum creatinine has been used as one of the markers of renal dysfunction [21].
Cystatin C, a low molecular weight protein consisting of 122 amino acids, is ubiquitously produced by all nucleated cells in the body. It functions as an inhibitor of cysteine proteinases [22]. The serum concentration of cystatin C is the same in adults and children over 1 year old [23]. Indeed, cystatin C exhibits remarkable characteristics. Despite its low molecular weight and positive charge, it manages to traverse the glomerular membrane freely and subsequently undergoes reabsorption in the proximal tubule. This unique behavior contributes to its diagnostic relevance in renal function assessment [24]. The initial measurement of cystatin C in the blood shows the amount of glomerular filtration [25]. An increase in serum cystatin C is associated with a decrease in GFR and is therefore an indicator of kidney damage [26]. However, our research, despite the scarcity of existing studies regarding this protein’s role as a predictor in diabetic nephropathy, seeks to investigate the fluctuations in serum cystatin C levels specifically among patients diagnosed with end-stage diabetic nephropathy.
| Materials and Methods | ▴Top |
This cross-sectional study examined variations in serum cystatin C levels among patients with end-stage diabetic nephropathy who were referred to Dr. Ganjavian Dezful Hospital in 2017. The study involved four distinct groups: a healthy group, a diabetes group, a nephropathy group, and a diabetic nephropathy group, all of whom sought care at Dr. Ganjavian Dezful Hospital during the same year. While the studied groups shared similar intervening variables, the diseases under investigation differed. The healthy group comprised 50 individuals, the diabetes group had 39 participants, the nephropathy group included 49 subjects, and the diabetic nephropathy group consisted of 41 patients. Random sampling was employed across all groups. Diabetic status was determined based on the 2016 American Diabetes Association (ADA) guidelines, which consider fasting plasma blood sugar levels exceeding 126 mg/dL (confirmed by two measurements) and specific diabetes symptoms (such as excessive thirst, overeating, frequent urination, or plasma glucose levels exceeding 200 mg/dL). Additionally, indicators of advanced nephropathy included a low GFR of 30 mL/min and urine protein levels of +2. Disease diagnoses within each group were established through consultation and approval by internal medicine and metabolic disease specialists, as well as nephrologists.
This study was performed in line with the principles of the Declaration of Helsinki. The Ethics Committee of Dezful University of Medical Sciences approved and supervised the conduction of this study under the ethics code of IR.DUMS.REC.1397.003.
Sampling
Upon selecting and enrolling the samples for our study, we meticulously documented demographic information and clinical symptoms. To investigate the association between diagnostic analytes and cystatin C levels in the serum of our study participants, we collected 10 mL of whole blood from each subject. The blood was drawn into anticoagulant-free test tubes to facilitate serum separation. Subsequently, we measured various serum variables, including blood sugar, hemoglobin A1c (HbA1c), blood urea nitrogen (BUN), creatinine, albumin, aspartate aminotransferase (AST or SGOT), alanine aminotransferase (ALT or SGPT), alkaline phosphatase (ALP), sodium (Na), potassium (K), calcium (Ca), and cystatin C. After separation, the sera were stored at -70 °C until further analysis. Diagnostic data were obtained using the BT1200 biochemical analyzer, meticulously calibrated for each analyte, at the central laboratory of Dr. Ganjavian Dezful Hospital. Serum cystatin C levels were quantified using the enzyme-linked immunosorbent assay (ELISA) method, employing a specific human cystatin C serum ELISA kit (Abcam, Cambridge, USA).
Determination of serum cystatin C level
In this study, in order to determine the serum level of cystatin C in the studied samples in the case and control groups, blood sampling was done. Then the sera were separated from the blood samples of the subjects through centrifugation for 15 min at 2,500 rpm.
Measurement of the serum level of cystatin C in the studied samples was done by ELISA method using a commercial human cystatin C serum ELISA kit (Abcam, Cambridge, USA). The collected serum samples were measured according to the manufacturer’s instructions.
Data analysis method and statistical analysis
Initially, after collecting the data, we proceeded with the coding process. Subsequently, we utilized SPSS version 16 software and employed statistical techniques for data analysis. Descriptive statistical methods, including the construction of frequency distribution tables and calculation of central and dispersion indices, were applied to characterize the study subjects and objectives. Additionally, to assess differences among study groups and test hypotheses, inferential statistical methods were applied. These included the Kolmogorov-Smirnov test, Levene’s test, Chi-square test, Fisher’s exact test, independent t-test, two-way analysis of variance (ANOVA), and the Mann-Whitney U test.
| Results | ▴Top |
Demographic indicators
This cross-sectional study was conducted in 2017 with the aim of investigating changes in the amount of cystatin C in the serum of patients with end-stage diabetic nephropathy referred to Dr. Ganjavian Dezful Hospital in the healthy group, the diabetes group, the nephropathy group, and the diabetic nephropathy group, with a sample size of 179 people (90 men and 89 women). The number of samples was 50 (27 men and 23 women) in the healthy group, 39 (20 men and 19 women) in the diabetes group, 49 (30 men and 19 women) in the nephropathy group, and 41 (27 men and 14 women) in the diabetic nephropathy group. The results showed that the four studied groups were not significantly different in terms of gender composition (P = 0.513). Also, the average age of the samples was 43.16 ± 15.36 years in the healthy group, 41.03 ± 12.22 years in the diabetes group, 44.80 ± 13.75 years in the nephropathy group, and 43.07 ± 8.07 years in the diabetic nephropathy group. The results showed that the four studied groups were not significantly different in terms of age (P = 0.601).
Differences on laboratory parameters
The average level of fasting blood sugar and the percentage of glycosylated hemoglobin were 91.78 ± 9.16 and 5.34 ± 0.59 mg/dL in the healthy group, 294.56 ± 76.85 mg/dL in the diabetes group, 122.55 ± 24.44 and 8.31 ± 0.89 mg/dL in the nephropathy group, and 176.50 ± 40.77 and 7.93 ± 0.64 mg/dL in the diabetic nephropathy group. The results showed that there was a significant difference in terms of the level of fasting blood sugar between the control group, the nephropathy and diabetic nephropathy groups (P < 0.001), but the average difference in the case of glycosylated hemoglobin between the diabetes and diabetic nephropathy groups was not significant (P > 0.05) (Fig. 1).
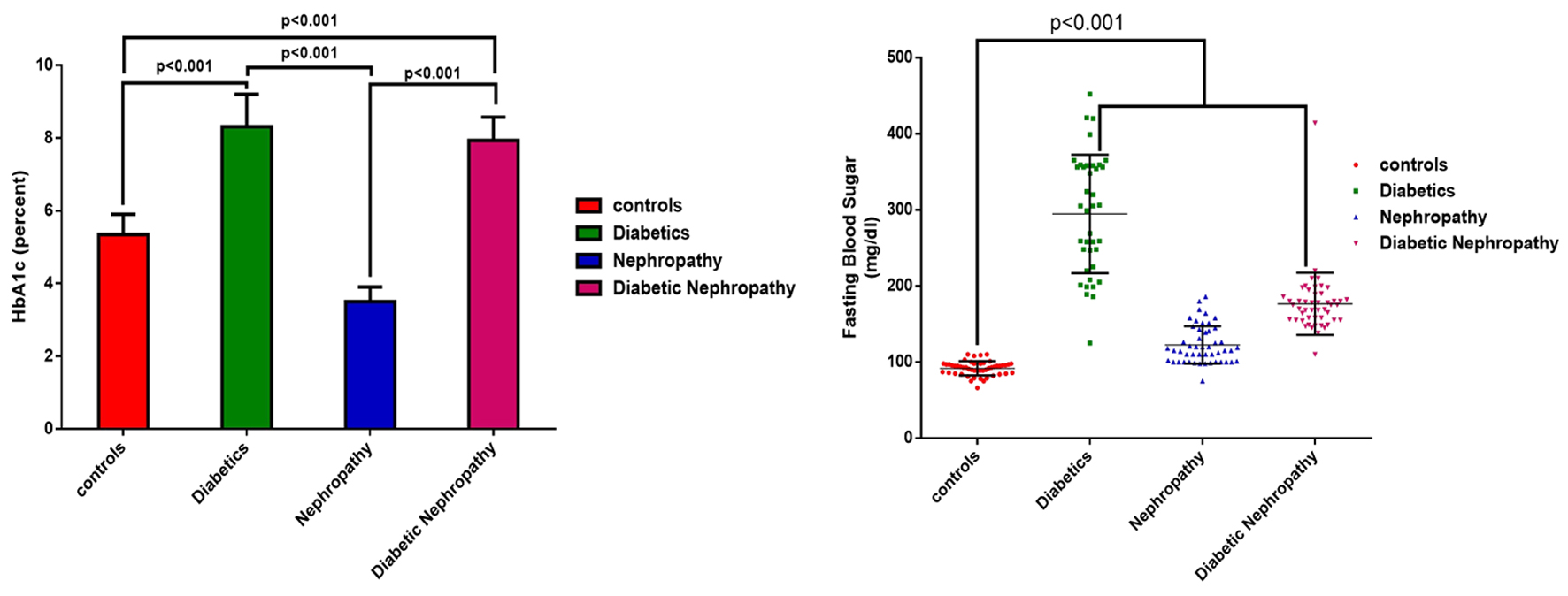 Click for large image | Figure 1. The average level of fasting blood sugar and the percentage of glycosylated hemoglobin of the studied samples by group. |
Data analysis to compare BUN and creatinine parameters of the groups showed that there was a significant difference between the healthy group and each of the nephropathy and diabetic nephropathy groups (P < 0.001), as well as between the whole healthy, nephropathy and diabetic nephropathy groups. Diabetic nephropathy group showed the absence of significant difference in serum albumin levels (P > 0.05). The difference between the mean albumin of the control group and the diabetes group was 0.8732 g/dL (P > 0.05, mean difference: 0.8732, 95% confidence interval (CI): -0.02843 to 1.775), with the nephropathy group of 0.165 g/dL (P > 0.05, mean difference: 0.1650, 95% CI: -0.7242 to 1.054), and with diabetic nephropathy group of 0.424 g/dL (P > 0.05, mean difference: 0.4244, 95% CI: -0.4240 to 1.273). The results showed that in the groups with diabetes, the average difference of albumin was more than that in the control group (Fig. 2).
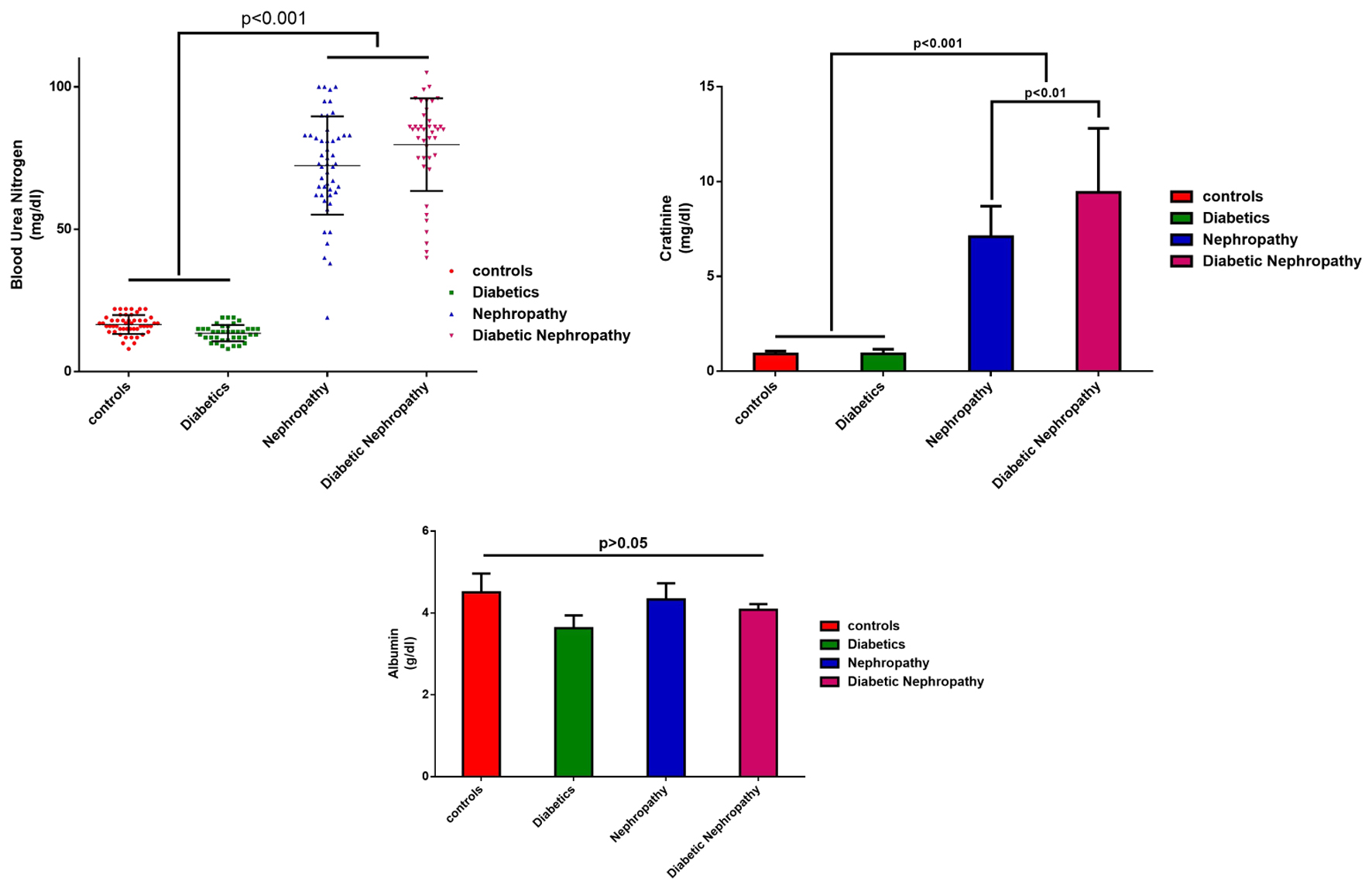 Click for large image | Figure 2. Average serum levels of urea nitrogen, creatinine and blood albumin of the studied samples by group. |
Analysis of biochemical data showed that the mean serum level of AST in the diabetic nephropathy group was significantly lower than those in the other groups (P < 0.001). Also, the mean serum ALT level in the diabetic nephropathy group was significantly lower than those in the control group and the nephropathy group (P < 0.001). Also, there was no significant difference in blood ALP levels between the control group and the diabetes, nephropathy and diabetic nephropathy groups (P < 0.01). The serum level of blood ALP in the control group was significantly lower than those in the other groups. The serum level of ALP in the diabetes group was significantly higher than that in the nephropathy group (P = 0.045) (Fig. 3).
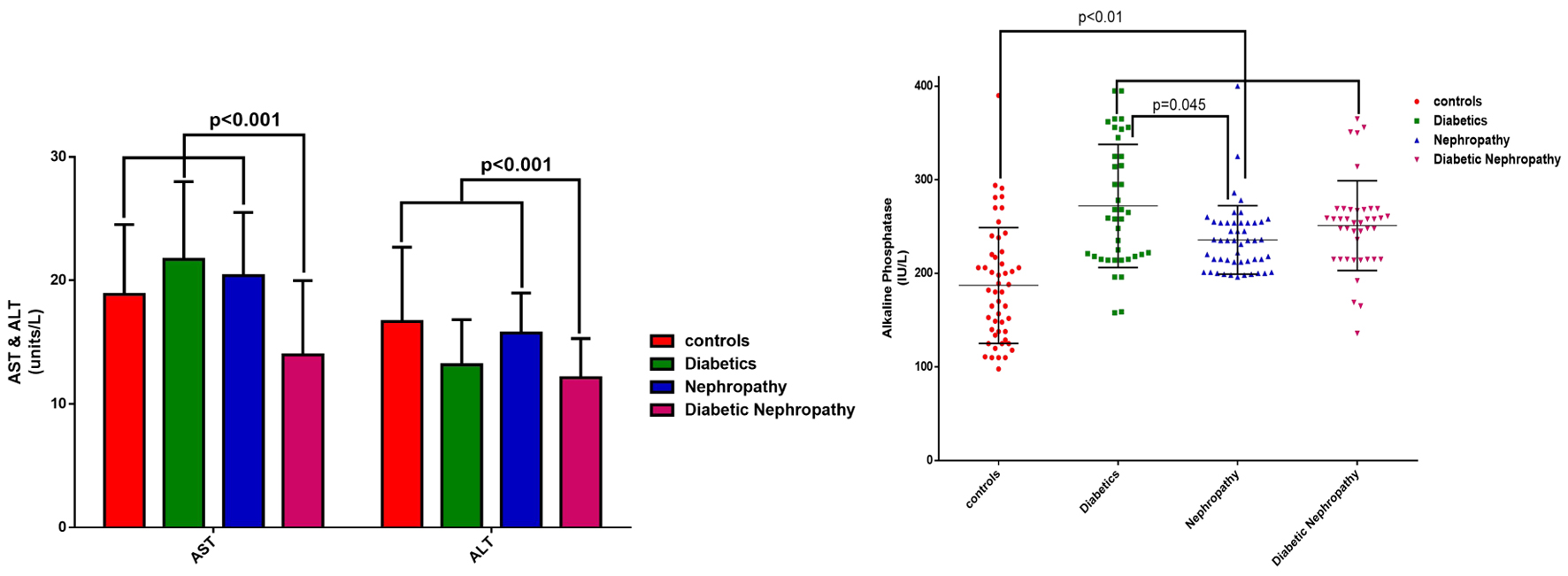 Click for large image | Figure 3. Average serum level of ALT, AST and ALP of the examined samples by group. ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase. |
Similar profiles of differences were found for serum sodium levels, which showed significant changes between the diabetic nephropathy group and each of the other groups (P < 0.001). The mean serum level of potassium in the diabetic nephropathy and nephropathy groups was significantly higher than those in the diabetes and control groups (P < 0.001). No significant changes were found in potassium (P > 0.05).
Cystatin C serum level analysis
The results of the investigations showed that the average blood serum level of cystatin C was 0.738 ± 0.127 mg/L in the healthy group, 0.760 ± 0.084 mg/L in the diabetes group, 1.208 ± 0.239 mg/L in the nephropathy group, and 1.249 ± 0.255 mg/L in the diabetic nephropathy group. The mean serum level of cystatin C in the diabetic nephropathy and nephropathy groups was significantly higher than those in the diabetes and control groups (P < 0.001). No significant difference was observed between the diabetes group and the control group in terms of serum cystatin C level (P > 0.05). Also, the difference in the mean serum cystatin C level between the diabetic nephropathy and nephropathy groups was not significant (P > 0.05) (Fig. 4).
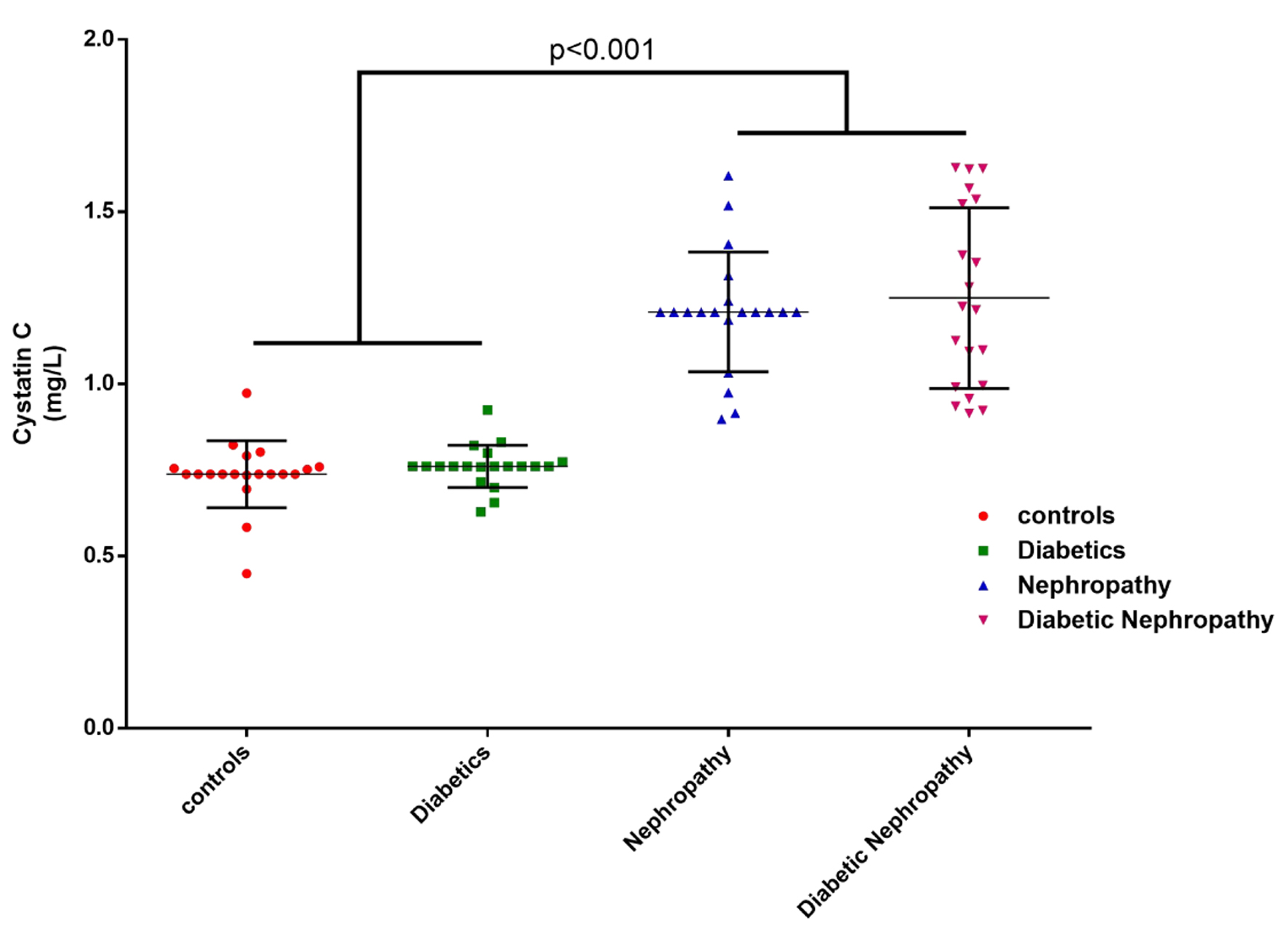 Click for large image | Figure 4. The mean serum level of cystatin C in the blood of the examined samples by group. |
In the analysis of factors related to kidney damage, the relationship between laboratory indicators of albumin, creatinine, BUN, and cystatin C was investigated. The results of the analysis showed that the amount of albumin among the studied groups has no significant difference. With the occurrence of nephropathy, the average difference between albumin and creatinine decreases significantly (P < 0.001) compared to the control and diabetes groups. But the average difference between albumin and cystatin C was not significant among the groups (P > 0.05). Also, the average difference of BUN to cystatin C, creatinine and albumin, with the occurrence of nephropathy and diabetic nephropathy, compared to the control and diabetes groups, has increased significantly (P < 0.001). However, this difference in mean BUN to cystatin C, creatinine and albumin was not significantly different between the groups. Also, the results showed that the average difference between creatinine and cystatin C in the control and diabetes groups was 0.168 and 0.157 mg/dL, respectively, which was not statistically significant. But this difference was significant in nephropathy and diabetic nephropathy groups (P < 0.001) (Fig. 5) (Table 1).
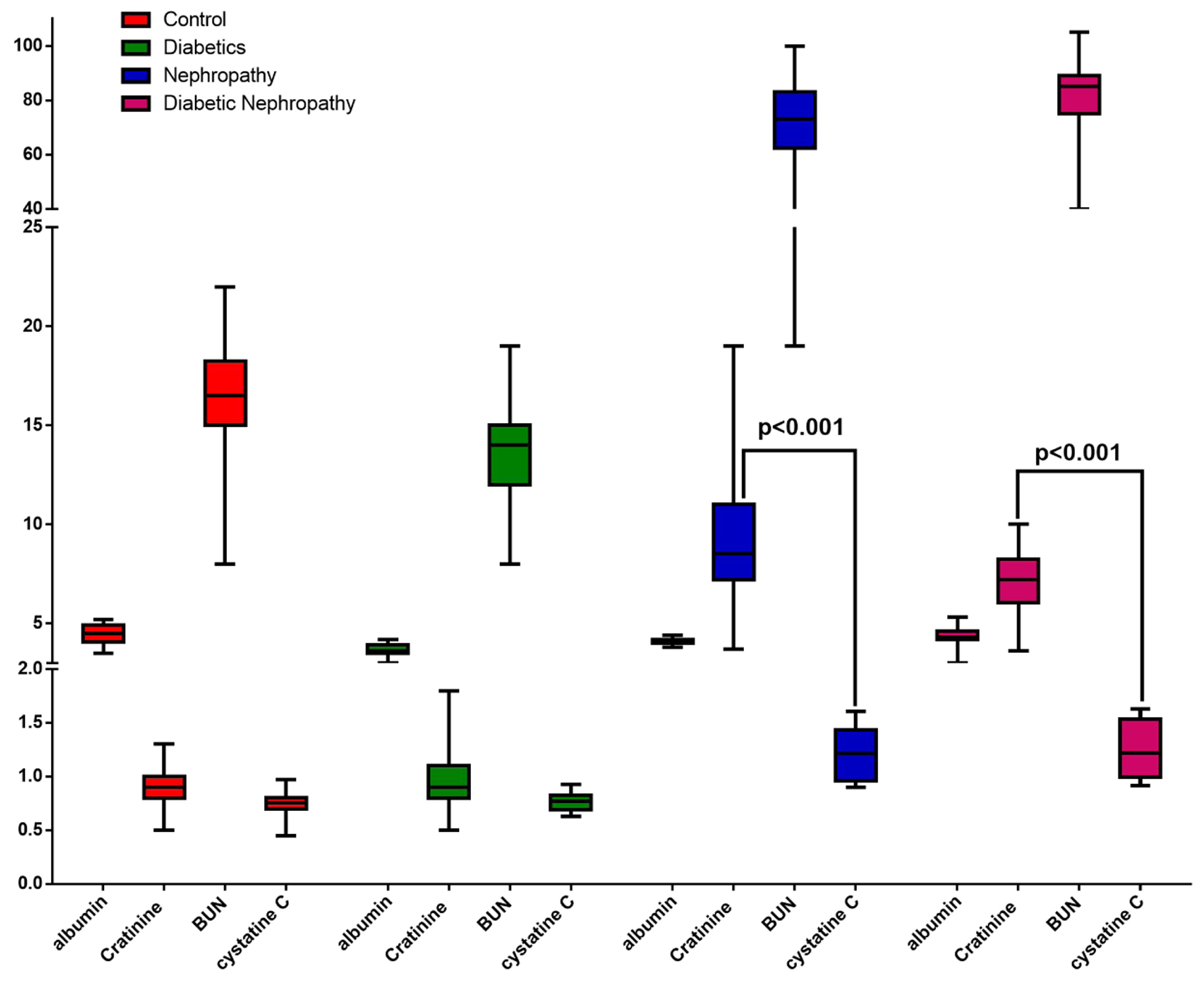 Click for large image | Figure 5. The average serum level of creatinine, albumin and cystatin C of the examined samples by group. |
 Click to view | Table 1. Difference in the Mean Serum Levels of Creatinine, Albumin and Cystatin C Blood Factors of the Examined Samples by Group Using Tukey’s Multiple Comparisons Test |
| Discussion | ▴Top |
Diabetic nephropathy, a progressive kidney disorder, manifests in individuals with both type 1 and type 2 diabetes. The risk of developing this condition is heightened by factors such as high blood pressure and hereditary kidney disease [27]. Although the precise etiology remains unclear, elevated blood sugar levels and hypertension are implicated in the pathogenesis of diabetic nephropathy [28-30]. In contemporary times, the prevalence of metabolic diseases has escalated significantly, leading to various complications. Consequently, researchers have placed substantial emphasis on identifying predictive factors associated with these conditions [31].
The study findings reveal significant differences in average fasting blood sugar levels among various groups. Specifically, the fasting blood sugar level in control subjects is the lowest and significantly distinct from that in individuals with diabetes and diabetic nephropathy. Additionally, there is a notable increase in the average percentage of glycosylated hemoglobin between the diabetes and diabetic nephropathy groups compared to the control and nephropathy groups. However, this difference between the two diabetic groups (diabetes and diabetic nephropathy) was not statistically significant.
The study findings indicate a substantial disparity in BUN serum levels across the examined groups. Specifically, the average BUN level in samples from both the nephropathy and diabetic nephropathy groups was significantly higher than that in the control and diabetes groups. Additionally, the average blood serum creatinine level in samples from the nephropathy and diabetic nephropathy groups was markedly elevated compared to the control and diabetes groups.
Subsequent to the examination of blood serum cystatin C levels, the findings revealed the following average serum concentrations: healthy group: 0.738 ± 0.127 mg/L; diabetes group: 0.760 ± 0.084 mg/L; nephropathy group: 1.208 ± 0.239 mg/L; diabetic nephropathy group: 1.249 ± 0.255 mg/L.
This finding shows that the occurrence of nephropathy and kidney problems leads to a significant increase in blood serum cystatin C levels. However, diabetes itself does not have a specific effect on serum cystatin C levels. Specifically, the average serum cystatin C level in the diabetic nephropathy and nephropathy groups is significantly higher than those in the diabetes and control groups. Interestingly, there is no significant difference in mean serum cystatin C levels between the two groups of diabetic nephropathy and nephropathy. Furthermore, no significant difference is observed between the diabetes group and the control group in terms of serum cystatin C levels. Thus, having diabetes does not appear to substantially impact the amount of cystatin C.
In their 2013 study, Jeon and colleagues evaluated the association between urinary cystatin C levels and the progression of type 2 diabetic nephropathy. The study included 237 type 2 diabetic patients who were followed up for 29 months. The researchers found that both the urinary cystatin C-to-creatinine ratio (CCR) and urinary non-albumin protein-to-creatinine ratio (NAPCR) were significantly different based on the degree of albuminuria. After adjusting for clinical factors, both urinary CCR and NAPCR were associated with a decline in the estimated glomerular filtration rate (eGFR). The study suggests that urinary cystatin C and NAP may serve as predictors of diabetic nephropathy progression [32].
A study by Bilgin et al [33] was conducted on 90 diabetic patients in 2021 with the aim of comparing the level of ferritin, cystatin C and high-sensitivity C-reactive protein (hs-CRP) in blood in type II diabetes patients according to the urine albumin level. They concluded that the mean ferritin level in patients with macro albuminuria is significantly higher than that in patients with normal urine albumin and the mean level of cystatin C in patients with micro albuminuria and macro albuminuria is significantly higher than that in patients with normal urine albumin, but the mean hs-CRP in patients was not significantly different according to the level of urinary albumin. In addition, the average level of glycosylated hemoglobin in patients with macro albuminuria was significantly higher than that in patients with normal urinary albumin and micro albuminuria. Also, the results showed that cystatin C alone can explain about 7% of diabetic nephropathy [33].
In an investigation conducted by Neelaveni et al, the serum concentration of cystatin C exhibited a direct and significant correlation with urinary albumin levels. This association suggests that the elevation in cystatin C may occur during the tubular phase before the onset of glomerular dysfunction. Consequently, both serum and urine cystatin C levels could serve as measurable indicators of subclinical tubular disorders, potentially identifying renal dysfunction prior to the manifestation of albuminuria [34].
In 2022, Aldenbratt et al investigated the utility of serum cystatin C as a measure of renal function in diabetic patients [35]. The study, titled “Cystatin C and renal function estimation: the search for better measurements of renal function in diabetic patients”, compared serum cystatin C levels with conventional estimates based on serum creatinine measurements. The findings strongly support the early detection of renal function decline using cystatin C levels. This research has important implications for optimizing early intervention strategies in diabetic nephropathy [36].
In their study, cystatin C demonstrated a stronger association with GFR compared to creatinine, using the Cockcroft formula, and the Modification of Diet in Renal Disease (MDRD) equation [35].
The concentration of cystatin C exhibits a stepwise increase that significantly correlates with the decline in GFR, indicating an inverse relationship. This finding supports the utility of cystatin C for early detection of kidney function decline. The study suggests that cystatin C is highly effective for measuring kidney function and may play a crucial role in early diagnosis, prevention, and treatment strategies for diabetic nephropathy [35, 37].
In their study, Tanabe et al highlighted that cystatin C serves as an endogenous marker for glomerular filtration. Consequently, its serum concentration is intricately linked to the GFR. Notably, diabetes exerts a notable impact on cystatin C levels: it leads to an 8.5% increase in serum cystatin C levels while concurrently causing a 3.9% decrease in serum creatinine levels [25]. The study findings revealed significant associations between serum cystatin C levels and several biomarkers. Specifically, an elevated level of CRP was positively correlated with cystatin C levels. Conversely, a decrease in white blood cell count and serum albumin was also linked to higher cystatin C levels. Additionally, creatinine levels exhibited an inverse relationship with cystatin C [25]. Furthermore, the study findings indicated that age, gender, and race exerted a more pronounced impact on creatinine levels compared to cystatin C. However, it is essential to recognize that cystatin C is influenced by factors beyond GFR. These additional factors should be taken into account when estimating GFR based on serum cystatin C levels [25].
Conclusion
The findings from this study indicate that diabetes does not significantly impact the concentration of cystatin C. Instead, this biomarker appears to be influenced by kidney dysfunction and the occurrence of nephropathy. Furthermore, the study highlights that serum cystatin C level may serve as a valuable warning sign and predictive factor for nephropathy in diabetic patients. However, it is essential to recognize that alterations in blood serum creatinine levels also play a crucial role in monitoring diabetic individuals.
Acknowledgments
This work was supported by Dezful University of Medical Sciences under the contract number of IR.RNU.REC.
Financial Disclosure
No funding was received for conducting this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author Contributions
Dr. Moradzadegan conceived of the presented idea. Mr. Zadabbas developed the theory and performed the computations. Dr. Moradzadegan and Dr. Golbashirzadeh verified the analytical methods. Dr. Moradzadegan and Dr. Golbashirzadeh investigate supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16(5):442-449.
doi pubmed - Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7-11.
doi pubmed - Vatashchuk MV, Bayliak MM, Hurza VV, Storey KB, Lushchak VI. Metabolic syndrome: lessons from rodent and drosophila models. Biomed Res Int. 2022;2022:5850507.
doi pubmed - Silvani L. Fatigue to fit: connecting the dots on energy and chronic diseases. 2022.
- Dewanjee S, Chakraborty P, Bhattacharya H, Chacko L, Singh B, Chaudhary A, Javvaji K, et al. Altered glucose metabolism in Alzheimer's disease: Role of mitochondrial dysfunction and oxidative stress. Free Radic Biol Med. 2022;193(Pt 1):134-157.
doi pubmed - Rodriguez-Sanchez B, Daugbjerg S, Pena-Longobardo L, Oliva-Moreno J, Aranda-Reneo I, Cicchetti A, et al. Does the inclusion of societal costs change the economic evaluations recommendations? A systematic review for multiple sclerosis disease. Eur J Health Econ. 2023;24(2):247-277.
- Kwan JT, Lanzo E, Ramsey DJ, Kalra A, Athappilly-Rolfe GK. Papilledema and retinopathy lead to diagnosis of IgA nephropathy: a case report. Ther Adv Rare Dis. 2023;4:26330040231152957.
doi pubmed - Min JW, Kim HD, Park SY, Lee JH, Park JH, Lee A, Ra H, et al. Relationship between retinal capillary nonperfusion area and renal function in patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2020;61(14):14.
doi pubmed - Krinock MJ, Singhal NS. Diabetes, stroke, and neuroresilience: looking beyond hyperglycemia. Ann N Y Acad Sci. 2021;1495(1):78-98.
doi pubmed - Mautone Gomes H, Silveira AK, Gasparotto J, Bortolin RC, Terra SR, Brum PO, Gelain DP, et al. Effects of coconut oil long-term supplementation in Wistar rats during metabolic syndrome - regulation of metabolic conditions involving glucose homeostasis, inflammatory signals, and oxidative stress. J Nutr Biochem. 2023;114:109272.
doi pubmed - Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, Gong Z, et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10(1):333.
doi pubmed - Hammoud SH, AlZaim I, Al-Dhaheri Y, Eid AH, El-Yazbi AF. Perirenal adipose tissue inflammation: novel insights linking metabolic dysfunction to renal diseases. Front Endocrinol (Lausanne). 2021;12:707126.
doi pubmed - Kuvat N, Tanriverdi H, Armutcu F. The relationship between obstructive sleep apnea syndrome and obesity: A new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J. 2020;14(7):595-604.
doi pubmed - Kucmierz J, Frak W, Mlynarska E, Franczyk B, Rysz J. Molecular interactions of arterial hypertension in its target organs. Int J Mol Sci. 2021;22(18):9669.
doi pubmed - Zhu M, Wang H, Chen J, Zhu H. Sinomenine improve diabetic nephropathy by inhibiting fibrosis and regulating the JAK2/STAT3/SOCS1 pathway in streptozotocin-induced diabetic rats. Life Sci. 2021;265:118855.
doi pubmed - Mehraee P, Nazarpou P, Ghanbari A. Designing a nursing care plan based on Faye Glenn Abdellah model in patients with diabetes type 2. 2020.
- Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - a metabolic and reproductive disorder. Biomed Pharmacother. 2021;143:112183.
doi pubmed - Kravets I, Mallipattu SK. The role of podocytes and podocyte-associated biomarkers in diagnosis and treatment of diabetic kidney disease. J Endocr Soc. 2020;4(4):bvaa029.
doi pubmed - Zsom L, Zsom M, Salim SA, Fulop T. Estimated glomerular filtration rate in chronic kidney disease: a critical review of estimate-based predictions of individual outcomes in kidney disease. Toxins (Basel). 2022;14(2):127.
doi pubmed - Akpinar K, Aslan D, Fenkci SM. Assessment of estimated glomerular filtration rate based on cystatin C in diabetic nephropathy. J Bras Nefrol. 2021;43(3):340-348.
doi pubmed - Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, Tang WHW, et al. Evaluation of kidney function throughout the heart failure trajectory - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(4):584-603.
doi pubmed - Lalmanach G, Kasabova-Arjomand M, Lecaille F, Saidi A. Cystatin M/E (Cystatin 6): a Janus-Faced cysteine protease inhibitor with both tumor-suppressing and tumor-promoting functions. Cancers (Basel). 2021;13(8):1877.
doi pubmed - Ebert N, Shlipak MG. Cystatin C is ready for clinical use. Curr Opin Nephrol Hypertens. 2020;29(6):591-598.
doi pubmed - Xin C, Xie J, Fan H, Sun X, Shi B. Association between serum cystatin c and thyroid diseases: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;12:766516.
doi pubmed - Tanabe A, Hirata A, Kuwabara K, Kubo S, Higashiyama A, Hirata T, Sugiyama D, et al. Association between visceral fat accumulation and decline in the estimated glomerular filtration rate based on cystatin C in the Japanese urban population: the KOBE study. Endocr J. 2023;70(1):97-106.
doi pubmed - Malmgren L, Oberg C, den Bakker E, Leion F, Siodmiak J, Akesson A, Lindstrom V, et al. The complexity of kidney disease and diagnosing it - cystatin C, selective glomerular hypofiltration syndromes and proteome regulation. J Intern Med. 2023;293(3):293-308.
doi pubmed - Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3-15.
doi pubmed - Bekmuratov L. Cardiovascular diseases in patients with diabetes mellitus. Ta'lim Va Rivojlanish Tahlili Onlayn Ilmiy Jurnali. 2023;3(1):193-198.
- DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17(5):319-334.
doi pubmed - Mohammadnezhadostad F, Shojaei D, Ghazipoor H. Investigation of nursing and medical services in patients with diabetes, abdominal pain and high blood pressure. Journal of Pharmaceutical Negative Results. 2023;14(2):3725-3741.
- Genco RJ, Graziani F, Hasturk H. Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol 2000. 2020;83(1):59-65.
doi pubmed - Kim BB, Chung SH, Yoon HS, Hahn WH, Bae CW, Choi YS. Decreased cystatin C-estimated glomerular filtration rate is correlated with prolonged hospital stay in transient tachypnea of newborn infants. Pediatr Neonatol. 2016;57(3):195-200.
doi pubmed - Bilgin S, Kurtkulagi O, Atak Tel BM, Duman TT, Kahveci G, Khalid A, Aktas G. Does C-reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes. 2021;15(6):1071-1074.
doi pubmed - Neelaveni K, Mallela AR. Overview of pathophysiology, diagnosis, biomarkers, treatment and recent advances in the management of diabetic nephropathy. International Journal of Research in Endocrinology. Diabetes And Metabolic Disorders. 2019;1(1):1-13.
- Aldenbratt A, Lindberg C, Johannesson E, Hammarsten O, Svensson MK. Estimation of kidney function in patients with primary neuromuscular diseases: is serum cystatin C a better marker of kidney function than creatinine? J Nephrol. 2022;35(2):493-503.
doi pubmed - Pucci L, Triscornia S, Lucchesi D, Fotino C, Pellegrini G, Pardini E, Miccoli R, et al. Cystatin C and estimates of renal function: searching for a better measure of kidney function in diabetic patients. Clin Chem. 2007;53(3):480-488.
doi pubmed - Yang YS, Peng CH, Lin CK, Wang CP, Huang CN. Use of serum cystatin C to detect early decline of glomerular filtration rate in type 2 diabetes. Intern Med. 2007;46(12):801-806.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
