| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 14, Number 3, June 2024, pages 128-148
Dapagliflozin-Saxagliptin Combination - The Quest for Optimal Glycemic Control With Cardio-Renal Protection in Type 2 Diabetes Mellitus: An Expert Consensus in Indian Settings
Sujoy Ghosha, k, Subhash K. Wangnoob, Sachin Chittawarc, Suresh Damodharand, Yogesh Kadame, Pramila Kalraf, K.P. Suresh Kumarg, I. Periyandavarh, S.K. Sharmai, Abdul Hamid Zargarj
aManipal Hospitals, Dhakuria, Kolkata, West Bengal, India
bIndraprastha Apollo Hospital, New Delhi, India
cGandhi Medical College, Bhopal, Madhya Pradesh, India
dHarvey Specialty Clinic, Coimbatore, Tamil Nadu, India
ePoona Diabetes Center, Pune, Maharashtra, India
fDepartment of Endocrinology, Ramaiah Medical College and Hospitals, Bengaluru, Karnataka, India
gKauvery Hospital, Chennai, Tamil Nadu, India
hSaroja Diabetes Center, Chennai, Tamil Nadu, India
iGalaxy Specialty Centre, Jaipur, Rajasthan, India
jCentre for Diabetes and Endocrine Care, Srinagar, Jammu and Kashmir, India
kCorresponding Author: Sujoy Ghosh, Manipal Hospitals Dhakuria Block-A, Scheme-L11, P-4&5, Gariahat Rd, Dhakuria, Ward Number 90, Kolkata, West Bengal 700029, India
Manuscript submitted March 1, 2024, accepted May 2, 2024, published online June 29, 2024
Short title: DAPA-SAXA FDC for T2DM in Indian Settings
doi: https://doi.org/10.14740/jem946
- Abstract
- Introduction
- Methodology
- Combination Therapy in the Management of T2DM
- Rationale for Combining DAPA and SAXA
- Clinical Evidence for Improved Glycemic Control With the Combination Therapy of DAPA and SAXA
- Safety of DAPA and SAXA in Patients With T2DM
- Cardiovascular Safety of SAXA
- Cardio-Renal Risk Reduction With DAPA + SAXA Combination
- Use of DAPA + SAXA in Indian Patients With T2DM
- Conclusions
- References
| Abstract | ▴Top |
The combination of dapagliflozin (DAPA; a sodium-glucose cotransporter-2 inhibitor (SGLT2i)) and saxagliptin (SAXA; a dipeptidyl peptidase-4 inhibitor (DPP4i)) added on to metformin targets multiple pathophysiological pathways and provides a synergistic effect on glycemic control. Notably, both DAPA and SAXA have demonstrated cardiovascular safety and shown to slow the progression of declining renal function in patients with type 2 diabetes mellitus (T2DM) having comorbid cardiovascular or renal diseases. Together, DAPA + SAXA has an acceptable tolerability profile, comparable with the individual agents and with a low propensity for hypoglycemia. The addition of DAPA + SAXA to metformin has been associated with low frequency of urinary tract and genital infections, attributed to the complementary effects of combining an SGLT2i and a DPP4i. This review compiles insights from a group of leading experts from India, summarizing concise clinical practice recommendations for the use of a fixed-dose combination of DAPA (10 mg) + SAXA (5 mg) in Indian patients with T2DM. The review encompasses available evidence and clinical experiences, highlighting the benefits of this combination for comprehensive glycemic control and enhanced cardio-renal protection in the management of T2DM.
Keywords: Dipeptidyl peptidase-4 inhibitor; Cardiovascular comorbidities; Chronic kidney disease; Fixed-dose combination; Sodium-glucose cotransporter-2 inhibitor; Type 2 diabetes mellitus
| Introduction | ▴Top |
Type 2 diabetes mellitus (T2DM) is a global health problem, with Southeast Asia at the epicenter. India is often regarded as the diabetic capital of the world and is central to the burden of T2DM in the Southeast Asian region as well as globally. Per the 2021 International Diabetes Federation (IDF) estimates, there are 537 million adults living with T2DM globally, with 74.2 million residing in India [1]. In the 2023, the Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) cross-sectional study, conducted among 113,043 individuals from urban and rural India, reported the overall weighted prevalence of diabetes as 11.4% and prediabetes as 15.3%. The study highlighted the escalating prevalence of metabolic non-communicable diseases, including hypertension, obesity, and dyslipidemia, emphasizing the need for optimal management of T2DM with comprehensive effects [2].
India faces several challenges around the management of T2DM, which includes the economic transition, rampant urbanization, and declining nutritional value, coupled with a general lack of awareness and literacy about T2DM, its management, complications, and long-term impact. Additionally, distinct Asian-Indian phenotypic features such as young age of onset, high visceral fat, waist circumference, waist-hip ratio, high levels of insulin resistance and early β-cell dysfunction despite low body mass index (BMI) heighten the clinical risk of T2DM in Indians [3, 4]. In the Investigation of Glycosylated Hemoglobin on Therapy in Indian Diabetics (TIGHT) study, nearly 77% of patients with T2DM receiving oral hypoglycemic agents with or without insulin had poor glycemic control (uncontrolled glycated hemoglobin (HbA1c) ≥ 7%) [5]. Other issues that confound the management of T2DM in India include delayed diagnosis, longer duration of disease and poor glycemia management, which are also associated with greater odds of developing severe microvascular and macrovascular complications in this population [6-9].
The Asian-Indian phenotype in T2DM is also predisposed to an increased risk of developing cardiovascular and renal diseases [9]. In an observational study from India, a large majority of the 5,080 patients with newly diagnosed T2DM were grouped in the high-risk category for developing cardiovascular diseases (2,007 patients (39.5%) classified as “high-risk” and 3,073 (60.5%) classified as “very high-risk”) [10]. Previous studies have also demonstrated high prevalence of cardiovascular disorders in patients with T2DM [11, 12]. A high prevalence of renal disorders also co-exists in Indian patients with T2DM. The SEEK study, a large cross-sectional study (n = 5,588) screening patients for early signs of renal diseases across India, reported that 31.6% patients with chronic kidney disease (CKD) have T2DM [13]. A similar study reporting the prevalence of renal dysfunction in normoalbuminuric Indian patients with T2DM (n = 3,534) concluded that more than one-third of the patients assessed developed CKD [14].
Adequate glycemic control is fundamental to achieving the larger goals of T2DM management, which include prevention of chronic complications, managing cardiovascular risk factors, improving the patient’s quality of life, and avoiding hypoglycemia. Compelling evidence supports the use of combination of oral antihyperglycemic agents with different mechanisms of action and wide-ranging clinical effects to achieve superior glycemic control and delay progressive cardiometabolic deteriorations [15, 16]. Optimized fixed-dose combinations (FDCs) of oral antihyperglycemic agents have shown to achieve the desired glycemic targets with acceptable safety and tolerability and additional benefits of improved adherence and reduced cost [17].
The FDC of once daily dapagliflozin (DAPA, 10 mg), a sodium-glucose cotransporter-2 inhibitor (SGLT2i) and saxagliptin (SAXA, 5 mg), a dipeptidyl peptidase-4 inhibitor (DPP4i) was approved for the treatment of T2DM in adults by the US Food and Drug Administration in 2017 [18, 19]. Following this, it was approved for T2DM in adults by the Drug Controller General of India (DCGI, Central Drugs Standard Control Organization (CDSCO)) in September 2019 [17, 20]. The FDC of DAPA and SAXA has demonstrated optimal bioequivalence to the combination therapy administered as two separate medications. Thus, although no phase 3 studies investigating the efficacy of the FDC have been conducted, several clinical studies have demonstrated potent and durable efficacy of the combination in lowering HbA1c and fasting plasma glucose (FPG) [21-24]. The FDC of DAPA + SAXA added to background metformin (MET) therapy might be particularly useful in Indian patients for achieving clinically meaningful glycemic control and improving associated metabolic disruptions (cardio-renal benefits) specific to patients with T2DM of the Asian-Indian phenotype [17].
Although several international diabetes guidelines advocate the use of optimum combination therapies, there are no specific clinical recommendations elucidating the use of DAPA + SAXA and their cardio-renal benefits in the Indian population. The present review provides clinical practice recommendations for the use of the DAPA + SAXA FDC in Indian patients with T2DM, focusing on their complementary mechanism of action, combined physiologic effects and available clinical evidence supporting their clinical benefits.
| Methodology | ▴Top |
A group of 10 experts from India convened a consensus meeting on May 12, 2023, which was held virtually to discuss the use of the FDC of DAPA + SAXA in Indian patients with T2DM. Discussions during this meeting were facilitated by a questionnaire intended to gather more structured information on the current clinical practice related to the use of the FDC in Indian patients with T2DM. Reported here are the statements recorded during the consensus meeting that were based on the clinical experiences of the expert panel along with supporting evidence from the literature. A comprehensive literature search was performed on PubMed and Google Scholar databases, using relevant search terms such as “diabetes”, “type 2 diabetes mellitus”, “fixed-dose combination”, “DPP4-inhibitor”, “SGLT2-inhibitor”, “dapagliflozin”, “saxagliptin” and “India”. The search was strengthened by using a combination of free text and Medical Subject Headings (MeSH) terms and Boolean operators to combine keywords. Primarily, clinical trials, systematic reviews and real-world evidence (RWE) studies served as primary data source.
| Combination Therapy in the Management of T2DM | ▴Top |
Over the years, the treatment standards for T2DM have evolved with a greater emphasis now on early detection, treatment initiation and intensification to dampen the progressive decline in β-cell function and the persistent increase in blood glucose levels. Early initiation of combination therapy is therefore recommended to improve prognosis in patients with T2DM. International guidelines for the management of diabetes mellitus from the American Diabetes Association (ADA) and American Association of Clinical Endocrinologists-American College of Endocrinology (AACE) advocate a stepwise intensification approach for glycemic control (Table 1) [15, 25, 26]. The AACE provides a broader choice of initial treatments and advocates a more individualized approach based on HbA1c levels. Both groups encourage early initiation of treatment with combination of complementary pharmacotherapies to delay onset of diabetic complications and treatment failure. Aligned with these guidelines, the Research Society for the Study of Diabetes in India (RSSDI) also advocates initiation of combination therapy if the HbA1c is 1.5 above the target (< 7%) in adult Indian patients with T2DM (Table 1) [27].
 Click to view | Table 1. Summary of Recommendation of T2DM Management Pertaining to Pharmacologic Agents by ADA, AACE and RSSDI |
In general, all treatment guidelines provide recommendations that focus on cardiovascular and renal safety, prevention of hypoglycemia, weight gain, as well as offer convenience of administration (oral agents, single-pill choices or once-a-day therapy) for improved adherence and long-term management [28, 29]. Thus, optimal treatment may require drugs with complementary mechanisms of action, which can target multiple pathophysiological defects [29, 30].
FDCs have been shown to be associated with improved medication adherence compared with free-drug combination regimen [30, 31]. In T2DM, studies have shown improved outcomes with combination therapy as opposed to monotherapy [32-35].
Do you perceive any challenge while prescribing FDCs?
Concurring with the available evidence, the majority of experts on the panel only recognized the lack of flexibility in dose adjustments and alterations as one of the challenges with prescribing FDCs [36-38]. A recent real-world study in patients with T2DM reported a significant (P < 0.001) improvement in adherence with the use of FDC versus the same drugs taken individually, with a safety profile comparable to that of the monotherapy [38] (Fig. 1).
 Click for large image | Figure 1. Combination therapy in T2DM. T2DM: type 2 diabetes mellitus. |
| Rationale for Combining DAPA and SAXA | ▴Top |
Eight major metabolic processes involving multiple organs that impact glucose metabolism have been identified as key players in the pathophysiology of T2DM. Together, these are called the ominous octet and comprise decreased insulin secretion, decreased incretin effect, increased lipolysis, increased glucagon secretion, increased glucose absorption, increased hepatic glucose production, neurotransmitter dysfunction and decreased glucose uptake (Fig. 2) [29, 39, 40]. The ominous octet reflects the multifactorial characteristic of T2DM, thus corroborating the use of therapeutics with effects at multiple physiological levels and early initiation of such therapies to prevent or slow the progressive damage of β cells and other detrimental downstream effects [29].
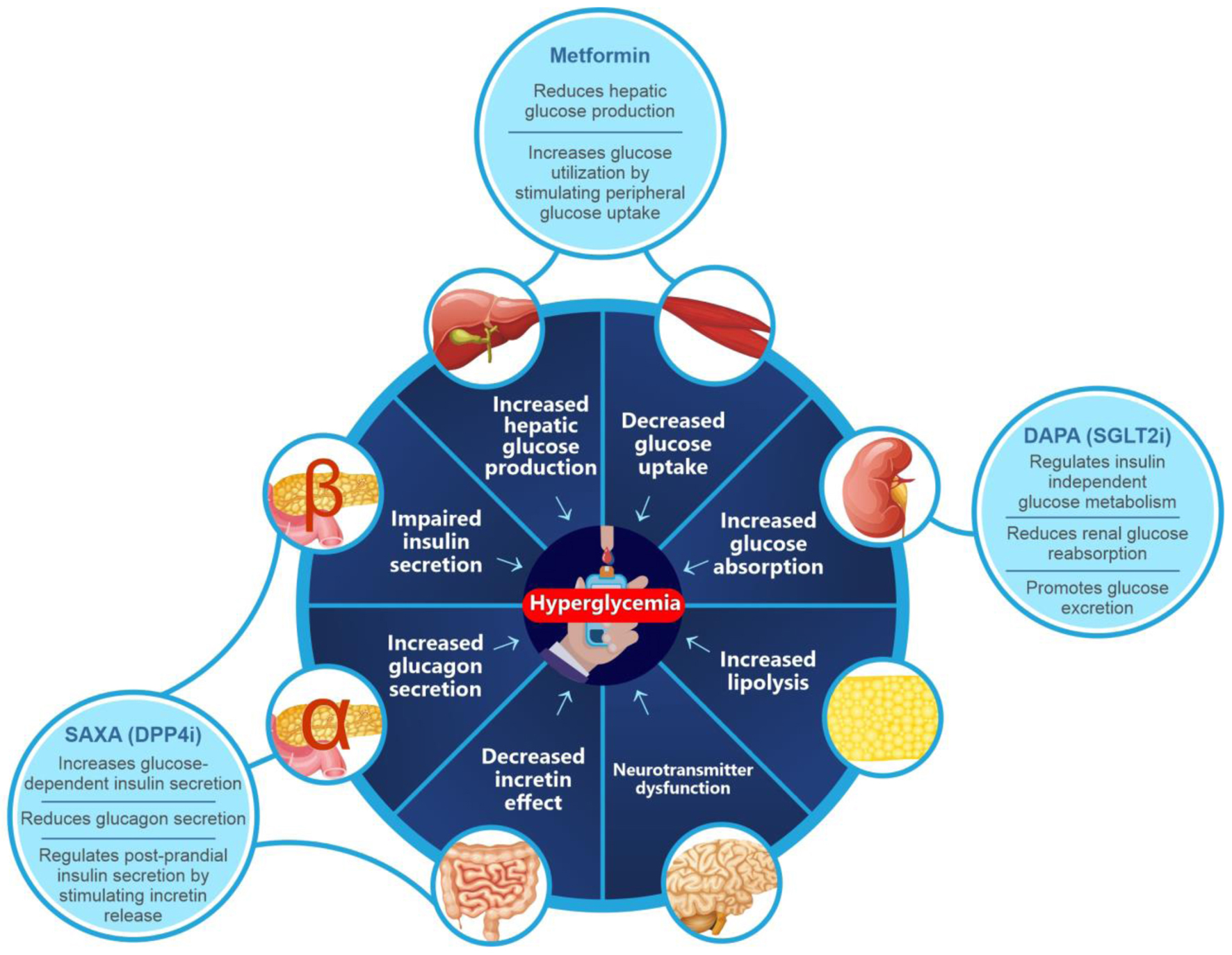 Click for large image | Figure 2. The ominous octet. DPP4i: dipeptidyl peptidase-4 inhibitor; SGLT2i: sodium-glucose cotransporter-2 inhibitor. |
DAPA, an SGLT2i, reduces renal glucose reabsorption and promotes glucose excretion, thus targeting the kidney-mediated regulation of insulin-independent, glucose metabolism in the ominous octet [28, 41]. A large body of evidence from clinical trials and RWE studies have demonstrated clinically relevant outcomes with DAPA in lowering HbA1c, FPG and postprandial plasma glucose (PPG), and minimizing the occurrence of hypoglycemia, cardiovascular events and all-cause mortality [42-46]. FOREFRONT, an RWE study conducted in India, reported that treatment with DAPA significantly (P < 0.001) reduced the HbA1c level by 1% at 3 months and by 1.49% at 6 months. Meaningful reductions in body weight (mean (standard deviation (SD)) change from baseline: -1.86 kg (3.04), P < 0.001) and improvement in the systolic blood pressure (SBP) and diastolic blood pressure (mean (SD) change from baseline: -3.77 (12.22) and -1.46 (8.30) mm Hg, respectively) were also observed in the study [47]. Other studies of DAPA in Indian patients with T2DM have reported adequate glycemic control and favorable metabolic effects (Table 2) [21, 22, 24, 43, 44, 47-65].
 Click to view | Table 2. Summary of the Studies Investigating the Outcomes of DAPA, DAPA + SAXA, and SAXA in Patients With T2DM |
DDP4is such as SAXA are involved in glucose-dependent insulin secretion, glucose-dependent decrease in glucagon secretion, improving β-cell sensitivity/function and inhibiting the degradation of incretin hormones, collectively resulting in the lowering of HbA1c and FPG levels [28]. Thus, SAXA’s insulin-dependent mode of action targets the gut, pancreas and liver to achieve glucose lowering effect [28]. In a multicenter, randomized, double-blind, placebo-controlled, parallel-group study in Indian patients with T2DM, SAXA showed clinically meaningful reductions in HbA1c with no new safety concerns, aside the known adverse event profile of SAXA [50]. In the ONTARGET-INDIA study conducted in 1,109 patients with T2DM inadequately controlled on MET alone, SAXA in combination with MET as a first add-on significantly reduced HbA1c levels, with an acceptable tolerability profile and no new risks being identified [49]. Additionally, significantly elevated (P < 0.001) circulating plasma DPP4 levels have been observed in non-obese Asian Indian patients with T2DM (receiving treatment with MET) vs. non-obese, non-diabetic patients. The elevated level of DPP4 was associated with metabolic markers of obesity such as increasing waist-to-hip ratio, total intra-abdominal adipose volume, and excess liver span [66]. Thus, treatment with DPP4is, such as SAXA, can produce improvements in metabolic derailments beyond glycemic control.
Taken together, these complementary mechanisms of action of DAPA + SAXA, when administered with MET (reduces hepatic glucose production and increases glucose utilization, thereby reducing plasma glucose levels) [67], can efficiently target six of the eight pathophysiological defects of T2DM, making it a potentially potent and effective combination [28, 39, 68]. The mean half-lives of oral DAPA and SAXA necessitate once daily administration and have an overall acceptable pharmacokinetic profile, no clinically meaningful drug interactions [69, 70]. Pharmacodynamic studies have further confirmed the complementary mode of action of the two agents [71]. The combination of DAPA + SAXA was shown to improve β-cell function, as evaluated by the homeostasis model assessment (HOMA-2) method [72]. The pharmacokinetic and pharmacodynamic attributes of DPP4i and SGLT2i make their combination feasible and clinically viable. However, combining SGLT2i with glucagon-like peptide 1 (GLP-1) receptor agonists, another class of antihyperglycemic drugs with complementary mechanism of action, may prove challenging due to differences in route of administration (majority of GLP-1 agonists are subcutaneous) and the absence of a convenient FDC [73]. These findings suggest that DAPA + SAXA is an optimal combination with a synergistic mechanism of action that produces clinically meaningful pharmacodynamic effects in patients with T2DM. Furthermore, among the various combinations of SGLT2i and DPP4i currently available in India, the DAPA + SAXA combination has extensive data evaluating the cardiovascular and renal safety and benefits of the individual agents (Table 3) [22, 24, 53, 54, 57, 59, 61, 62, 65, 74-89].
 Click to view | Table 3. Comparison of Key Outcomes for SGLT2i + DPP4i Fixed-Dose Combinations Approved in India |
| Clinical Evidence for Improved Glycemic Control With the Combination Therapy of DAPA and SAXA | ▴Top |
Early initiation of triple therapy (add-on dual therapy to MET) as opposed to a stepwise accentuation strategy could be considered in patients failing to achieve glycemic goals. Studies of empagliflozin/linagliptin single-pill combination, SAXA add-on to DAPA and MET, and DAPA add-on to SAXA and MET have shown sustained glycemic control for up to 52 weeks with added benefits of weight control and improvements in blood pressure (Fig. 3) [28].
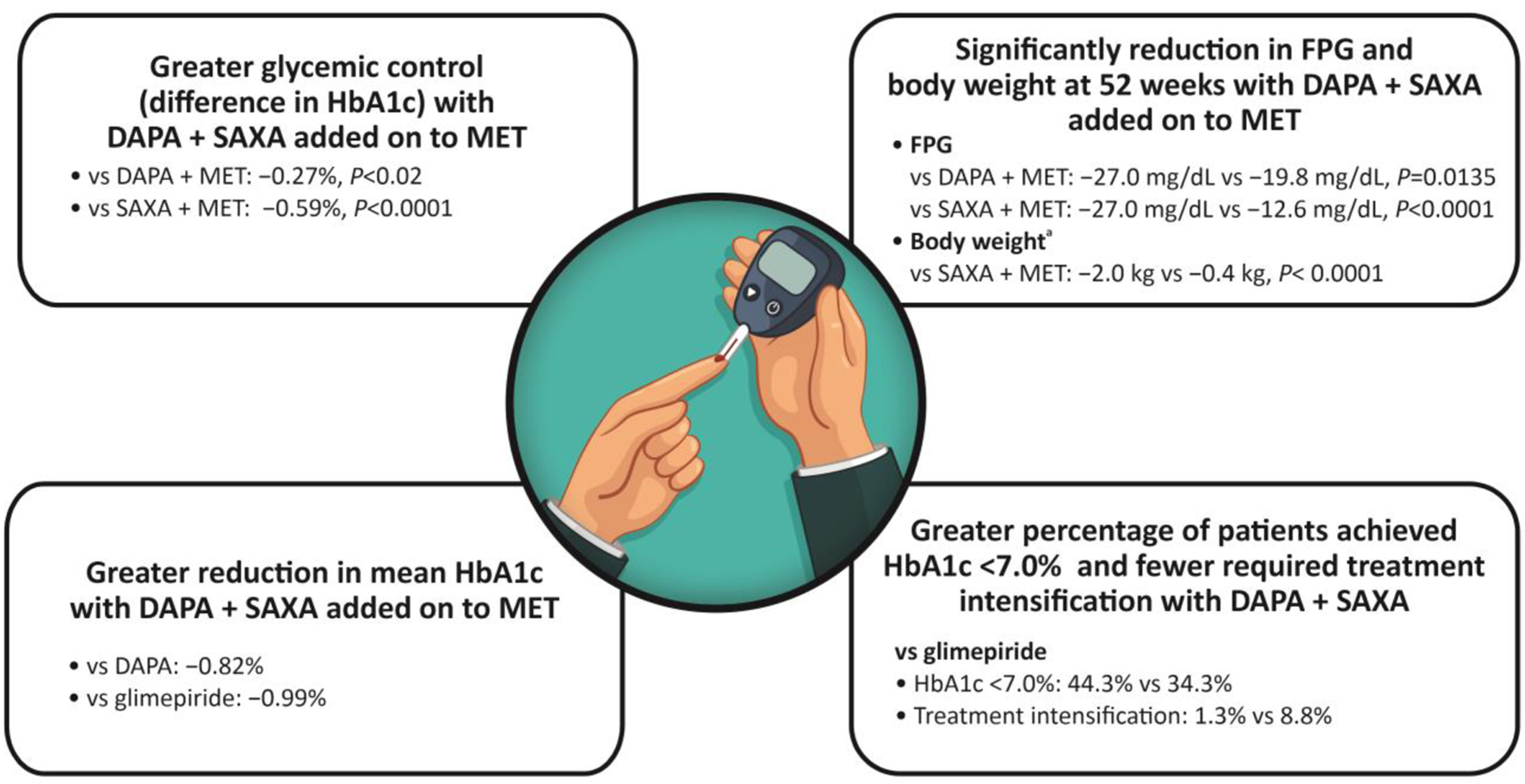 Click for large image | Figure 3. Clinical evidence for improved glycemic control with DAPA + SAXA. aDifferences in the change in total body weight between DAPA + SAXA + MET and DAPA + MET were not available. DAPA: dapagliflozin; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; MET: metformin; SAXA: saxagliptin. |
Concurrent dual addition of DAPA + SAXA to ongoing MET therapy was associated with greater glycemic control (1.5%) as compared with treatment with DAPA + MET (difference -0.27%, P < 0.02) and SAXA + MET (difference -0.59%, P < 0.0001) in patients with uncontrolled T2DM. This improvement in HbA1c levels was associated with meaningful reductions in SBP and body weight in patients with advanced disease and considerable β-cell dysfunction, representative of inadequately controlled T2DM [53].
These findings were further substantiated in a longer 52-week, double-blind, randomized trial wherein triple therapy with a combination of low-dose DAPA (5 mg) + SAXA (5 mg) added on to MET significantly reduced FPG (-27.0 mg/dL vs. -19.8 mg/dL (DAPA + MET; P = 0.0135) vs. -12.6 mg/dL (SAXA + MET; P < 0.0001)) and body weight (-2.0 kg vs. -0.4 kg (SAXA + MET; P < 0.0001)) when compared with dual therapy of SAXA or DAPA added-on to MET in patients with uncontrolled T2DM [90].
The combination of DAPA (10 mg) + SAXA (5 mg) and MET has also demonstrated improved glycemic control compared with glimepiride plus MET. The mean HbA1c change from baseline was -1.20% with DAPA + SAXA and -0.82% with DAPA, vs. -0.99% with glimepiride. There were also significant reductions in the FPG and PPG in the DAPA + SAXA group compared with glimepiride [51]. In another long-term, 52-week, multicenter, double-blind, active-controlled study, efficacy and safety of DAPA (10 mg) + SAXA (5 mg) was compared with glimepiride (1 - 6 mg) in patients with T2DM. More patients achieved HbA1c < 7.0% (44.3% vs. 34.3%; P = 0.044), and fewer patients required treatment intensification (1.3% vs. 8.8%; P = 0.002) with DAPA + SAXA than with glimepiride. The combination of DAPA and SAXA also improved body weight and other metabolic parameters in patients inadequately controlled on MET [22]. These findings supported the use of DAPA + SAXA as an oral alternative in insulin-naive patients with T2DM.
Is the glycemic efficacy of the FDC (DAPA + SAXA) equal to insulin, with benefits in terms of weight and SBP reduction?
Based on their clinical experience in Indian patients with T2DM, 50% of the experts noted that the combination of DAPA + SAXA can potentially produce effects that are comparable with insulin along with additional benefits of improved weight and blood pressure management. The oral combination of DAPA + SAXA versus insulin resulted in non-inferior reductions in HbA1c (adjusted mean ± SE change, -1.7±0.1% vs. -1.5±0.1%; P = 0.118), along with beneficial reductions in body weight (between-group difference, -3.64 kg (95% confidence interval (CI): -4.20 to -3.09); P < 0.001) and a lower frequency of hypoglycemia (20.9% vs. 13.1%, P = 0.008) versus insulin [23, 24]. Thus, in line with clinical recommendations, the DAPA + SAXA FDC can be initiated instead of progressing directly to insulin therapy, in case of inadequate glycemic control with ongoing therapy [91] (Fig. 4).
 Click for large image | Figure 4. Glycemic efficacy of DAPA + SAXA. DAPA: dapagliflozin; SAXA: saxagliptin. |
| Safety of DAPA and SAXA in Patients With T2DM | ▴Top |
The safety of DAPA and SAXA as monotherapies has been well-established and the DAPA + SAXA FDC has a safety profile comparable to its mono components, including their propensity for reducing the risk of hypoglycemia [42-45]. Among the DPP4is, SAXA has shown to be well tolerated in patients with renal impairment [52, 92]. DAPA has also shown to be generally well-tolerated in broader patient populations and associated with non-serious incidences of genital and urinary tract infections [93].
In a year-long study of DAPA + SAXA versus glimepiride, no episodes of severe hypoglycemia were observed in the DAPA + SAXA group and no patients discontinued the treatment due to hypoglycemia [22]. Triple therapy with DAPA + SAXA added on to MET was well tolerated during 52 weeks of treatment; the incidence of adverse events in patients receiving triple therapy was similar to the sitagliptin add-on group [94]. In a study of DAPA versus placebo as add-on to SAXA plus MET, adverse events were similar in DAPA (66%) and placebo (71%) groups. Genital infections occurred more often with DAPA (6%) than with placebo (1%); frequency of urinary tract infections was similar between the two groups (9% vs. 10%) [21].
No new safety signals were observed in patients with T2DM who were administered SAXA as an add-on to DAPA and MET. Hypoglycemia was infrequent in both groups (≤ 2.5%), with no major episodes. The rate of urinary tract infections was similar in the SAXA and placebo add-on groups (7.8% vs. 7.4%). The incidence of genital infections was lower (3.3%) with SAXA added on to DAPA and MET, as compared with the placebo add-on group (6.2%) [58]. Notably, urinary tract and genital infections have been reported to occur less frequently with triple therapy of DAPA + SAXA added on to MET, and this has been attributed to the complementary effects of the combination of an SGLT2i and a DPP4i [21, 58]. Also the small risk of fractures and amputations, which are reported with SGLT2is, has not been observed with the combination of DAPA + SAXA [95].
Rosenstock et al have shown that treatment with a triple combination of DAPA + SAXA + MET leads to significant increase in high-density lipoprotein (HDL) cholesterol (4.4% (95% CI: 1.1-7.8%), P = 0.009) versus SAXA + MET and reductions in triglycerides (-8.5% (95% CI: -15.9% to -0.4%), P = 0.04) when compared with DAPA + MET [53]. Combination of DAPA + SAXA significantly decreased liver fat (P < 0.007) and adipose tissue volume (P < 0.01) versus glimepiride, and reduced serum liver enzyme levels, suggestive of a favorable metabolic profile in patients with T2DM inadequately controlled on MET therapy [56].
Does the FDC have an acceptable safety and tolerability including hypoglycemia risk?
Supported by their clinical experience, all experts agreed that the FDC has an acceptable safety and tolerability and achieves adequate glycemic control with fewer incidences of hypoglycemia in Indian patients with T2DM. This concurs with several studies that report lower incidences of hypoglycemia in patients treated with a combination of DAPA + SAXA when compared with other oral antihyperglycemics [23, 24, 51] (Fig. 5).
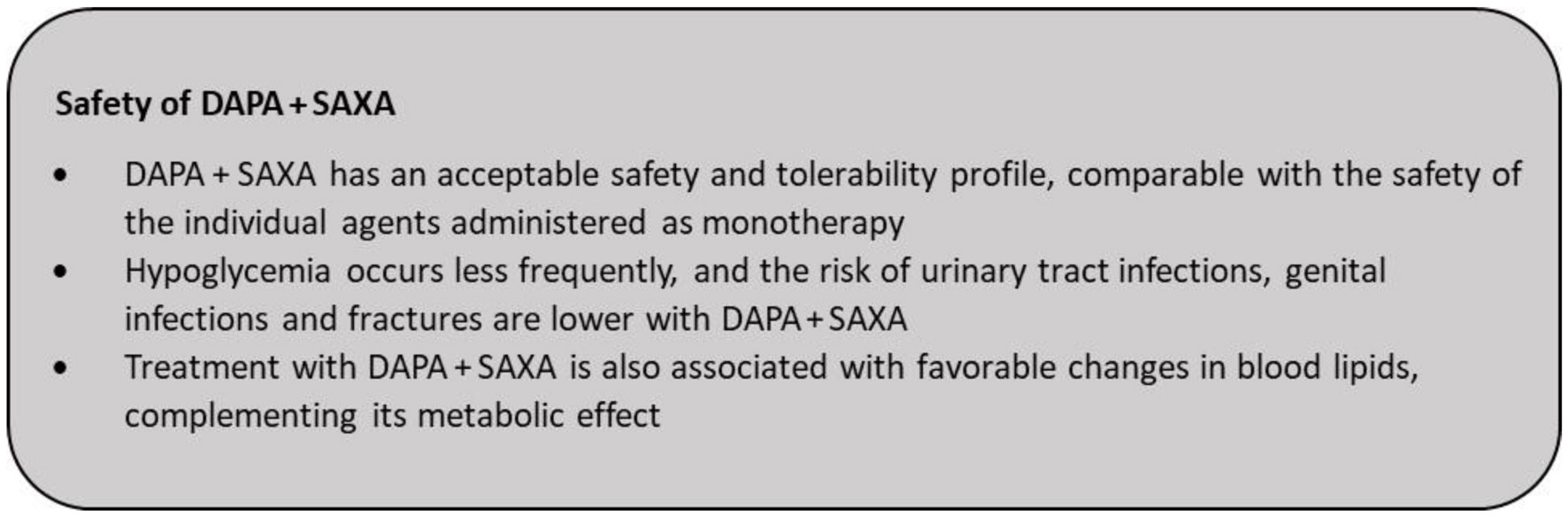 Click for large image | Figure 5. Safety of DAPA + SAXA. DAPA: dapagliflozin; SAXA: saxagliptin. |
| Cardiovascular Safety of SAXA | ▴Top |
Findings from several studies reveal the protective effects of most DPP4is against cardiovascular diseases driven by various effects including reduced inflammation, lipid levels, adiposity, decreased plaque development and endothelium-mediated vasodilatation [96-100]. In a pooled analysis of eight clinical studies, cardiovascular events, including death, myocardial infarction, and stroke were observed in 1.1% of patients treated with SAXA and 1.8% treated with a comparator (placebo, MET, or up-titrated glyburide) (relative risk (RR): 0.44, 95% CI: 0.24 - 0.82) [101]. Another retrospective analysis of 20 phase 2/3 studies of SAXA evaluating the incidence of major adverse cardiovascular event (MACE), reported a 25% reduction in the risk of MACE with SAXA versus the control treatments [102].
The SAXA Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) study enrolled 16,492 patients with T2DM at a risk or a history of cardiovascular disease to evaluate the cardiovascular outcomes for SAXA vs. placebo added on to usual care [103]. The 2-year all-cause mortality rates were similar between SAXA and placebo-treated patients (4.9% and 4.2%, respectively; hazard ratio (HR): 1.11, 95% CI: 0.96 - 1.27; P = 0.15) [103]. As with other DPP4is, treatment with SAXA met the primary composite endpoint of cardiovascular safety (MACE) by demonstrating no increased risk of cardiovascular death, nonfatal myocardial infarction and nonfatal ischemic stroke (HR: 1.00, 95% CI: 0.89 - 1.12) [103]. The incidence of cardiovascular death was also comparable between the SAXA and placebo arms (HR: 1.03, 95% CI: 0.87 - 1.22) [103]. SAXA was reported to be neutral even in the composite secondary endpoint. However, an increase (27%, P = 0.007) in the rate of hospitalization for heart failure (hHF) was noted, which was one of the six components of the composite of the secondary endpoint. Notably, the risk of heart failure was highest in patients with CKD (estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2), elevated N-terminal pro-B-type natriuretic peptides (NT-proBNP) levels and those with a history of previous heart failure, suggesting an interplay of several clinical factors (Fig. 6) [60]. The risk of hHF was similar for SAXA and placebo at 6 months (2.4% vs. 2.1%, HR: 1.11 (95% CI: 0.91 - 1.36), P = 0.31) and 12 months (1.7% vs. 1.5%, HR: 1.09 (95% CI: 0.85 - 1.39), P = 0.51). A time-varying coefficients model was developed to further evaluate the attenuating effects of SAXA on the risk of hospitalization as a result of heart failure over time. The analysis revealed that the risk of hospitalization with SAXA attenuated over time at 10 to 11 months (at around 314 days, log HR surpasses 0) after randomization, indicating a possible absence of an underlying molecular pathogenesis for this effect [60]. Additionally, the observation of increased heart failure risk should be interpreted with caution as this is mainly derived from one study designed to assess MACE, with hHF being one component of the composite secondary endpoint. Findings may also be impacted by the heterogeneous definition of the event (heart failure requiring hospitalization) [60, 104]. Contrasting these findings from the SAVOR-TIMI 53 study, the pooled analysis of 20 SAXA studies also reported a lower incidence of heart failure (HR: 0.55 (95% CI: 0.27 - 1.12)) in patients receiving SAXA vs. the control groups [102]. Furthermore, a meta-analysis of studies, including the SAVOR-TIMI 53 trial, revealed that SAXA did not increase the rate of heart failure as compared to either sulfonylurea or placebo (RR: 0.99, 95% CI: 0.89 - 1.10) [105]. The recent Mechanistic Evaluation of Glucose-lowering Strategies in Patients With Heart Failure (MEASURE-HF) study was conducted to further investigate findings from the SAVOR-TIMI 53 study [106, 107]. It was designed to determine the effects of SAXA (vs. sitagliptin and placebo) on left ventricular structure, function and NT-proBNP levels in patients with T2DM and established symptomatic heart failure. Overall, there were no significant differences in left ventricular end systolic volume index, ejection fraction and mass, and NT-proBNP levels between SAXA and the placebo arm and consistent changes between SAXA and the sitagliptin arm [106, 107]. Thus, SAXA treatment did not correlate with any unfavorable physiological changes in the heart that are typically suggestive of heart failure. Taken together these findings underscore a neutral effect of SAXA on the risk of adverse cardiovascular outcomes [104] (Fig. 7).
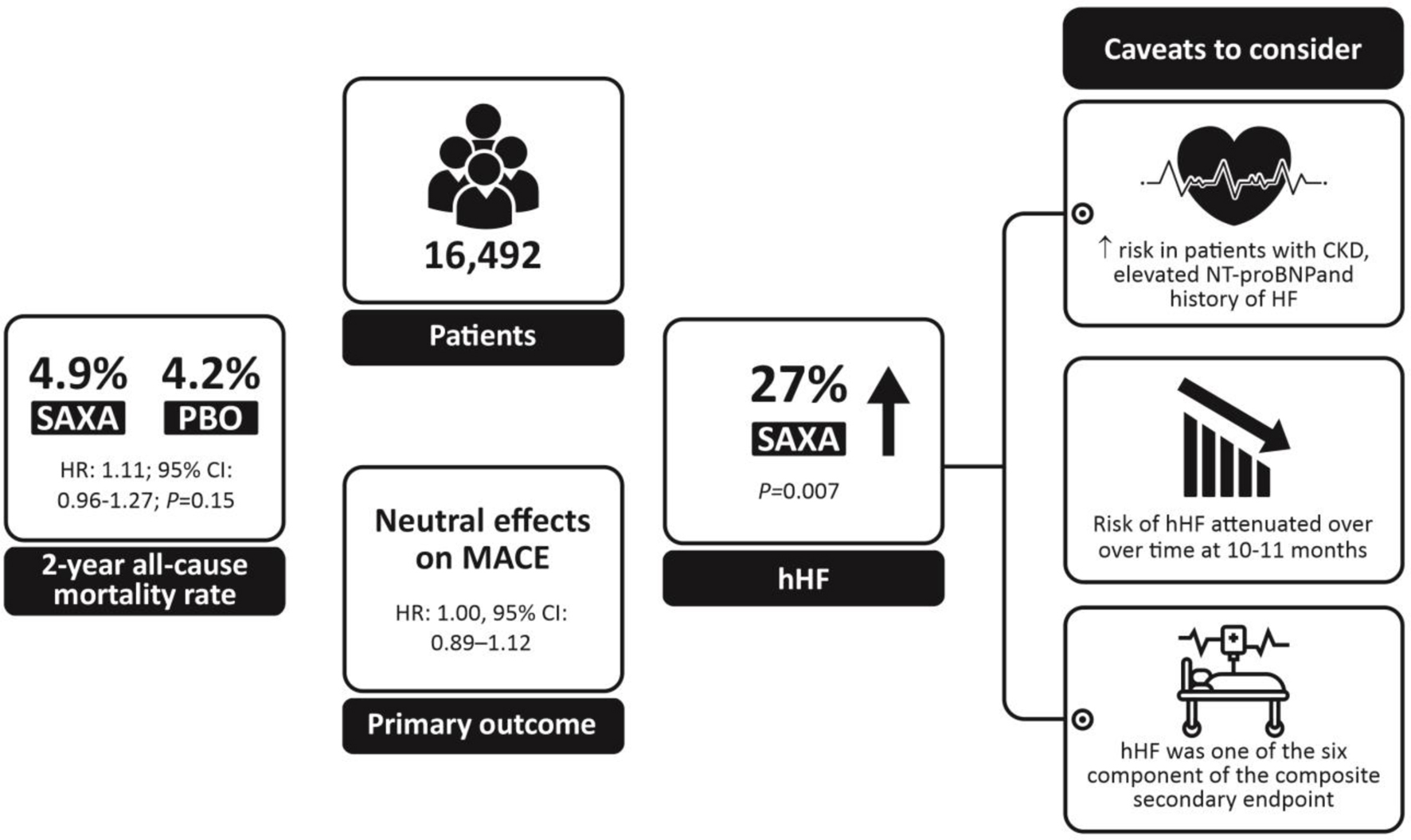 Click for large image | Figure 6. Summary of results from SAVOR-TIMI 53. CKD: chronic kidney disease; hHF: hospitalization for heart failure; MACE: major adverse cardiovascular event; NT-proBNP: N-terminal pro-B-type natriuretic peptides; PBO: placebo; SAXA: saxagliptin. |
Large RWE studies evaluating the risk for hHF also showed lack of association between the use of SAXA and an increased risk of hHF when evaluated against other select antihyperglycemic agents [108-110]. A population-based study of SAXA users (n = 78,553) reported that the risk of hHF was not higher with SAXA when compared with other antihyperglycemic agents (pioglitazone, second-generation sulfonylureas, or long-acting insulin formulations) [108]. A large Korean RWE study of 534,327 participants who were newly prescribed SAXA (n = 29,479), sitagliptin (n = 167,157), vildagliptin (n = 67,412), linagliptin (n = 220,672), or gemigliptin (n = 49,607), showed that SAXA was associated with lower risk of cardiovascular events as compared with other DPP4is [55]. In the French DIAPAZON epidemiological RWE study, 2.4% of the 1,033 patients with T2DM suffered a cardiovascular event but none was considered to be related with the use of SAXA [111].
Is the use of the FDC associated with lower risk of cardiovascular and renal adverse events?
The experts highlighted that use of DAPA + SAXA in Indian patients with T2DM was not associated with any major cardiovascular and renal adverse events. The combination of DAPA + SAXA is generally well tolerated, and no unexpected adverse events have been observed during routine clinical practice in Indian patients.
Is the use of the FDC associated with lower rates of treatment discontinuations due to cardiovascular and renal adverse events?
A majority of experts suggested that in their clinical practice, fewer patients discontinued the FDC treatment following cardiovascular or renal adverse events. Overall, these observations corroborate with findings from large clinical studies of DAPA + SAXA that report a low frequency of discontinuations due to adverse events [112].
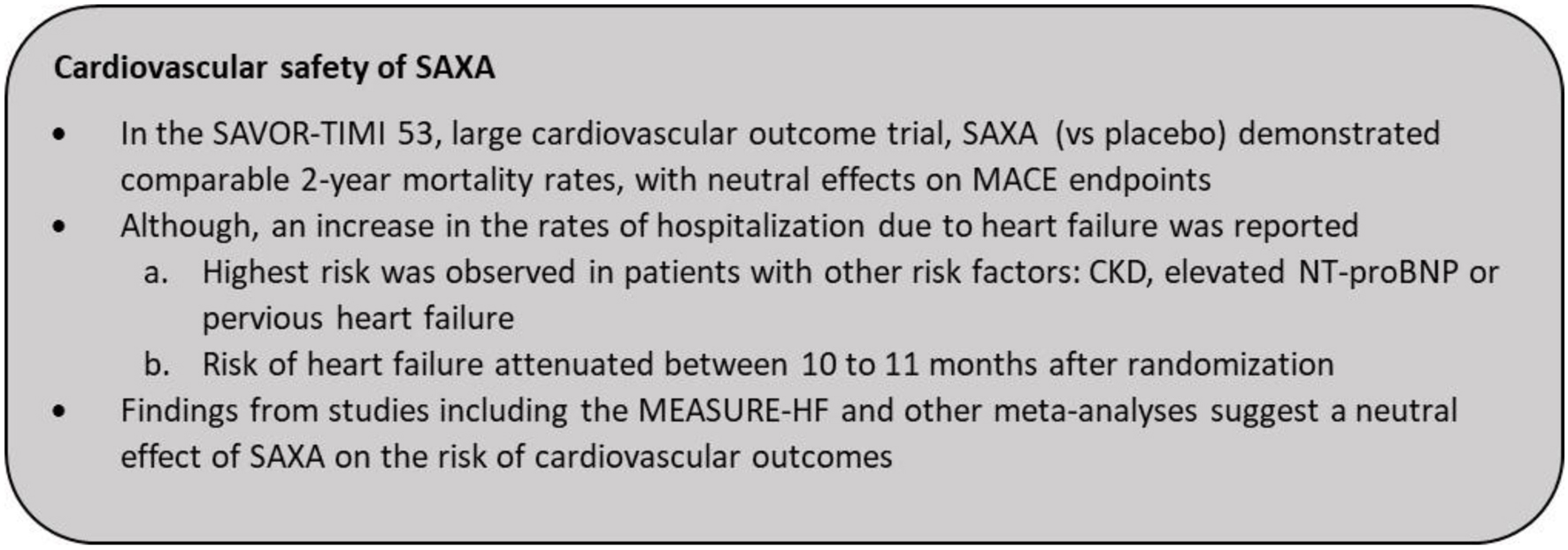 Click for large image | Figure 7. Cardiovascular safety of SAXA. SAXA: saxagliptin. |
| Cardio-Renal Risk Reduction With DAPA + SAXA Combination | ▴Top |
Individually, treatment with DAPA and SAXA has shown promising results in improving cardiovascular and renal outcomes in several large landmark studies [59, 62, 65, 92, 103, 113].
Cardiovascular risk reduction
In the Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial of 17,160 patients with T2DM who had or were at risk for atherosclerotic cardiovascular disease were randomized to receive either DAPA or placebo. There was a significant risk reduction in hHF (4.9% vs. 5.8%; HR: 0.83; 95% CI: 0.73 - 0.95; P = 0.005) and cardiovascular death with DAPA (HR: 0.98; 95% CI: 0.82 - 1.17) [62]. DAPA has also shown to dampen renal disease progression in patients with T2DM with relatively good baseline renal function [62, 113]. In the DAPA in Patients With Heart Failure and Reduced Ejection Fraction (DAPA-HF) trial (n = 4,744), DAPA significantly reduced the composite endpoint of worsening heart failure (hospitalization or urgent visit resulting in intravenous therapy for heart failure) or death from cardiovascular causes by 26% (P < 0.001) as compared with placebo [59]. In the DAPA on Left Ventricular Hypertrophy (DAPA-LVH) trial, patients with T2DM, and left ventricular hypertrophy were randomized to receive DAPA 10 mg once daily or placebo for 12 months; DAPA significantly reduced left ventricular mass compared with placebo with an absolute mean change of -2.82 g (95% CI: -5.13 to -0.51, P = 0.018). This was also accompanied by reductions in SBP, body weight, visceral and subcutaneous adipose tissue and insulin resistance, suggesting reverse remodelling in the left ventricular structure that may partly contribute to the cardio-protective effects of DAPA [54].
The phase 3 DAPA Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) study is the largest (n = 6,262) trial designed to evaluate the effects of DAPA on cardiovascular death, and hHF in patients with chronic heart failure and a left ventricular ejection fraction of more than 40%. At 2.3 years, treatment with DAPA was associated with reduced risk of worsening heart failure (HR: 0.79; 95% CI: 0.69 - 0.91) and cardiovascular deaths (HR: 0.88; 95% CI: 0.74 - 1.05) as compared with placebo, with lower incidences of heart failure events and symptoms [61].
In the Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors in the Real-world Setting (CVD-REAL), a large RWE study conducted across 13 countries, the initiation of SGLT2is (including DAPA, empagliflozin and canagliflozin) was associated with a significantly lower risk of hospitalization due to heart failure (HR: 0.66, 95% CI: 0.58 - 0.75; P < 0.001), all-cause death (ACD (HR: 0.52, 95% CI: 0.45 - 0.60; P < 0.001)), myocardial infraction (HR: 0.85, 95% CI: 0.78 - 0.92; P < 0.001), and stroke (HR: 0.78; 95% CI: 0.72 - 0.85; P < 0.001) when compared with other glucose lowering agents over a period of 1 year [57]. These outcomes were consistent across all patient subgroups of age, sex, comorbidities, geographic region and ethnicities. Notably, among the SGLT2is, DAPA contributed to 60% of total exposure time in patients [57]. An electronic medical records database-based RWE study in Taiwan also demonstrated better outcome of DAPA in terms of reduction of heart failure compared with empagliflozin [114]. Similar outcomes have also been reported in other extensions of the CVD-REAL studies conducted across several countries [57, 115, 116]. Lower risk of hospitalization due to heart failure, ACD and myocardial infarction have also been associated with SGLT2is in the CVD-REAL 2 study (DAPA being 75% of total exposure, and 235,064 new SGLT2i users) [84]. In their recent evaluation, real-world data from the Maccabi database in Israel (n = 5,307 for SGLT2is and n = 5,307 for other glucose lowering agents), initiation of SGLT2is versus other glucose lowering agents was associated with lower risk of hospitalization due to heart failure or death overall (HR: 0.57, 95% CI: 0.46 - 0.70; P < 0.001) and in patients with both reduced ejection fraction (HR: 0.61, 95% CI: 0.40 - 0.93) and preserved ejection fraction (HR: 0.55, 95% CI: 0.43 - 0.70) [117].
In the large SAVOR-TIMI 53 study, SAXA met the primary composite endpoint, demonstrating no heightened risk of MACE, including cardiovascular death, nonfatal myocardial infarction, and nonfatal ischemic stroke (HR: 1.00, 95% CI: 0.89 - 1.12) [103]. Retrospective analyses of SAXA studies have revealed a notable 25% reduction in the risk of MACE and a lower incidence (or absence of increased risk) of heart failure in patients receiving SAXA when compared with the control treatments (HR: 0.55; 95% CI: 0.27 - 1.12) [102, 105]. Several RWE studies evaluating the risk of hHF have also found no association between SAXA use or an increased risk of hHF when compared with other antihyperglycemic agents [108-110].
Renal risk reduction
Effect of DAPA on renal outcomes
Studies assessing cardiovascular safety of SGLT2is have suggested that these agents lower albuminuria and impede the progressive deterioration of kidney function over time [118]. Declining eGFR and worsening albuminuria are deemed as independent markers of adverse renal outcomes and cardiovascular death in patients with T2DM [119]. In a post-authorization safety study for DAPA, real-world data from three databases - one in the UK (Clinical Practice Research Datalink (CPRD)) and two in the USA (the HealthCore Integrated Research Database (HIRD)) and the Medicare database - were derived to compare hospitalization for acute kidney injury (hAKI) among DAPA initiators and other glucose lowering drugs. Results demonstrated a lower risk of hAKI in patients treated with DAPA compared with other glucose lowering drugs [120]. The long-term renoprotective effects of DAPA were further substantiated in the DAPA and Prevention of Adverse Outcomes in CKD (DAPA-CKD) study. At 2.4 years, treatment with DAPA in patients with CKD was associated with a significantly lower risk (P < 0.001) of a composite of a sustained decline in the eGFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes as compared with the placebo group, regardless of the presence or absence of T2DM [121].
Effect of SAXA on renal outcomes
Incretin modifying therapies, specifically DPP4is, have shown to ameliorate albuminuria and exert renal cell protection by reducing oxidative stress [65, 122-125]. Studies of SAXA added on to ongoing therapy in patients with T2DM and moderate or severe CKD or end-stage renal disease have demonstrated meaningful reductions in HbA1c and FPG with good tolerability [52, 92, 126]. The SAVOR-TIMI 53 study included patients with varying degrees of renal function and at the end of 2.1 years, the use of SAXA was associated with reduced urinary albumin/creatinine ratio (UACR) levels (vs. placebo), a predictor of declining renal and cardiovascular function. This reduction in UACR was observed in patients with normo-, micro-, and macroalbuminuria, without affecting eGFR. Patients treated with SAXA were less likely to have worsening UACR levels than patients on placebo [65]. There was no deterioration in renal safety outcomes such as doubling of serum creatinine, initiation of chronic dialysis, renal transplant and the composite endpoint of death. Notably, the majority of patients in SAVOR-TIMI 53 were treated with angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) and the addition of SAXA further improved UACR levels without any other adverse renal effects [65]. In patients with diabetic nephropathy receiving renin-angiotensin-aldosterone system blockade therapy, SAXA significantly lowered albuminuria at week 12 (-57.9%, P < 0.001) vs. standard antidiabetic agents [127]. SAXA showed superior efficacy to vildagliptin in transitioning patients to a lower albuminuria category, despite achieving similar reductions in UACR [127]. Thus, SAXA has shown clinically important advantages with regard to renal outcomes that could potentially mitigate progressive deterioration of renal function associated with chronic T2DM.
A combination of an SGLT2i and DPP4i is therefore purported to have beneficial effects on the kidney function. The DELIGHT study was the first prospective study to evaluate the efficacy of DAPA + SAXA in patients with T2DM and moderate-to-severe CKD, receiving ACEi or ARB therapy. The results showed significant lowering in albuminuria (38%, P < 0.0001) and HbA1c (0.58%, P < 0.0001) after 24 weeks of treatment compared with placebo [95]. The substantial magnitude of reduction in UACR suggests that the combination could potentially dampen the deterioration of kidney function and provide long-term renoprotection in T2DM patients with moderate-to-severe CKD [95]. Furthermore, the reduction in eGFR at week 1 and reversal of this effect at week 3 following discontinuation of the study medication, alludes to the activation of tubuloglomerular feedback, a mechanism suggested to curtail renal damage. The DAPA + SAXA combination had a manageable safety profile and was well tolerated in patients with eGFR as low as 25 mL/min/1.73 m2 [95].
Is the use of the DAPA + SAXA FDC associated with better cardiovascular and renal outcomes?
In view of the complementary cardiovascular and renal benefits of DAPA and SAXA, the panel members recommended the use of the combination in Indian patients with T2DM and early stages of heart failure. Results from the DAPA-HF, MEASURE-HF and DELIGHT studies and the more pragmatic real-world studies elucidate the clinical benefits of DAPA and SAXA in T2DM patients with CKD or heart failure that could guide clinicians to optimize treatment for these patients [59, 92, 95, 106, 107, 128] (Fig. 8).
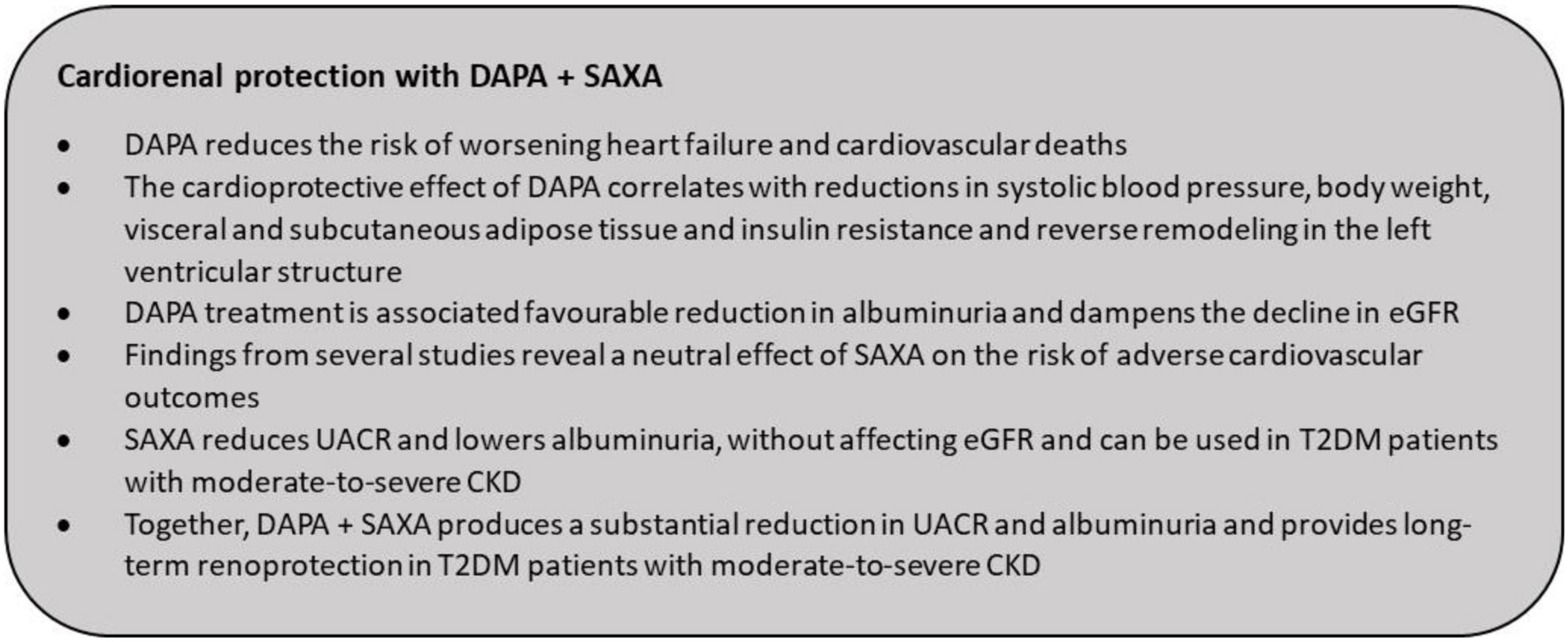 Click for large image | Figure 8. Cardiorenal protection with DAPA + SAXA. DAPA: dapagliflozin; SAXA: saxagliptin. |
| Use of DAPA + SAXA in Indian Patients With T2DM | ▴Top |
Landmark clinical trials and several real-world studies have illustrated the cardio and renal benefits conferred by the DAPA + SAXA combination while achieving durable glycemic control. Clinic and population-based data provide overwhelming evidence for the higher risk of chronic renal and cardiovascular diseases in South Asian patients with T2DM as compared with other ethnicities [6]. The large real-world TIGHT study revealed a high burden of CKD and heart failure in Indian patients with long-standing T2DM [5]. Collectively, these data indicate the need for more intensive multimodal treatment in Indian patients with T2DM. Understanding the prescribing practices of newer agents and combinations in the population will help maximize the benefits of these therapies. The following statements provide clinical experience-based recommendations for the use of DAPA + SAXA, which could potentially support individualization of treatment goals according to the specific needs of the Indian population (Fig. 9).
When do you recommend initiating treatment with DAPA-SAXA FDC?
The experts recommended the initiation of FDC based on the individual treatment goal and patient centric factors. The FDC can be introduced as part of treatment intensification for patients not meeting treatment goals (inadequately controlled on MET or other anti-hyperglycemic agents), regardless of the presence of cardio-renal comorbidities [26]. The FDC can also be used earlier at treatment initiation and in patients with cardiovascular and renal comorbidities to attain desirable glycemic control and arrest the progression of cardiovascular and renal complications [25-27, 129].
At what duration of diabetes, do your patients receive the FDC?
Aligned with the treatment initiation strategy, the experts recommended treatment with the FDC in patients who have been diagnosed with T2DM for < 1 to 5 years of T2DM, as an approach to achieve adequate glycemic control and mitigate cardiovascular and renal complications.
At what HbA1c levels do your patients receive the FDC?
The experts recommended prescribing FDC to patients with T2DM when their HbA1c levels are between 7.6% and 9.0%. This concurs with the RSSDI recommendation of initiating combination therapy in patients with HbA1c > 1.5 above the recommended goal of 7% [27].
What are the comorbidities in your patients receiving FDC?
Currently Indian patients with T2DM who are being prescribed the FDC in primary care have comorbid CKD and cardiovascular diseases. Supported by cardiovascular outcome trials and studies in CKD, the combination of DAPA + SAXA is expected to provide comprehensive vascular risk control and metabolic benefits that can potentially reduce the risk of advancing these comorbid conditions to renal or heart failure.
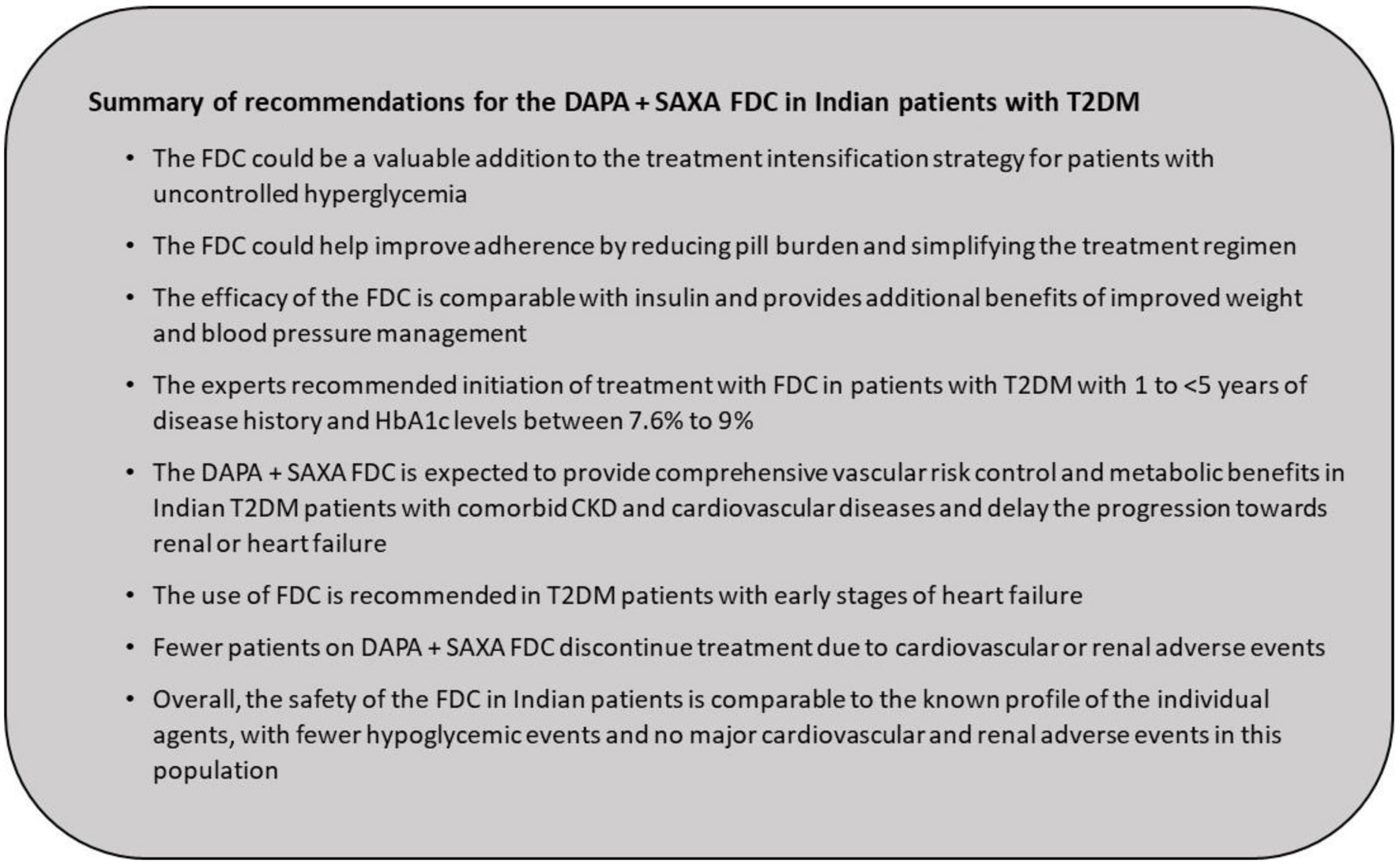 Click for large image | Figure 9. Summary of recommendations for the DAPA + SAXA FDC in Indian patients with T2DM. DAPA: dapagliflozin; FDC: fixed-dose combination; SAXA: saxagliptin; T2DM: type 2 diabetes mellitus. |
| Conclusions | ▴Top |
The statements provided in this paper can be used by Indian diabetologists and endocrinologists to aid treatment decisions that reflect the experience and opinion of experts in the field. Overall, available clinical evidence suggests that the inclusion of DAPA + SAXA FDC in the management of T2DM is associated with greater improvements in glycemic control, with benefits in weight management, lower risk of hypoglycemia and an acceptable safety profile. Together, these antihyperglycemic agents represent a synergistic combination, which is associated with improved cardiovascular outcomes and renoprotection. Together, these antihyperglycemic agents form a synergistic combination, contributing to improved cardiovascular outcomes and renoprotection, with DAPA exhibiting favorable effects on body weight, blood pressure, reduced risk of hHF, and clinically meaningful alterations in markers of declining renal function, complemented by SAXA’s effects on ischemic events and improvements in UACR. The FDC of DAPA + SAXA addresses patient preference for oral antihyperglycemic agents and could enhance patient adherence enabling improved long-term management of T2DM. The FDC of DAPA + SAXA may be a suitable therapy for the metabolically deranged Indian patients for improving the long-term cardiovascular and renal outcomes. The strategies and approaches presented in this paper should be implemented in accordance with the existing clinical practice guidelines and as determined appropriate by the treatment physician, considering the condition of the individual patient.
Acknowledgments
The authors would like to thank AstraZeneca Pharma India Limited for development of the manuscript in collaboration with Priya Ganpathy, MPH, CMPP (SIRO Clinpharm UK Limited) and Shreyasi Asthana, PhD (SIRO Clinpharm Pvt. Ltd, India) in accordance with GPP2022 guidelines (https://www.ismpp.org/gpp-2022).
Financial Disclosure
This study was funded by AstraZeneca Pharma India Limited.
Conflict of Interest
Authors Sujoy Ghosh, Subhash K. Wangnoo, Sachin Chittawar, Suresh Damodharan, Yogesh Kadam, Pramila Kalra, K.P. Suresh Kumar, I. Periyandavar, and S.K. Sharma have nothing to disclose. Abdul Hamid Zargar has received honoraria from Novo Nordisk, Eli Lilly, Johnson & Johnson, AstraZeneca, BI and Sanofi.
Author Contributions
All authors contributed to the study conception, scope, and design, and participated in the consensus meeting. All authors critically reviewed the manuscript and approved the final version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AACE: American Association of Clinical Endocrinologists-American College of Endocrinology; ACD: all-cause death; ACEi: angiotensin-converting enzyme inhibitor; ADA: American Diabetes Association; ARB: angiotensin receptor blocker; BMI: body mass index; CDSCO: Central Drugs Standard Control Organization; CKD: chronic kidney disease; CVD-REAL: Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors in the Real-world Setting; CPRD: Clinical Practice Research Datalink; DAPA: dapagliflozin; DAPA-CKD: DAPA and Prevention of Adverse Outcomes in CKD; DAPA-HF: DAPA in Patients With Heart Failure and Reduced Ejection Fraction; DAPA-LVH: DAPA on Left Ventricular Hypertrophy; DCGI: Drug Controller General of India; DECLARE-TIMI 58: Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58; DELIVER: DAPA Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure; DPP4: dipeptidyl peptidase-4; DPP4i: dipeptidyl peptidase 4 inhibitor; eGFR: estimated glomerular filtration rate; FDC: fixed-dose combination; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; HOMA-2: homeostasis model assessment; hHF: hospitalization for heart failure; HR: hazard ratio; ICMR-INDIAB: Indian Council of Medical Research-India Diabetes; IDF: International Diabetes Federation; MACE: major adverse cardiovascular event; MEASURE-HF: Mechanistic Evaluation of Glucose-lowering Strategies in Patients With Heart Failure; MET: metformin; NT-proBNP: N-terminal pro-B-type natriuretic peptides; PPG: postprandial plasma glucose; RSSDI: Research Society for the Study of Diabetes in India; SAXA: saxagliptin; SAVOR-TIMI 53: SAXA Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53; SGLT2: sodium-glucose cotransporter-2; SGLT2i: sodium-glucose cotransporter-2 inhibitor; T2DM: type 2 diabetes mellitus; TIGHT: The Investigation of Glycosylated Hemoglobin on Therapy in Indian Diabetics; UACR: urinary albumin/creatinine
| References | ▴Top |
- IDF Diabetes Atlas. 2021. Available from: https://diabetesatlas.org/regional-factsheets/?dlmodal=active&dlsrc=https%3A%2F%2Fdiabetesatlas.org%2Fidfawp%2Fresource-files%2F2021%2F11%2FIDFDA10-global-fact-sheet.pdf.
- Anjana RM, Unnikrishnan R, Deepa M, Pradeepa R, Tandon N, Das AK, Joshi S, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474-489.
doi pubmed - Hills AP, Arena R, Khunti K, Yajnik CS, Jayawardena R, Henry CJ, Street SJ, et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6(12):966-978.
doi pubmed - Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12(6):357-370.
doi pubmed - Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res Care. 2019;7(1):e000654.
doi pubmed pmc - Misra A, Sattar N, Tandon N, Shrivastava U, Vikram NK, Khunti K, Hills AP. Clinical management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6(12):979-991.
doi pubmed - Gupta R, Misra A. Epidemiology of microvascular complications of diabetes in South Asians and comparison with other ethnicities. J Diabetes. 2016;8(4):470-482.
doi pubmed - Rosenstock J, Chuck L, Gonzalez-Ortiz M, Merton K, Craig J, Capuano G, Qiu R. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naive type 2 diabetes. Diabetes Care. 2016;39(3):353-362.
doi pubmed - Das A, Unnikrishnan A, Saboo B, Mohan V, Vijay V. Indian guidance on cardiovascular and renal comorbidity management in type-2 diabetes mellitus. Journal of The Association of Physicians of India. 2018.
- Unnikrishnan AG, Sahay RK, Phadke U, Sharma SK, Shah P, Shukla R, Viswanathan V, et al. Cardiovascular risk in newly diagnosed type 2 diabetes patients in India. PLoS One. 2022;17(3):e0263619.
doi pubmed pmc - Mohan V, Shah S, Saboo B. Current glycemic status and diabetes related complications among type 2 diabetes patients in India: data from the A1chieve study. J Assoc Physicians India. 2013;61(1 Suppl):12-15.
pubmed - Mohan V, Venkatraman JV, Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: the Indian scenario. J Diabetes Sci Technol. 2010;4(1):158-170.
doi pubmed pmc - Singh AK, Farag YM, Mittal BV, Subramanian KK, Reddy SR, Acharya VN, Almeida AF, et al. Epidemiology and risk factors of chronic kidney disease in India - results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114.
doi pubmed pmc - Jayakumari S, Gomez R, Dipin S, Jayakumar RV, Vijayakumar K, Sreenath R, Ajeesh T, et al. Prevalence of normoalbuminuric chronic kidney disease among individuals with type 2 diabetes mellitus from India. Indian J Med Res. 2022;156(4&5):632-639.
doi pubmed pmc - Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
doi pubmed pmc - Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
doi pubmed pmc - Chadha M, Das AK, Deb P, Gangopadhyay KK, Joshi S, Kesavadev J, Kovil R, et al. Expert opinion: optimum clinical approach to combination-use of SGLT2i + DPP4i in the Indian diabetes setting. Diabetes Ther. 2022;13(5):1097-1114.
doi pubmed pmc - AstraZeneca. FDA approves once-daily QTERN (dapagliflozin and saxagliptin) tablets for adults with type-2 diabetes. https://www.astrazeneca.com/media-centre/press-releases/2017/fda-approves-once-daily-qtern-dapagliflozin-and-saxagliptin-tablets-for-adults-with-type-2-diabetes-240217.html. Accessed May 29, 2023.
- AstraZeneca. AstraZeneca receives Complete Response Letter from US FDA for saxagliptin/dapagliflozin fixed-dose-combination. https://www.astrazeneca.com/media-centre/press-releases/2015/astrazeneca-receivescomplete-response-letter-from-us-16102015.html. Accessed May 29, 2023.
- AstraZeneca India receives marketing permission for QTERN®, once-daily anti-diabetes treatment for adults with type 2 diabetes. https://www.astrazeneca.in/media/press-releases/2019/astrazeneca-india-receives-marketing-permission-for-qtern.html#!. Accessed on May 29, 2023.
- Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, Hansen L, Chen H, Johnsson E, Garcia-Sanchez R, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(11):1134-1137.
doi pubmed - Frias JP, Gonzalez-Galvez G, Johnsson E, Maaske J, Testa MA, Simonson DC, Dronamraju N, et al. Efficacy and safety of dual add-on therapy with dapagliflozin plus saxagliptin versus glimepiride in patients with poorly controlled type 2 diabetes on a stable dose of metformin: Results from a 52-week, randomized, active-controlled trial. Diabetes Obes Metab. 2020;22(7):1083-1093.
doi pubmed pmc - Vilsboll T, Ekholm E, Johnsson E, Dronamraju N, Jabbour S, Lind M. Dapagliflozin plus saxagliptin add-on therapy compared with insulin in patients with type 2 diabetes poorly controlled by metformin with or without sulfonylurea therapy: a randomized clinical trial. Diabetes Care. 2019;42(8):1464-1472.
doi pubmed - Vilsboll T, Ekholm E, Johnsson E, Garcia-Sanchez R, Dronamraju N, Jabbour SA, Lind M. Efficacy and safety of dapagliflozin plus saxagliptin versus insulin glargine over 52 weeks as add-on to metformin with or without sulphonylurea in patients with type 2 diabetes: A randomized, parallel-design, open-label, Phase 3 trial. Diabetes Obes Metab. 2020;22(6):957-968.
doi pubmed pmc - ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140-S157.
doi pubmed pmc - Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, Isaacs SD, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm - 2023 update. Endocr Pract. 2023;29(5):305-340.
doi pubmed - Makkar B, Kumar V, Saboo B, Agarwal S. RSSDI Clinical Practice Recommendations for the Management of Type 2 Diabetes Mellitus 2022. International journal of diabetes in developing countries. 2022;42(Suppl 1):1-143.
- Dey J. SGLT2 inhibitor/DPP-4 inhibitor combination therapy - complementary mechanisms of action for management of type 2 diabetes mellitus. Postgrad Med. 2017;129(4):409-420.
doi pubmed - Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
doi pubmed pmc - Arya DS, Chowdhury S, Chawla R, Das AK, Ganie MA, Kumar KMP, Nadkar MY, et al. Clinical benefits of fixed dose combinations translated to improved patient compliance. J Assoc Physicians India. 2019;67(12):58-64.
pubmed - Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713-719.
doi pubmed - Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, Kountouri A, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. 2020;9(9):e015716.
doi pubmed pmc - van Ruiten CC, van der Aart-van der Beek AB, RG IJ, Nieuwdorp M, Hoogenberg K, van Raalte DH, Heerspink HJL. Effect of exenatide twice daily and dapagliflozin, alone and in combination, on markers of kidney function in obese patients with type 2 diabetes: A prespecified secondary analysis of a randomized controlled clinical trial. Diabetes Obes Metab. 2021;23(8):1851-1858.
doi pubmed pmc - Ji L, Dong X, Li Y, Li Y, Lim S, Liu M, Ning Z, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab. 2021;23(2):404-414.
doi pubmed pmc - Giugliano D, Longo M, Caruso P, Di Fraia R, Scappaticcio L, Gicchino M, Petrizzo M, et al. Feasibility of simplification from a basal-bolus insulin regimen to a fixed-ratio formulation of basal insulin plus a GLP-1RA or to basal insulin plus an SGLT2 inhibitor: BEYOND, a randomized, pragmatic trial. Diabetes Care. 2021;44(6):1353-1360.
doi pubmed pmc - Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab. 2009;11(6):527-533.
doi pubmed - Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65(5):795-796.
doi pubmed pmc - Bohm AK, Schneider U, Aberle J, Stargardt T. Regimen simplification and medication adherence: Fixed-dose versus loose-dose combination therapy for type 2 diabetes. PLoS One. 2021;16(5):e0250993.
doi pubmed pmc - Sharma MD. Potential for combination of dipeptidyl peptidase-4 inhibitors and sodium-glucose co-transporter-2 inhibitors for the treatment of type 2 diabetes. Diabetes Obes Metab. 2015;17(7):616-621.
doi pubmed pmc - Kelly SD, Neary SL. Ominous octet and other scary diabetes stories: the overview of pathophysiology of type 2 diabetes mellitus. Physician Assistant Clinics. 2020;5(2):121-33.
- Brunton SA. The potential role of sodium glucose co-transporter 2 inhibitors in the early treatment of type 2 diabetes mellitus. Int J Clin Pract. 2015;69(10):1071-1087.
doi pubmed pmc - Sriphrapradang C, Thewjitcharoen Y, Buranapin S, Sawanyawisuth K, Yenseung N, Ubonchareon W, Limpijankit L, et al. Effectiveness and safety of sodium-glucose co-transporter-2 inhibitors in Thai adults with type 2 diabetes mellitus: a real-world study. Curr Med Res Opin. 2020;36(10):1601-1610.
doi pubmed - Hassoun A, Dhanwal DK, Nafach J, Ajaz Y, Khan AM, Ben Nakhi A, AlArouj M, et al. Real-world assessment of efficacy and safety parameters for dapagliflozin in management of type 2 diabetes mellitus: REWARD study. Dubai Diabetes and Endocrinology Journal. 2022;28(1):25-34.
- McGurnaghan SJ, Brierley L, Caparrotta TM, McKeigue PM, Blackbourn LAK, Wild SH, Leese GP, et al. The effect of dapagliflozin on glycaemic control and other cardiovascular disease risk factors in type 2 diabetes mellitus: a real-world observational study. Diabetologia. 2019;62(4):621-632.
doi pubmed - Morales C, Bellido V, Tejera C, Goni F, Palomares R, Sevillano C, Bellido D, et al. DAPA-RWE: a retrospective multicenter study comparing dapagliflozin and sitagliptin in patients with Type 2 diabetes treated under routine clinical practice in Spain. J Comp Eff Res. 2021;10(10):815-821.
doi pubmed - Toulis KA, Willis BH, Marshall T, Kumarendran B, Gokhale K, Ghosh S, Thomas GN, et al. All-cause mortality in patients with diabetes under treatment with dapagliflozin: a population-based, open-cohort study in the health improvement network database. J Clin Endocrinol Metab. 2017;102(5):1719-1725.
doi pubmed - Viswanathan V, Singh KP. Use of dapagliflozin in the management of type 2 diabetes mellitus: a real-world evidence study in indian patients (FOREFRONT). Diabetes Technol Ther. 2019;21(8):415-422.
doi pubmed - Sethi BK, Kalra S, Bhattacharya S, Kumar A, Rai M, Srivastava MK, A S, et al. Real-world evidence of generic dapagliflozin: relevance and results from indian multicenter retrospective study. Journal of Diabetology. 2022;13(3):242-248.
- Kalra S, Bajaj S, Unnikrishnan AG, Baruah MP, Sahay R, Hardik V, Kumar A. Therapeutic experience of saxagliptin as first add-on after metformin in Indian type 2 diabetes patients: a non-interventional, prospective, observational study (ONTARGET-INDIA). Indian J Endocrinol Metab. 2019;23(3):312-317.
doi pubmed pmc - Kumar KMP, Jain SM, Tou C, Schutzer K-M. Saxagliptin as initial therapy in treatment-naive Indian adults with type 2 diabetes mellitus inadequately controlled with diet and exercise alone: a randomized, double-blind, placebo-controlled, phase IIIb clinical study. International Journal of Diabetes in Developing Countries. 2014;34(4):201-209.
- Muller-Wieland D, Kellerer M, Cypryk K, Skripova D, Rohwedder K, Johnsson E, Garcia-Sanchez R, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(11):2598-2607.
doi pubmed pmc - Nowicki M, Rychlik I, Haller H, Warren M, Suchower L, Gause-Nilsson I, Schutzer KM. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract. 2011;65(12):1230-1239.
doi pubmed - Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, Iqbal N. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376-383.
doi pubmed - Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020;41(36):3421-3432.
doi pubmed pmc - Ha KH, Kim B, Shin HS, Lee J, Choi H, Kim HC, Kim DJ. Comparative cardiovascular risks of dipeptidyl peptidase-4 inhibitors: analyses of real-world data in Korea. Korean Circ J. 2018;48(5):395-405.
doi pubmed pmc - Johansson L, Hockings PD, Johnsson E, Dronamraju N, Maaske J, Garcia-Sanchez R, Wilding JPH. Dapagliflozin plus saxagliptin add-on to metformin reduces liver fat and adipose tissue volume in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22(7):1094-1101.
doi pubmed pmc - Khunti K, Kosiborod M, Kim DJ, Kohsaka S, Lam CSP, Goh SY, Chiang CE, et al. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors vs other glucose-lowering drugs in 13 countries across three continents: analysis of CVD-REAL data. Cardiovasc Diabetol. 2021;20(1):159.
doi pubmed pmc - Matthaei S, Aggarwal N, Garcia-Hernandez P, Iqbal N, Chen H, Johnsson E, Chin A, et al. One-year efficacy and safety of saxagliptin add-on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016;18(11):1128-1133.
doi pubmed - McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
doi pubmed - Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579-1588.
doi pubmed - Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089-1098.
doi pubmed - Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
doi pubmed - Fuchigami A, Shigiyama F, Kitazawa T, Okada Y, Ichijo T, Higa M, Hiyoshi T, et al. Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY-CVR). Cardiovasc Diabetol. 2020;19(1):1.
doi pubmed pmc - Morales C, Merino-Torres JF, Moreno-Moreno P, Lainez M, Tejado I, Yoldi A, Gutierrez Medina S, et al. Effectiveness and safety of dapagliflozin in real-life patients: data from the DAPA-RWE Spanish multicentre study. Drugs Context. 2022;11:2021-11-5.
doi pubmed pmc - Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69-76.
doi pubmed - Anoop S, Misra A, Bhatt SP, Gulati S, Pandey RM, Mahajan H. High circulating plasma dipeptidyl peptidase- 4 levels in non-obese Asian Indians with type 2 diabetes correlate with fasting insulin and LDL-C levels, triceps skinfolds, total intra-abdominal adipose tissue volume and presence of diabetes: a case-control study. BMJ Open Diabetes Res Care. 2017;5(1):e000393.
doi pubmed pmc - LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev. 2021;42(1):77-96.
doi pubmed pmc - Abdul-Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP-4 inhibitor fit in the management of type 2 diabetes? Diabetes Care. 2015;38(3):373-375.
doi pubmed - Boulton DW, Kasichayanula S, Keung CF, Arnold ME, Christopher LJ, Xu XS, Lacreta F. Simultaneous oral therapeutic and intravenous (1)(4)C-microdoses to determine the absolute oral bioavailability of saxagliptin and dapagliflozin. Br J Clin Pharmacol. 2013;75(3):763-768.
doi pubmed pmc - Vakkalagadda B, Lubin S, Reynolds L, Liang D, Marion AS, LaCreta F, Boulton DW. Lack of a Pharmacokinetic interaction between saxagliptin and dapagliflozin in healthy subjects: a randomized crossover study. Clin Ther. 2016;38(8):1890-1899.
doi pubmed - Scheen AJ. Pharmacokinetic drug evaluation of saxagliptin plus dapagliflozin for the treatment of type 2 diabetes. Expert Opin Drug Metab Toxicol. 2017;13(5):583-592.
doi pubmed - Ekholm E, Hansen L, Johnsson E, Iqbal N, Carlsson B, Chen H, Hirshberg B. Combined treatment with saxagliptin plus dapagliflozin reduces insulin levels by increased insulin clearance and improves beta-cell function. Endocr Pract. 2017;23(3):258-265.
doi pubmed - Anderson JE. Combining Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors to Target Multiple Organ Defects in Type 2 Diabetes. Diabetes Spectr. 2020;33(2):165-174.
doi pubmed pmc - Ahsan S. Abstract# 1004069: Effectiveness of remogliflozin and vildagliptin combination in type 2 diabetes mellitus patients uncontrolled on triple oral drug therapy. Endocrine Practice. 2021;27(6):S62.
- DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38(3):384-393.
doi pubmed - Gupta A, Malhotra P, Jamwal V, Khalse M. A retrospective analysis of fixed combination of empagliflozin and linagliptin in addition to the existing treatment for its clinical effectiveness in adults with type 2 diabetes: a real-world clinical experience. J Assoc Physicians India. 2021;69(7):11-12.
pubmed - Jabbour SA, Hardy E, Sugg J, Parikh S, Study G. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37(3):740-750.
doi pubmed - Kawamori R, Haneda M, Suzaki K, Cheng G, Shiki K, Miyamoto Y, Solimando F, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: Glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018;20(9):2200-2209.
doi pubmed pmc - Khaladkar K, Mohan B, Khaladkar K, Suryawanshi S, Barkatestrong/Strong H. Efficacy and safety of a fixed dose combination of remogliflozin etabonate and vildagliptin in patients with type-2 diabetes mellitus: a randomized, active-controlled, double-blind, Phase III study. J Assoc Physicians India. 2022;70(4):11-12.
pubmed - Matthaei S, Catrinoiu D, Celinski A, Ekholm E, Cook W, Hirshberg B, Chen H, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38(11):2018-2024.
doi pubmed - Petchiappan V, Mathew E, Jose J, Fardan M, Chidambaram Y, Thangavelu S. Use of fixed-dose combination therapy with remogliflozin and vildagliptin as an add-on drug in improving the glycemic control of type 2 diabetes mellitus: an observational study. Journal of Pharmacology and Pharmacotherapeutics. 2023;14(1):72-78.
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461.
doi pubmed - Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-242.
doi pubmed - Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628-2639.
doi pubmed - McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W, Lewsey JD, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail. 2018;6(1):8-17.
doi pubmed - Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424.
doi pubmed - Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79.
doi pubmed pmc - Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
doi pubmed - Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155-1166.
doi pubmed pmc - Rosenstock J, Perl S, Johnsson E, Garcia-Sanchez R, Jacob S. Triple therapy with low-dose dapagliflozin plus saxagliptin versus dual therapy with each monocomponent, all added to metformin, in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(9):2152-2162.
doi pubmed pmc - Blonde L, Dipp S, Cadena D. Combination Glucose-Lowering Therapy Plans in T2DM: Case-Based Considerations. Adv Ther. 2018;35(7):939-965.
doi pubmed - Nowicki M, Rychlik I, Haller H, Warren ML, Suchower L, Gause-Nilsson I, Investigators DC. Saxagliptin improves glycaemic control and is well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetes Obes Metab. 2011;13(6):523-532.
doi pubmed - Cahn A, Raz I, Bonaca M, Mosenzon O, Murphy SA, Yanuv I, Rozenberg A, et al. Safety of dapagliflozin in a broad population of patients with type 2 diabetes: Analyses from the DECLARE-TIMI 58 study. Diabetes Obes Metab. 2020;22(8):1357-1368.
doi pubmed - Handelsman Y, Mathieu C, Del Prato S, Johnsson E, Kurlyandskaya R, Iqbal N, Garcia-Sanchez R, et al. Sustained 52-week efficacy and safety of triple therapy with dapagliflozin plus saxagliptin versus dual therapy with sitagliptin added to metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(4):883-892.
doi pubmed pmc - Pollock C, Stefansson B, Reyner D, Rossing P, Sjostrom CD, Wheeler DC, Langkilde AM, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(6):429-441.
doi pubmed - Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-2222.
doi pubmed pmc - Fisman EZ, Tenenbaum A. Antidiabetic treatment with gliptins: focus on cardiovascular effects and outcomes. Cardiovasc Diabetol. 2015;14:129.
doi pubmed pmc - Schernthaner G, Duran-Garcia S, Hanefeld M, Langslet G, Niskanen L, Ostgren CJ, Malvolti E, et al. Efficacy and tolerability of saxagliptin compared with glimepiride in elderly patients with type 2 diabetes: a randomized, controlled study (GENERATION). Diabetes Obes Metab. 2015;17(7):630-638.
doi pubmed - Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114(11):1788-1803.
doi pubmed - Kajikawa M, Maruhashi T, Hidaka T, Matsui S, Hashimoto H, Takaeko Y, Nakano Y, et al. Effect of Saxagliptin on Endothelial Function in Patients with Type 2 Diabetes: A Prospective Multicenter Study. Sci Rep. 2019;9(1):10206.
doi pubmed pmc - Frederich R, Alexander JH, Fiedorek FT, Donovan M, Berglind N, Harris S, Chen R, et al. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med. 2010;122(3):16-27.
doi pubmed - Iqbal N, Parker A, Frederich R, Donovan M, Hirshberg B. Assessment of the cardiovascular safety of saxagliptin in patients with type 2 diabetes mellitus: pooled analysis of 20 clinical trials. Cardiovasc Diabetol. 2014;13:33.
doi pubmed pmc - Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326.
doi pubmed - Mannucci E, Nreu B, Montereggi C, Ragghianti B, Gallo M, Giaccari A, Monami M, et al. Cardiovascular events and all-cause mortality in patients with type 2 diabetes treated with dipeptidyl peptidase-4 inhibitors: An extensive meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;31(10):2745-2755.
doi pubmed - Men P, Li XT, Tang HL, Zhai SD. Efficacy and safety of saxagliptin in patients with type 2 diabetes: A systematic review and meta-analysis. PLoS One. 2018;13(5):e0197321.
doi pubmed pmc - Mechanistic Evaluation of Glucose-lowering strategies in patients with heart failure (MEASURE-HF). Available from: https://clinicaltrials.gov/ct2/show/NCT02917031.
- Pitt B, Reicher B, Gilbert R, Scirica B, Swedberg K, Langkilde A, Monyak J, et al. Effect of saxagliptin on LV structure and function in patients with type 2 diabetes and heart failure: results of Measure-HF. Journal of Cardiac Failure. 2022;28(5, Supplement):S92-S93.
- Toh S, Hampp C, Reichman ME, Graham DJ, Balakrishnan S, Pucino F, Hamilton J, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164(11):705-714.
doi pubmed pmc - Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, Hu N, et al. A Multicenter Observational Study of Incretin-based Drugs and Heart Failure. N Engl J Med. 2016;374(12):1145-1154.
doi pubmed - Fu AZ, Johnston SS, Ghannam A, Tsai K, Cappell K, Fowler R, Riehle E, et al. Association between hospitalization for heart failure and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care. 2016;39(5):726-734.
doi pubmed - Balkau B, Charbonnel B, Penfornis A, Chraibi N, Lahouegue A, Faure C, Thomas-Delecourt F, et al. The Use of Saxagliptin in People with Type 2 Diabetes in France: The Diapazon Epidemiological Study. Diabetes Ther. 2017;8(5):1147-1162.
doi pubmed pmc - Del Prato S, Rosenstock J, Garcia-Sanchez R, Iqbal N, Hansen L, Johnsson E, Chen H, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: Post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab. 2018;20(6):1542-1546.
doi pubmed pmc - Raz I, Mosenzon O, Bonaca MP, Cahn A, Kato ET, Silverman MG, Bhatt DL, et al. DECLARE-TIMI 58: Participants' baseline characteristics. Diabetes Obes Metab. 2018;20(5):1102-1110.
doi pubmed - Shao SC, Chang KC, Hung MJ, Yang NI, Chan YY, Chen HY, Kao Yang YH, et al. Comparative risk evaluation for cardiovascular events associated with dapagliflozin vs. empagliflozin in real-world type 2 diabetes patients: a multi-institutional cohort study. Cardiovasc Diabetol. 2019;18(1):120.
doi pubmed pmc - Cavender MA, Norhammar A, Birkeland KI, Jorgensen ME, Wilding JP, Khunti K, Fu AZ, et al. SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018;71(22):2497-2506.
doi pubmed - Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 Inhibitors). Circulation. 2017;136(3):249-259.
doi pubmed pmc - Lam CSP, Karasik A, Melzer-Cohen C, Cavender MA, Kohsaka S, Norhammar A, Thuresson M, et al. Association of sodium-glucose cotransporter-2 inhibitors with outcomes in type 2 diabetes with reduced and preserved left ventricular ejection fraction: Analysis from the CVD-REAL 2 study. Diabetes Obes Metab. 2021;23(6):1431-1435.
doi pubmed pmc - Kluger AY, Tecson KM, Barbin CM, Lee AY, Lerma EV, Rosol ZP, Rangaswami J, et al. Cardiorenal outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: a systematic review. Rev Cardiovasc Med. 2018;19(2):41-49.
doi pubmed - Jardine MJ, Hata J, Woodward M, Perkovic V, Ninomiya T, Arima H, Zoungas S, et al. Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis. 2012;60(5):770-778.
doi pubmed - Johannes CB, Beachler DC, Layton JB, Danysh HE, Ziemiecki R, Arana A, Dinh J, et al. Post-authorization safety study of hospitalization for acute kidney injury in patients with type 2 diabetes exposed to dapagliflozin in a real-world setting. Drug Saf. 2023;46(2):157-174.
doi pubmed pmc - Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436-1446.
doi pubmed - Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85(3):579-589.
doi pubmed - Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J. 2011;58(1):69-73.
doi pubmed - Wanner C, Cooper ME, Johansen OE, Toto R, Rosenstock J, McGuire DK, Kahn SE, et al. Effect of linagliptin versus placebo on cardiovascular and kidney outcomes in nephrotic-range proteinuria and type 2 diabetes: the CARMELINA randomized controlled trial. Clin Kidney J. 2021;14(1):226-236.
doi pubmed pmc - Taylor OM, Lam C. The effect of dipeptidyl peptidase-4 inhibitors on macrovascular and microvascular complications of diabetes mellitus: a systematic review. Curr Ther Res Clin Exp. 2020;93:100596.
doi pubmed pmc - Perl S, Cook W, Wei C, Iqbal N, Hirshberg B. Saxagliptin Efficacy and Safety in Patients With Type 2 Diabetes and Moderate Renal Impairment. Diabetes Ther. 2016;7(3):527-535.
doi pubmed pmc - Mohsen M, Elberry AA, Mohamed Rabea A, Abdelrahim MEA, Hussein RRS. Saxagliptin and vildagliptin lowered albuminuria in patients with diabetes and hypertension independent on glycaemic control. Int J Clin Pract. 2021;75(3):e13769.
doi pubmed - Sharma A, Cooper LB, Fiuzat M, Mentz RJ, Ferreira JP, Butler J, Fitchett D, et al. Antihyperglycemic therapies to treat patients with heart failure and diabetes mellitus. JACC Heart Fail. 2018;6(10):813-822.
doi pubmed - Blonde L, Umpierrez GE, Reddy SS, McGill JB, Berga SL, Bush M, Chandrasekaran S, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: developing a diabetes mellitus comprehensive care plan-2022 update. Endocr Pract. 2022;28(10):923-1049.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
