
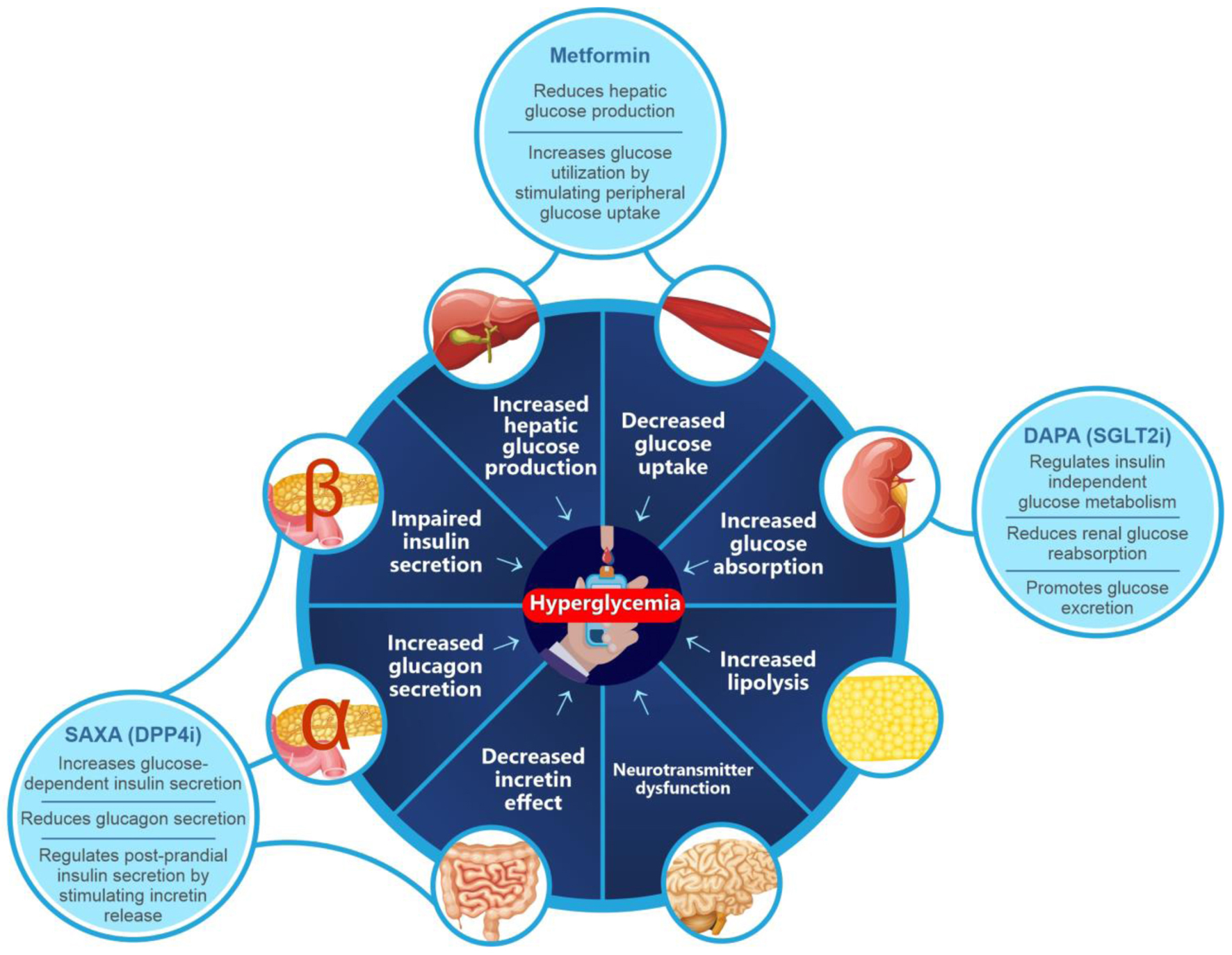
Figure 1. Combination therapy in T2DM. T2DM: type 2 diabetes mellitus.
| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 14, Number 3, June 2024, pages 128-148
Dapagliflozin-Saxagliptin Combination - The Quest for Optimal Glycemic Control With Cardio-Renal Protection in Type 2 Diabetes Mellitus: An Expert Consensus in Indian Settings
Figures


Tables
| ADA [25] | AACE [26] | RSSDI [27] |
|---|---|---|
| ADA: American Diabetes Association; AACE: American Association of Clinical Endocrinology; AGi: alpha-glucosidase inhibitors; DPP4i: dipeptidyl peptidase-4 inhibitor; GLP-1 RA: glucagon-like peptide-1 receptor agonist; GIP: gastric inhibitory polypeptide; GLP-1: glucagon-like peptide-1; HbA1c: glycated hemoglobin; NASH: nonalcoholic steatohepatitis; RSSDI: Research Society for the Study of Diabetes in India; SGLT2i: sodium-glucose cotransporter-2 inhibitor; T2DM: type 2 diabetes mellitus; TZD: thiazolidinediones. | ||
|
|
|
| Author and trial name | Type of trial | Country | Patients and intervention | Key outcomes |
|---|---|---|---|---|
| ACR: albumin to creatinine ratio; AE: adverse event, BSA: body surface area; BW: body weight; BMI: body mass index; CI: confidence interval; CVD: cardiovascular disease; DAPA: dapagliflozin; DBP: diastolic blood pressure; DPP4i: dipeptidyl peptidase-4 inhibitor; DKA: diabetic ketoacidosis; ESRD: end-stage renal disease; FPG: fasting plasma glucose; GLIM: glimepiride; HF: heart failure; hHF: hospitalization for heart failure; HR: hazard ratio; HOMA-IR: homeostatic model assessment of insulin resistance; eGFR: estimated glomerular filtration rate; MET: metformin; NYHA: New York heart association; LINA: linagliptin; PPG: post-prandial plasma glucose; INS: insulin; ITT: intent to treat; MI: myocardial infraction; oGLD: other glucose lowering drugs; UTI: urinary tract infection; LDL: low-density lipoprotein; MACE: major adverse cardiovascular event; RWE: real-world evidence; RCT: randomized controlled trial; SGLT2i: sodium-glucose cotransporter-2 inhibitor; PBO: placebo; SAXA: saxagliptin; SBP: systolic blood pressure; SITA: sitagliptin; VILD: vildagliptin; SU: sulfonylurea; SCAT: subcutaneous adipose tissue; TEAE: treatment emergent adverse events; UTI: urinary tract infection; VAT: visceral adipose tissue. | ||||
| Wiviott et al, 2019 [62] (DECLARE TIMI 58) | RCT | Multinational | Patients with T2DM (n = 17,160; n = 10,186 without atherosclerotic CVD) treated with DAPA 10 mg vs. PBO |
|
| McGurnaghan et al, 2019 [44] | RWE | Scotland, UK | Patients with T2DM (n = 8,566) treated with DAPA as per recommended dose |
|
| Viswanathan et al, 2019 [47] (FOREFRONT) | RWE | India | Patients with T2DM (n = 1,941, treated with DAPA) as per recommended dose |
|
| McMurray et al, 2019 [59] (DAPA-HF) | RCT | United Kingdom (UK) | Patients with T2DM and NYHA class II, III, or IV HF and an ejection fraction of 40%, treated with DAPA 10 mg (n = 2,373) vs. PBO (n = 2,371) |
|
| Fuchigami et al, 2020 [63] (DIVERSITY-CVR) | RCT | Japan | Patients with T2DM treated with DAPA 5 - 10 mg (n = 170) vs. SITA 50 - 100 mg (n = 170) |
|
| Brown et al, 2020 [54] (DAPA-LVH) | RCT | Scotland, UK | Patients with T2DM and LVH, treated with DAPA 10 mg (n = 66) |
|
| Khunti et al, 2021 [57] (CVD REAL) | RWE | Multinational | T2DM patients who were new users of SGLT2i (n = 440,599); DAPA contributed 60% of total exposure time |
|
| Hassoun et al, 2022 [43] (REWARD) | RWE | UAE and Kuwait | Patients with T2DM treated with DAPA 10 mg (n = 511) |
|
| Morales et al, 2022 [64] (DAPA-RWE) | RWE | Spain | Patients with T2DM treated with DAPA (n = 594) and SITA (n = 452) |
|
| Sethi et al, 2022 [48] | RWE | India | Patients with T2DM treated with DAPA as an add-on to other antihyperglycemic agents with or without INS (n = 1,935) |
|
| Solomon et al, 2022 [61] (DELIVER) | RCT | Multinational | Patients with T2DM with HF and LVEF of more than 40% treated with DAPA 10 mg (n = 3,131) and PBO (n = 3,132) |
|
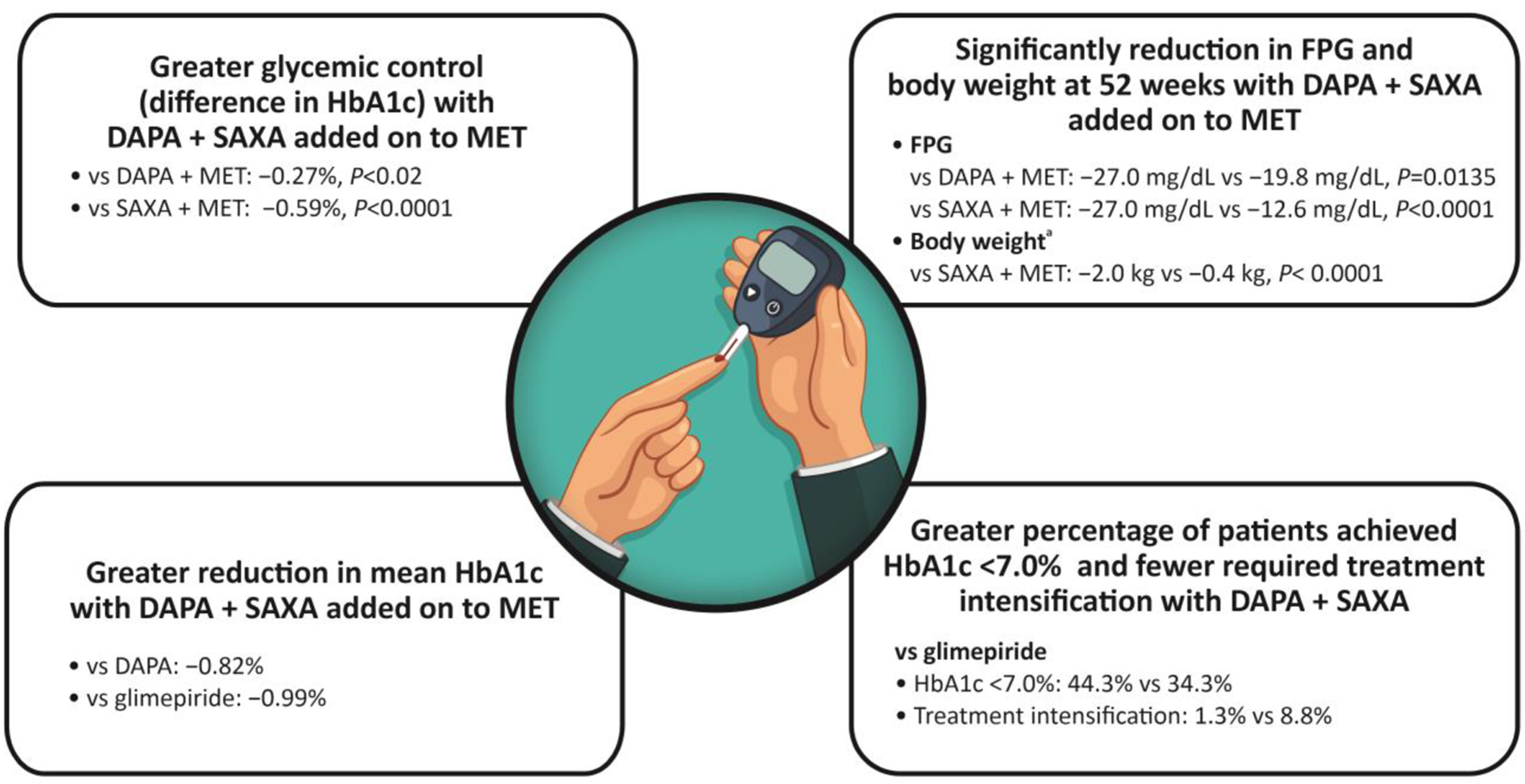
| Rosenstock et al, 2015 [53] | RCT | Multinational | Patients with T2DM treated with DAPA (10 mg) + SAXA (5 mg) + MET (n = 179) vs. SAXA + MET or PBO (n = 176) vs. DAPA + MET or PBO (n = 179) |
|
| Mathieu et al, 2016 [21] | RCT | Multinational | Patients with T2DM treated with PBO + SAXA + MET (n = 158) vs. DAPA 10 mg + SAXA + MET (n = 158) |
|
| Matthaei et al, 2016 [58] | RCT | Multinational | Patients with T2DM treated with DAPA 10 mg + MET + SAXA 5 mg (n = 153) vs. DAPA 10 mg + MET + PBO (n = 162) |
|
| Muller et al, 2018 [51] | RCT, DapaZu study | Germany, Czech Republic, Hungary, Poland and Slovakia | Patients with T2DM treated with MET + DAPA 10 mg (n = 314) vs. MET + DAPA 10 mg + SAXA 5 mg (n = 312) vs. MET + GLIM 1 to 6 mg (titrated) (n = 313) |
|
| Vilsboll et al, 2020 [24] | RCT | Multinational | Patients with T2DM treated with DAPA 10 mg + SAXA 5 mg (n = 306) vs. INS 100 U/mL (n = 294) |
|
| Frias et al, 2020 [22] | RCT | Multinational | Patients with T2DM treated with DAPA 10 mg + SAXA 5 mg (n = 227) vs. GLIM 1 - 6 mg (titrated; n = 217) |
|
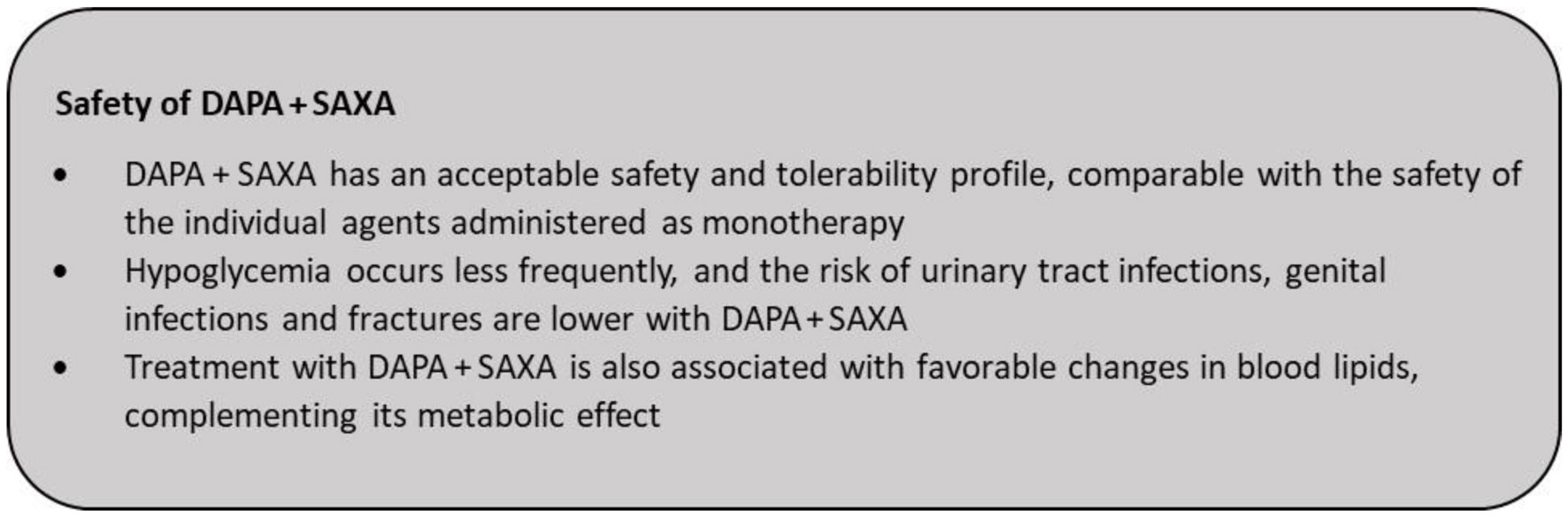
| Johansson et al, 2020 [56] | RCT | Multinational | Patients with T2DM treated with DAPA 10 mg + SAXA 5 mg + MET (n = 46) vs. GLIM + MET (n = 36) | > 30% reduction in liver fat (P = 0.007) and > 10% reduction in adipose tissue volumes (P < 0.01) with DAPA + SAXA + MET vs. GLIM + MET |
| Nowicki et al, 2011 [52] | RCT | Multinational | Patients with T2DM and renal impairment (moderate, severe or ESRD on hemodialysis) treated with SAXA 2.5 mg (n = 85) vs. PBO (n = 85) |
|
| Kumar et al, 2014 [50] | RCT | India | Treatment-naive pts with T2DM treated with SAXA (n = 107) vs. PBO (n = 106) |
|
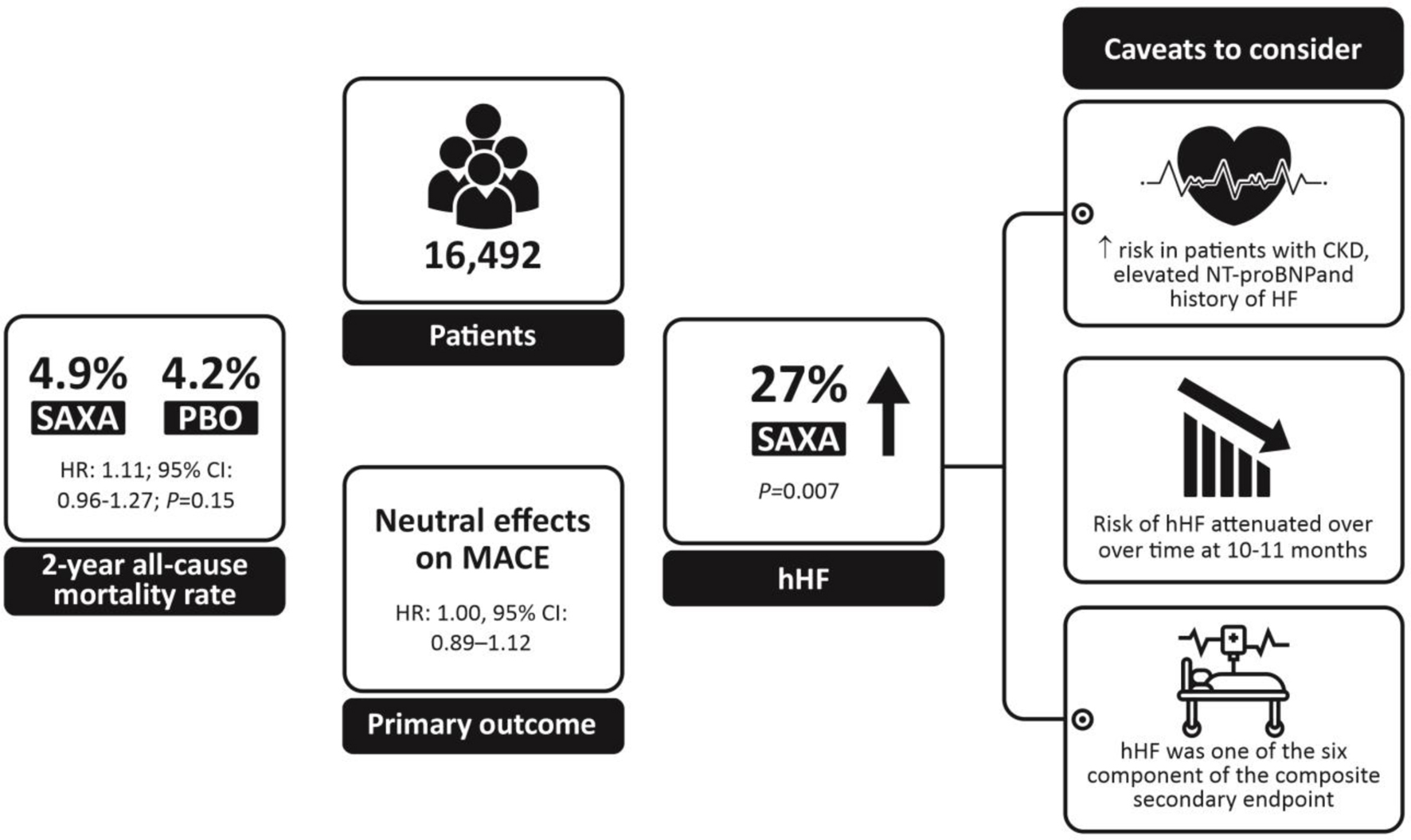
| Scirica et al, 2014 [60] (SAVOR-TIMI) | RCT | Multinational | Patients with T2DM and a history of, or at risk of, cardiovascular events treated with SAXA 5 mg or 2.5 mg (n = 8,280) vs. PBO (n = 8,212) |
|
| Mosenzon et al 2016 [65] (SAVOR-TIMI) | RCT | Multinational | Patients with T2DM and history of established CVD or multiple risk factors for CVD (n = 16,492) treated with SAXA 5 mg or 2.5 mg vs. PBO |
|
| Ha et al, 2018 [55] | RWE | Korea | Patients with T2DM who are newly prescribed DPP4i, SITA (n = 167,157), VILD (n = 67,412), SAXA (n = 29,479), LINA (n = 220,672), or GEMI (n = 49,607) | CVD risk: HR (95% CI); VILD, 0.97 (0.94 - 1.01), P = 0.163; SAXA, 0.76 (0.71 - 0.81), P < 0.001; LINA, 0.95 (0.92 - 0.98), P < 0.001; GEMI, 0.84 (0.80 - 0.88), P < 0.001 |
| Kalra et al, 2019 [49] (ONTARGET-INDIA) | RWE | India | Patients with T2DM inadequately controlled on MET treated with SAXA 5 mg (n = 1,109) |
|
| Outcomes | DAPA + SAXA [22, 24, 53, 80] | Empagliflozin + linagliptin [75, 76, 78] | Remogliflozin + vildagliptin [74, 79, 81] | DAPA + sitagliptin [77] |
|---|---|---|---|---|
| CARMELINA: Cardiovascular and Renal Microvascular Outcome Study With Linagliptin; CARLINA: Cardiovascular Outcome Study of Linagliptin; CVOT: Cardiovascular Outcome Trial; CVD REAL: Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors; DAPA: dapagliflozin; DAPA-HF: Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DAPA-LVH: Dapagliflozin on Left Ventricular Hypertrophy; DECLARE-TIMI: Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction; DELIVER: Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure; EMA: European Medicines Agency; EMPA-REG: Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose; FDA: Food and Drug Administration; FDC: fixed-dose combination; NA: not available; SAXA: saxagliptin; SAVOR-TIMI: Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus-Thrombolysis in Myocardial Infarction; TECOS: Trial Evaluating Cardiovascular Outcomes With Sitagliptin; VIVIDD: Vildagliptin in Ventricular Dysfunction Diabetes. | ||||
| Mean reduction in HbA1c, % | -1.37 to -1.5 | -0.93 to -1.19 | -1.2 to -1.8 | 0.0 to -0.4 |
| Mean reduction in FPG, mg/dL | -35.8 to -38 | -35.3 to -47.11 | -32.5 to -47.37 | -23.2 to -25.7 |
| Mean reduction in body weight, kg | -1.9 to -3.1 | -1.53 to -3.0 | NA | -1.4 to -2.5 |
| Genital infections, % | 0 to 5.30 | 1.1 to 8.6 | NA | 9.8 |
| Urinary tract infections, % | 0.6 to 6.5 | 0 to 10.2 | NA | 6.7 |
| Hypoglycemia, % | 1 to 6.2 | 0 to 3.6 | None | 5.3 |
| CVOT trials | SAVOR-TIMI [65], DECLARE-TIMI [62], DAPA-HF [59], DAPA-LVH [54], CVD REAL [57, 84], DELIVER [61] | EMPA-REG [88], CARMELINA [87], CAROLINA [89], EMPEROR-Preserved [82], EMPEROR-Reduced [86] | VIVIDD [85] | TECOS [83] |
| FDC US/EU approval status | ||||
| US FDA approval | Yes | Yes | No | No |
| EMA approval | Yes | Yes | No | No |