| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 14, Number 3, June 2024, pages 103-127
Role of Ketogenic Diets and Intermittent Fasting in Neurologic Diseases, Cancers, and Obesity: A Systematic Review of Human Studies
Nikhila Chelikama, p, Sai Anusha Akellab, p, Manisha Rakesh Lakhanpalc, q, Simmy Lahorid, Aryak Singhe, Sana Zafarf, Prashanth Gumpu Shivashankarg, Lokesh Manjanih, Ruslan Ilyassovi, Abdirazak Ibrahim Alij, Roghayeh Marandik, Rahul Gujarathil, Divesh Manjanim, Avinash Adigan, Urvish Patelo
aKansas City Heart Rhythm Institute (KCHRI), HCA Midwest Health System, Overland Park, KS 66211, USA
bDepartment of Medicine, Kakatiya Medical College, Warangal, India
cDepartment of Medicine, Emilio Aguinaldo College, Manila, Metro Manila, Philippines
dPramukhswami Medical College, Karamsad, Anand, Gujarat, India
eDepartment of Internal Medicine, Quinnipiac University Frank H. Netter MD School of Medicine/St. Vincent’s Medical Center Internal Medicine Residency Program, Bridgeport, CT, USA
fServices Institute of Medical Sciences, Lahore, Punjab, Pakistan
gAdichunchanagiri Hospital, BG Nagar, Bangalore, Karnataka, India
hDepartment of Medicine, Medstar Washington Hospital Center, Washington, DC, USA
iDepartment of Internal Medicine, Karaganda Medical University, Karaganda City, Kazakhstan
jDepartment of Internal Medicine, Jackson Park Hospital & Medical Center, Chicago, IL 60649, USA
kUniversity of California, Irvine, Orange, CA, USA
lDepartment of Hospital Medicine, University of Florida Health, Jacksonville, FL, USA
mUniversity of Medicine and Pharmacy, Timisoara, Romania
nDepartment of Internal Medicine, Huntsville Hospital, Huntsville, AL, USA
oDepartment of Public Health and Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
pThese authors contributed equally to this article.
qCorresponding Author: Manisha Rakesh Lakhanpal, Department of Medicine, Emilio Aguinaldo College, Manila, Metro Manila, Philippines
Manuscript submitted February 27, 2024, accepted May 6, 2024, published online June 29, 2024
Short title: KD and IF in Neurologic Diseases, Cancers and Obesity
doi: https://doi.org/10.14740/jem944
| Abstract | ▴Top |
Ketogenic diet (KD) and intermittent fasting (IF) are non-pharmacologic nutritional therapies with modest side effects such gastrointestinal discomfort, dyslipidemia, and hypomagnesemia for various medical conditions. KD and IF may help with weight loss, diabetes, cardiovascular disease, polycystic ovarian syndrome, cancer, and chronic neurological illnesses. We studied KD and IF’s impact on cancer, obesity, and neurodegenerative illnesses. We advised evidence-based KD and IF safety. KD is hard to maintain despite benefits. Thus, occasional KD adoption may help newly diagnosed overweight or obese type 2 diabetics lose weight and control blood glucose and lipids. KD is a high-fat, low-carbohydrate diet with 4:1 or 3:1 fat to carbohydrates and protein, thus peripheral tissues and the brain need fatty acids for energy. Most fasting energy comes from ketones. KD has been reliably used to treat refractory epilepsy since the 1920s. KD prevents epilepsy, stroke, severe brain injury, Alzheimer’s, and other neurological illnesses. KD has been used to treat obesity since the 1960s. Combining chemotherapy and radiation with KD may increase tumor cell sensitivity. Thus, KD can be used to treat obesity, cancer, and chronic neurological disease without medicine or side effects. As an adjuvant therapy, KD may offer new neuroprotection and neuroscience treatments. Antioxidant defense and inflammation reduction may alleviate Alzheimer’s and Parkinson’s risk. By targeting cancer cell metabolism, KD and IF may be used to treat cancer. Radiation and chemotherapy may be intensified when used in conjunction with KD. In aggressive brain cancer glioblastoma, KD is most promising. Ketosis suppresses hunger, aiding weight loss in overweight or obese patients. Among type 2 diabetes patients, it helps to manage blood glucose and cholesterol, but long-term adherence is difficult. If paired with exercise, IF may be more effective than calorie restriction for weight loss. Further prospective human trials are needed to assess KD and IF’s therapeutic efficacy and safety.
Keywords: Ketogenic diet; Intermittent fasting; Neurological disorders; Traumatic brain injury; Alzheimer’s disease; Behavioral disease; Cancer; Obesity
| Introduction | ▴Top |
In the USA, nearly 100 million Americans are affected by one of more than 1,000 neurological diseases. The US’s current annual economic burden of common neurological diseases is nearly 800 billion dollars. Additionally, the cost is expected to double by the year 2050 due to the rise in the elderly population and the subsequent rise in the incidence of neurological diseases [1]. In the breakdown of both direct (medical) and indirect (non-medical) costs, it was found that conditions such as Alzheimer’s disease (AD), dementia, chronic low back pain, stroke, traumatic brain injury (TBI), migraine headaches, epilepsy, multiple sclerosis, and Parkinson’s disease had the highest annual expenditure [1]. Strong evidence supports the average increase of 150 - 300 calories per day in the past 30 years, leading to more obesity and cardiovascular events in the USA with no change in physical activity [2]. Although many pharmacological methods could be controlled by neurological and other medical conditions, non-pharmacologic options such as exercise, mindfulness and meditation, and various diet plans are also available. Tables 1 and 2 describe commonly available diets and fasting methods. If adequately evaluated, such non-pharmacologic techniques’ effect will help reduce the annual economic burden due to chronic neurological and other diseases.
 Click to view | Table 1. Types of Diets and Fasting Methods |
 Click to view | Table 2. Various Types of Fasting Methods |
In the USA, cancer is the second leading cause of death (one in every five), followed by heart disease. In 2020, about 1,603,844 new cancer cases were reported in the USA, and 602,347 patients died of cancer [3]. It is critical to find new treatment modalities that could increase the efficacy of current treatment and decrease tumor cell growth. Tumor cells are not flexible with their primary energy source and require glucose [4]. Tumor cells are characterized by higher glycolytic and pentose phosphate activity even in the presence of oxygen, and their high glucose uptake corresponds with poor prognosis in some tumors [5, 6].
The increasing prevalence of obesity has led to higher rates of morbidity and mortality related to various diseases such as diabetes, cancer, cardiovascular disorders, and cerebrovascular disorders. Consequently, healthcare costs have also risen significantly. To address this issue, effective obesity control measures are crucial to reduce or avoid the need for extensive medical expenses [7]. The induction of ketosis through a low-carbohydrate diet led to lower blood glucose levels, resulting in reduced insulin secretion stimulation and a decreased stimulus for fat accumulation. Higher levels of β-hydroxybutyrate were associated with a more significant loss of visceral adipose tissue, clinically significant as visceral adiposity is linked to metabolic syndrome and cardiovascular disease [8].
The ketogenic diet (KD) and intermittent fasting (IF) are helpful nutritional interventions with minor reported side effects such as gastrointestinal symptoms, hyperuricemia, hypomagnesemia, renal calculi, and dyslipidemia, which are transient and easy to manage [9]. Recently, studies have given strong evidence of KD’s therapeutic implications in weight loss, diabetes, cardiovascular disorder, polycystic ovarian syndrome, cancer, and chronic neurological disorders [10]. Also, the American Heart Association (AHA) recommended that the intake of added sugar should vary from five teaspoons per day (or 80 calories) for an average adult woman with daily 1,800 calories expenditure, to nine teaspoons per day (or 144 calories) for an average adult man with daily 2,200 calories expenditure [2]. We aimed to review the role of KD and IF in neurologic diseases, cancer, and obesity in this article.
| Methods | ▴Top |
Endpoints
The primary aim of our study was to evaluate the effectiveness of KD in various chronic conditions like neurological disorders, cancer, and obesity.
Search strategy and selection criteria
We performed a systematic review of previously published studies using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines from last 10 years. We used PubMed to find observational or interventional studies.
We used keywords (ketogenic diet (Title/Abstract) OR one meal a day (Title/Abstract) OR (Atkin diet (Title/Abstract) OR DASH (Title/Abstract) OR Paleo (Title/Abstract) OR (intermittent fasting (Title/Abstract) OR modified Atkins diet (Title/Abstract) OR OMAD (Title/Abstract)) to identify various types of diet and used keywords (obesity (Title/Abstract) OR cancer (Title/Abstract) OR epilepsy (Title/Abstract) OR chronic conditions (Title/Abstract) OR stroke (Title/Abstract) OR Alzheimer’s disease (Title/Abstract) OR migraine (Title/Abstract) OR neurological disorders (Title/Abstract) OR neuroprotection (Title/Abstract) OR tumor (Title/Abstract) OR cardiorespiratory fitness (Title/Abstract) OR hyperinsulinemic state (Title/Abstract) OR diabetes (Title/Abstract)) to identify various chronic conditions.
Any literature other than case reports, review articles, and animal studies were excluded. Non-English literature, non-full text, and non-human studies were excluded. Flow diagram of the study selection process is described in Figure 1.
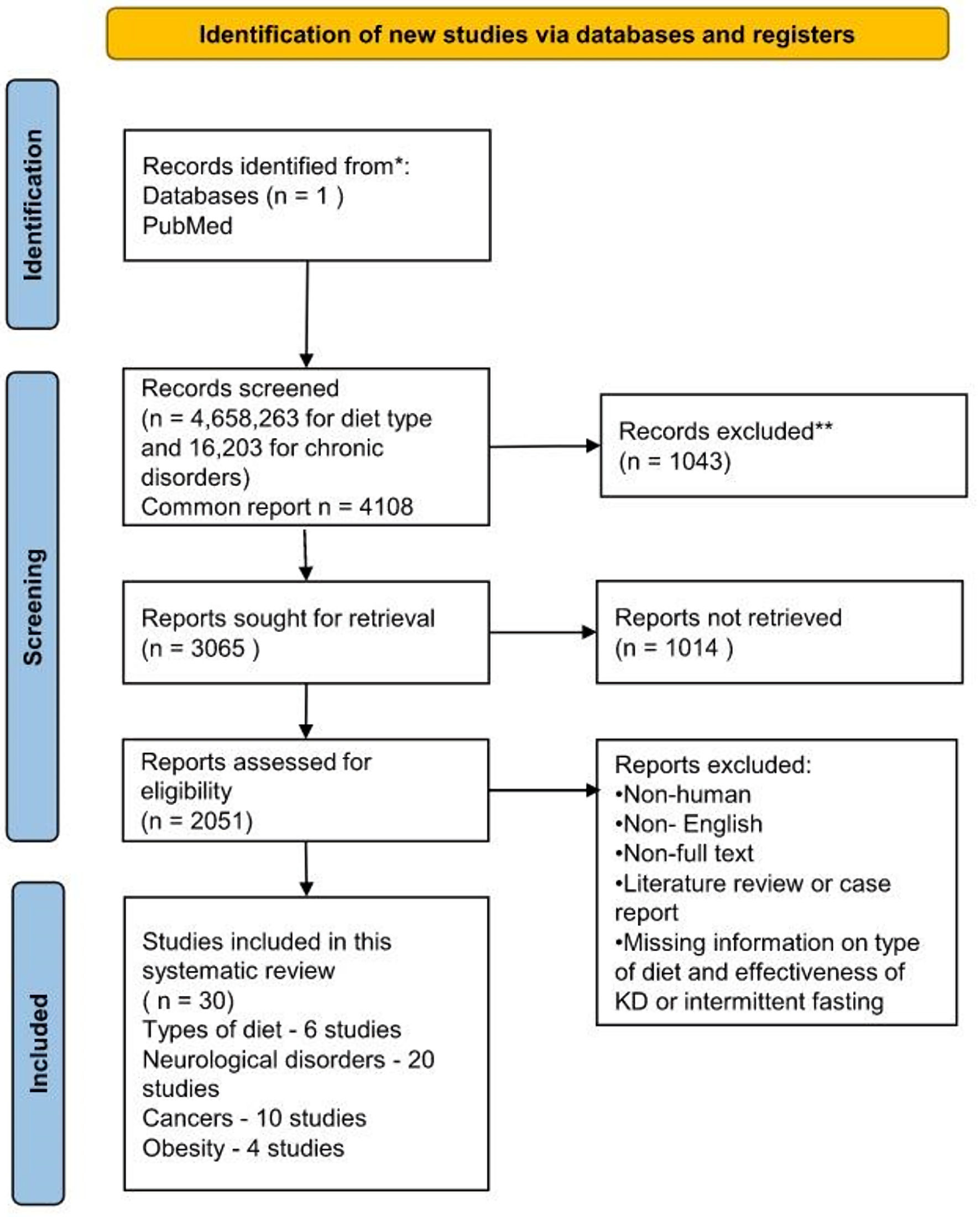 Click for large image | Figure 1. Flow diagram of the study selection process. KD: ketogenic diet. |
Study selection
All studies were identified using the search strategy described above and screened independently for their eligibility, and any disagreement was resolved through discussion with senior authors. Studies describing observational and interventional studies were considered for full-text evaluation.
Data extraction
A data extraction form on an Excel sheet was used to extract data from the included studies for the assessment of literature synthesis. Extracted information included: study setting (study name, year of publication), type of the diet, mechanism of action for the diet, effectiveness of KD and IF, chronic conditions, and cancers. Two authors extracted the data independently and the differences identified were resolved through discussion with the senior author.
Ethical approval
Though this article does not contain any studies with direct involvement of human participants or animals performed by any of the authors, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
| Results | ▴Top |
Out of 4,658,263 articles on various types of diet and 16,203 articles on chronic disorders and cancers, 4,108 articles had information on the effectiveness of any diet to control or improve chronic disorders, of which 2,051 studies have specific information on the effectiveness of the KD and IF in various disorders. After applying inclusion and exclusion criteria, we found six studies describing types of diet, 20 studies on neurological disorders, 10 on cancers, and four on obesity.
Types of diet
The KD is a high-fat, low-carbohydrate dietary regimen characterized by limited carbohydrate intake (20 - 50 g/day), leading to fatty acids becoming the primary source of cellular energy production, particularly in the brain [11-14]. Its potential benefits include weight loss, reversal/control of type 2 diabetes, improved lipid profiles, and favorable effects on neurological disorders such as epilepsy and Alzheimer’s disease. However, it is associated with various adverse effects such as muscle cramps, bad breath, changes in bowel habits, and keto-flu [11-14]. Modified versions of the KD, such as the Atkins and modified Atkins diets (MADs), offer less restrictive alternatives, showing similar benefits in seizure reduction for epilepsy patients with fewer adverse effects [15-19]. Other diets like the DASH and Paleo diets focus on different nutritional approaches, with the former aimed at reducing blood pressure and cardiovascular risks, and the latter emphasizing whole foods similar to those consumed in the Paleolithic era [20-22]. IF, another dietary strategy, offers benefits in controlling type 2 diabetes and improving glucose tolerance, but it comes with potential risks such as nausea, vomiting, and metabolic acidosis [23-27]. Table 1 [11-30] and Table 2 [31, 32] describe commonly available diets and fasting methods [11, 14].
Role of KD in neuroprotection and neurological disorders
The provided articles cover a wide range of clinical conditions and the effects of KD on these conditions [13, 33-51]. For epilepsy, various studies indicate promising outcomes, with significant reductions in seizure frequency observed in both children and adults following KD treatment [33-37]. Additionally, KD shows potential benefits in conditions such as stroke and mitochondrial disorders like mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS), where it may improve mitochondrial dysfunction and lead to better seizure control [38]. In Alzheimer’s disease, studies suggest that KD and medium-chain triglyceride (MCT) supplementation could improve cognitive function, especially in individuals without the apolipoprotein E 4 (APOE4) allele [13, 39]. Moreover, KD demonstrates efficacy in migraine treatment, with reductions in attack frequency and duration observed in several studies [43, 45, 46]. Other neurological conditions such as Parkinson’s disease and amyotrophic lateral sclerosis (ALS) also show potential for improvement with KD intervention [47-50]. Furthermore, the KD may have implications in metabolic disorders, as evidenced by its association with decreased risk factors for cognitive decline and dementia in elderly individuals [51]. Overall, the research underscores the diverse therapeutic potential of the KD across various neurological and metabolic conditions. The human studies showing the beneficial effects of KD are mentioned in Table 3 [13, 33-51].
 Click to view | Table 3. Studies Showing Neuroprotective Effects of KD |
Role of KD in cancer management
The articles reviewed encompass various cancer types and investigate the role of KD and other dietary interventions in cancer treatment [6, 52, 53-59]. Results suggest that KD may counteract the detrimental effects of radiotherapy and chemotherapy on body composition in head and neck cancer patients and contribute synergistically to pathological tumor response in non-metastasized rectal cancer patients [52, 53]. Additionally, KD and IF show promising metabolic changes and potential prognostic benefits in brain tumor patients [6]. Moreover, KD during radiotherapy appears safe and may improve quality of life (QOL) and metabolic health in breast cancer patients [55], while a combination of chemotherapy and KDs may improve biochemical parameters and overall survival in locally advanced breast cancer patients without substantial side effects [56, 57]. These findings suggest that KD may play a role in cancer treatment as a safe and achievable component, potentially improving treatment outcomes and QOL for some cancer patients. (Table 4) [5, , 6, 52, 54,55].
 Click to view | Table 4. Role of KD and IF in Different Types of Cancer Patients |
Role of KD and IF in obesity
The KD demonstrates effectiveness in weight loss therapy through various mechanisms such as appetite suppression, increased satiety effect of proteins, modification in hormone levels, increased lipolysis, and metabolic rate. Studies indicate that very low-calorie KDs (VLCKDs) can promote hepatic fat mobilization and potentially reverse mild renal impairment. However, long-term adherence to KD can be challenging, prompting the consideration of periodic KDs for managing conditions like type 2 diabetes mellitus. On the other hand, IF has emerged as an effective strategy for weight loss, with variations like IF1 and IF2 showing significant reductions in body weight, fat mass, and glucose levels. Time-restricted eating (TRE) combined with exercise training has also shown promise in reducing fat mass and visceral fat, particularly in overweight and older populations. Combining IF with exercise programs yields more favorable outcomes compared to diet alone therapy. Overall, both KD and IF offer potential benefits for obesity management, with further research needed to explore their long-term safety and effectiveness. (Table 5) [10, 60-68].
 Click to view | Table 5. Ketogenic Diet and Intermittent Fasting in Obesity |
| Discussion | ▴Top |
Dietary interventions greatly influence regulating metabolic disorders and associated comorbidities such as hypertension, diabetes, and hyperlipidemia. In the context of hypertension, the Dietary Approaches to Stop Hypertension (DASH) regimen has demonstrated efficacy in reducing blood pressure by as much as 11 mm Hg among hypertensive individuals [20]. Atkin’s diet, which is high in protein and fat and low in carbohydrates, has been proven to improve satiety, glycemic control, and lipid profile. Noteworthy is its potential to mitigate seizure frequency in refractory epilepsy. However, symptoms like nausea, dizziness, constipation, and headache commonly occur with the Atkins diet [15]. Hence, alternatively, the MAD with carbohydrate restriction to 10 g/day while encouraging high-fat foods shows better compliance and tolerability [28]. Another diet known for reducing the risk of modern-day ailments like cardiovascular events and diabetes is the Paleo diet. Rich in proteins and long-chain polyunsaturated fatty acids, this regimen’s potential benefits are to be acknowledged, but with caution among osteoporosis patients due to its inherent calcium deficiency [21]. The ketogenic diet, characterized by high fat, low carbohydrate, is proven to be prudent in weight loss, better metabolic profile, and emerging utility in various neurological disorders like epilepsy and dementia. Its potential to reduce the incidence of major adverse cardiovascular events by reversing type 2 diabetes, improving lipid profile, and weight loss is widely documented. However, compliance with this diet is impaired due to adverse effects like muscle cramps and changes in bowel habits. Furthermore, the potential for stimulating inflammation and, therefore, precipitating biological aging must be considered with the long-term use of a ketogenic diet.
Mechanism of action and types of ketogenic diet
KD is a high-fat, low-carbohydrate diet (20 - 50 g/day), in which carbohydrates are nearly eliminated, thus enabling fatty acids to become the required obligatory source of cellular energy production by peripheral tissues and the brain [10]. Under normal dietary conditions, the brain utilizes glucose as an energy source; however, ketone bodies are the primary energy source during fasting. Under fasting conditions, fatty acids from the body fat are oxidized in the mitochondria to produce acetyl CoA, which is further used to synthesize ketone bodies such as β-hydroxybutyrate and acetoacetate and used as an energy source. KDs mimic the metabolic state of fasting and maintain permanent ketosis [69]. Ketone bodies are implicated in symptomatic relief and disease-modifying activity in neurological disorders such as Alzheimer’s and Parkinson’s disease and maintain a neuroprotective role in stroke and TBI [69, 70]. The effectiveness of a KD for neurological disorders stems from the efficiency of ketones over glucose as an energy source for brain cells and because ketone bodies have a higher inherent energy [70, 71].
Many subclasses of KD could be utilized. The classic KD is the most restrictive yet offers the highest ketogenic potential, with a ratio of grams of fat to combined grams of carbohydrates and protein being 4:1 or 3:1 (other diet subclasses generally fall within 2:1 or 1:1) [9, 71]. The MCT diet contains a high ketogenic potential because MCTs are readily absorbed by enterocytes and rapidly converted to ketones by the body [71]. Unlike the classic KD, in which 60-80% of dietary energy is provided by long-chain fats, in the MCT diet, only 45% of dietary energy is provided through medium-chain fats such as octanoic acid and decanoic acid [71]. Therefore, the MCT diet allows a more extensive carbohydrate content; however, it is utilized less commonly than classic KD because of unpleasant gastrointestinal side effects [9]. Although the MCT diet is utilized less frequently, studies have shown that the MCT KD is used worldwide in treating drug-resistant epilepsy, especially in children [71]. Lastly, the MAD and low glycemic index treatment (LGIT) are diets, in which net daily carbohydrate consumption is limited to a specific amount per day and fat consumption is encouraged, ideally composed of 60-70% of total calories [72]. In the MAD, the net daily carbohydrate consumption is limited to 20 g for adolescents and adults, and 15 - 20g for pediatric patients. In LGIT, the daily carbohydrate consumption is limited to 40 - 60 g/day from foods that have a glycemic index < 50 for both pediatric and adult patients [72].
Neuroprotective role of KD in neurological disorders
Epilepsy
Epilepsy is a severe neurological disease that results from aberrant, synchronous depolarization of neurons in the central nervous system and affects 1% of the US population [1]. Epilepsy is successfully controlled by one or more antiepileptic drugs (AEDs), but still, 35% of patients have refractory epilepsy [73, 74]. Since the 1920s, studies have found that KD is an effective treatment of epilepsy in both children and adults [75-78]. A new variant of KD, particularly MAD and LGIT, provides a therapeutic mechanism, especially for individuals with medically intractable epilepsy, by reducing the onset of seizures, improving seizure frequency and severity, and improving these patients’ QOL of these patients [69, 72, 79-81]. A study by Agarwal et al found that epileptic children who started KD at a young age show a more favorable response, with > 50% seizure reduction at 12-month follow-up [79]. However, utilizing the KD in adults and adolescents also shows response rates comparable to those in children. Additionally, Seo et al found that 80% of patients who failed to achieve seizure control relied on a lower ratio diet (3:1) and improved with a higher ratio diet (4:1) [82]. A randomized controlled trial (RCT) by Neal et al reported that KD is significantly effective in treating drug-resistant epilepsy compared to no change in therapy. Considering the results of this study, we should consider KD with any AEDs in treating drug-resistant epilepsy [78].
There are two mechanisms by which KD affects seizure control: 1) Reducing the glucose concentration and increases fatty acid oxidation and ketones production, which offers a stabilized energy source to the brain in the form of ketones bodies and decrease the likelihood of disruptions in energy availability, which also decrease the possibility of seizures; 2) Alternating neurotransmitter releases and uptake, ketone bodies, specifically acetoacetate and β-hydroxybutyrate, decrease seizure activity by limiting γ-aminobutyric acid receptor-induced seizures. Also, KD with chronic ketosis may modify the tricarboxylic acid (TCA) cycle to limit reactive oxygen species (ROS) and boost energy production in the brain [69]. The specific actions of the KD in limiting ROS are due to their direct neuroinhibitory actions, such as: increasing gamma aminobutyric acid (GABA) synthesis in the brain, inducing the expression of neuronal uncoupling proteins (UCPs), upregulating numerous energy metabolism genes, and inducing mitochondrial biogenesis [69, 72]. The result of upregulating oxidative phosphorylation and limiting glucose also leads to neuron hyperpolarization by activating KATP channels, decreasing the onset of seizures [9, 69, 72].
Stroke
Cerebral stroke, particularly ischemic stroke, is the leading cause of chronic disability and the second leading cause of dementia in the USA [83]. A study by Shaafi et al reported the effect of KD on pathological conditions like oxidative stress, glutamate-mediated excitotoxicity, and apoptosis that occur in ischemic stroke [83]. It has been shown that hyperglycemia aggravates ischemic stroke while β-hydroxybutyrate provides a protective function [70, 83, 84]. In vitro study by Maalouf et al showed that ketone bodies can protect from excitotoxicity and increase the survival time of the patients, also ketone bodies, through NADH oxidation regulation, reduce free radical-induced oxidative stress in neuronal cells and also reduce some apoptotic biomarkers [85]. Ketone bodies can protect neurons from glutamate excitotoxicity by allowing efficient glutamate removal and conversion to GABA, improving the neurons’ free radical elimination ability, and reducing some apoptotic markers such as bax-mRNA and bax protein [84].
AD
AD is the leading cause of dementia, affecting as many as 2.4% of the US population [1]. AD etiopathogenesis has been linked to oxidative stress, neuroinflammation, mitochondrial impairment, hypometabolism, and blood-brain barrier disruption. Studies have shown that amyloid β accumulation is associated with toxic effects on the mitochondria, such as impaired energy homeostasis and impaired electron transport chain activity, which could consequently lead to cell death and cause synaptic damage seen in AD [10, 71]. KD acts to induce antioxidant and anti-inflammatory activity in patients with AD. KD also stimulates nuclear factor erythroid-derived 2 (NF-E2)-related factor 2 (Nrf2), which induces endogenous detoxification and helps to alleviate oxidative stress associated with AD [86]. Additionally, it decreases the production of amyloid precursor protein (APP), and therefore the β-amyloid peptide, and also helps to activate peroxisome proliferator-activated receptor gamma (PPARγ), which plays a role in decreasing systemic inflammation. In particular, β-hydroxybutyrate is one of the central ketone bodies detected in the blood following a KD and can cross the blood-brain barrier, lowering neuroinflammation by activating the hydroxyl-carboxylic acid receptor 2 (HCA2) and leading to memory improvement. Ketone bodies, β-hydroxybutyrate in particular, were also found to be therapeutic in protecting against the production of toxic Aβ plaques associated with AD [70, 86].
TBI
TBI occurs when a traumatic event leads to rapid brain movement within the skull, resulting in brain damage. In the USA, the annual incidence of TBI is 1.7 million, resulting in 12,000 deaths and 3.2 to 5.3 million persons with long-term disability. The elderly population ≥ 65 years accounts for 10% of these injuries, commonly due to falls and motor vehicle accidents [1]. Limited understanding of the pathophysiology of TBI made it challenging to develop its clinical treatment. Primary brain injury in TBI can be prevented with medical care and secondary brain injury can be targeted to improve the outcomes. Secondary TBI leads to metabolic cellular dysfunction, cerebral edema, free radical damage, oxidative damage, ischemic injury, cerebral glucose metabolism disruption, and programmed cell apoptosis. KD has a neuroprotective effect by inducing the state of ketosis and targeting the secondary brain injury phenomenon [87]. A review study by McDougall et al found that KD is an effective treatment therapy for TBI, where KD increases the ketone bodies in circulation and provides an alternative energy source to the brain. In addition, they are metabolically efficient and require less oxygen per adenosine triphosphate (ATP). KD inhibits cellular apoptosis and edema through this anti-inflammatory and antioxidative effect [88]. It has also been shown that KD could play a role in significantly reducing cerebral edema post-TBI, which is the leading cause of injury-related morbidity and mortality worldwide [88].
Migraine
Migraine is another disabling neurological disorder affecting 16.2% of the US population [1]. Migraine primarily arises due to brain excitatory-inhibitory imbalance leading to episodic activation and sensitization of the trigeminovascular pain pathway causing recurrent headaches and sensitivity to sensory stimuli [89]. The diet is considered a critical factor in migraines because some foods can trigger the attack without scientific evidence. Many pieces of evidence suggest that KD may be an effective treatment in different stages of migraine, reinstating metabolism and excitability of the brain and protecting against neuroinflammation and redox mechanisms [90]. A study by Yudkoff et al reported that KD could activate astrocytes metabolism, promoting glutamate conversion to glutamine, eventually converted to GABA, thus balancing the exciting and inhibitory neurotransmission in migraine and decreasing brain cortical excitability [91].
Brain tumor
Malignant brain tumors are devastating even after aggressive chemotherapy, radiation, and surgical resection. The average life expectancy of glioblastoma is 18 months after all treatments are available. Therefore, it is very critical to find new treatment modalities that increase the efficacy of current treatment and decrease the tumor cell growth, which can be achieved by using KD. The KD simulates fasting and leads to high production of acetyl CoA (ACA) by fatty acid oxidation. When the amount of acetyl-CoA exceeds the capacity of the TCA cycle to utilize it, the production of ketone bodies - βHB and ACA increases, which can be used as an energy source by the normal brain cells. However, tumor cells are not flexible with their primary energy source and require glucose. This metabolic dysregulation achieved by inducing KD may target the Warburg effect in highly glycolytic tumors, such as malignant gliomas [4].
ALS
ALS is a progressive neurodegenerative disorder in which metabolic dysfunction features upper and lower motor neuron demise, leading to muscle weakness culminating in paralysis and, finally, death due to respiratory paralysis [92]. Due to the multifactorial origin of ALS, no specific treatment is identified, and since there is mitochondrial involvement, KD can be an effective treatment modality. The metabolic dysfunction is characterized by altered glucose uptake, and the decreased levels of C4-intermediate, such as β-hydroxybutyrate of the TCA cycle [92]. These findings suggest that C4 ketones are an excellent source of alternative fuels that could help overcome problems associated with the reduced ability to use glucose as a fuel. Additionally, C4 ketones provide a variety of protective mechanisms, such as antioxidant and anti-inflammatory properties, which have been proved to be beneficial in ALS [92]. In previous studies, the use of KD and MAD/LGIT reduced the loss of lower motor neurons in the ventral horn of the spinal cord [80]. It also showed that the administration of KD led to higher motor neuron survival and improved motor function [10].
Autism spectrum disorder (ASD)
ASD is one of the most prevalent developmental disorders today, with symptoms appearing during the first years of life and continuing throughout life. ASD has been linked to multiple metabolic disorders and shares traits with diseases related to epilepsy, such as Landau-Kleffner, Dravet, and Rett syndromes. Therefore, the positive effects of KD on epilepsy, when taken into account, has the potential to alleviate specific symptoms related to ASD, especially in females [93].
Role of IF in neuroprotection and neurological disorders
Periods of deliberate fasting with restriction of solid food intake are being practiced worldwide. In the Western world, particularly the USA, the average calorie intake has risen along with the incidence of associated diseases such as cardiovascular disease, neurodegenerative disease, and obesity, with one-third of American adults and 20% of teenagers being obese [94]. IF is a recurring method of eating in which individuals go extended periods (16 - 48 h) with little or no energy intake and have intervening periods of regular food intake [95]. This method decreases food intake and body weight and improves brain functions and structures [95, 96]. IF can be practiced with or without KD. In humans, caloric restriction has been shown to reduce markers of oxidative stress, inflammation, and cardiovascular disease risk, while in animal models, it has been shown to protect neurons against degeneration [97]. Evidence suggests that IF prevents oxidative damage by diminished production of mitochondrial ROS, increased antioxidant defenses, and increased repair mechanisms for molecules damaged due to oxidation [94, 97]. Additionally, IF has been found to upregulate brain-derived neurotrophic factor (BDNF) in animal models by decreasing oxidative stress, increasing synaptic plasticity, neurogenesis, and cell survival [94, 98]. IF has also been associated with increasing heat shock protein 70 (HSP70), which offers neuroprotection via its anti-apoptotic role and downregulating mammalian target of rapamycin (mTOR). This kinase allows for positive effects such as delayed aging, synaptic plasticity, and neurodegeneration due to autophagy’s disinhibition [94]. Similarly, the presence of inflammation can worsen the outcome of obesity, stroke, and neurodegenerative diseases, and studies have shown that IF can reduce the concentration of inflammatory markers such as interleukin 6 (IL-6) and C-reactive protein (CRP) [94, 96, 98]. There are primarily two critical proteins involved in the anti-inflammatory effect exerted by IF, mTOR and SIRT1. As previously mentioned, mTOR is a significant player in inflammation and is downregulated by IF. At the same time, SIRT1 is a deacetylase that is upregulated by IF and inhibits NFκB, a central transcription factor responsible for expressing many genes associated with inflammation [94].
Several IF regimens are hypothesized to impact health outcomes: complete alternate-day fasting, modified fasting regime, time-restricted feeding, and religious fasting. Alternate day fasting involves days when no calories are consumed, followed by feeding days when foods and beverages are consumed as desired. In animal models, alternate-day fasting has been shown to reduce total plasma cholesterol and triglycerides and reduce liver steatosis and inflammation gene expression, and evidence in humans suggests that it can lead to modest weight loss and improvements in some metabolic parameters. On regularly scheduled fasting days, modified fasting regimens (also known as intermittent energy restriction) limit energy consumption to 20-25% of energy needs, resulting in weight loss with mixed effects on inflammatory markers [99]. A time-restricted feeding regime involves a daily fasting interval of 12 to 21 h. It has been associated with reduced body weight and inflammatory marker levels such as IL-6 and tumor necrosis factor α (TNF-α). Lastly, observational studies suggest that the religious fasting regimen results in transitory weight loss and mixed impacts on other biomarkers [99].
A health-promoting mechanism associated with IF includes the regulation of circadian rhythms. Because meal timing can significantly influence circadian rhythm, adopting an IF regime can exclude or reduce energy intake in the evening and nighttime, which could synchronize food ingestion with optimal postprandial hormone response times [95, 99]. This could lead to improved energy metabolism mechanisms and body weight regulation. Studies have also shown that IF can slow the progression of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and stroke because it upregulates the expression of antioxidant enzymes (heme oxygenase 1), neurotrophic factors such as BDNF and fibroblast growth factor 2 (FGF2), and protein chaperones (HSP70 and GRP78) [95]. Along with these factors, IF can suppress the inflammasome and reduce inflammation [95].
Role of KD and IF in cancers
Prior studies suggest that the increased glycolysis seen in cancer cells appears to be a response to protect against increased hydroperoxide-mediated oxidative stress caused by altered mitochondrial metabolism. Therefore, strategies to utilize this mechanism in cancer cells might help amplify the effects of radiation and chemotherapy [5]. This led to the suggestion that diets like KD or a MAD low in carbohydrates could selectively target the glycolytic tumors and thus complement the cancer therapy. For oncological purposes, KDs can be defined as a high-fat (usually ≥ 65% of energy intake), low-carbohydrate (≤ 50 g and day) diet that ideally also provides an adequate protein supply (about 1.5 g/kg per day). Thus, fatty acids become the required obligatory source of cellular energy and target the Warburg effect in highly glycolytic tumors [6, 52, 4].
The clinical trials described KD and IF’s role in brain cancer, non-metastasized rectal cancer, high-grade glioma (new and recurrent), head and neck squamous cell carcinoma, locally advanced and metastatic breast cancer, and ovarian and endometrial cancer [5, 6, 52, 54, 55, 57-59]. Results of these studies described KD as an accompanying measure for cancer patients undergoing standard-of-care therapy that helps improve the QOL, survival with no severe side effects, and acceptable safety and tolerability in advanced cancer patients. Chemotherapy and radiotherapy with KD may have enhanced antitumor effects (Table 4). Although the role of KD was described in depth, most RCT results were heterogeneous to provide quantitative measures of survival timeline, outcomes, and recovery. Thus, more prospective studies in humans are warranted to evaluate the potential therapeutic effectiveness and safety of KD.
Role of KD in obesity
There is strong supportive evidence in favor of KD being a very effective weight loss therapy, with some contrasting theories regarding its mechanism of action in weight loss. The most likely explanation for KD’s effectiveness in weight loss is its appetite-suppressing action of ketosis. In addition, reduced appetite due to higher satiety effect of proteins and modification in levels of hormones like ghrelin and leptin, which influence appetite, increased lipolysis, reduced respiratory quotient, increased metabolic rate to consume fats, and increased gluconeogenesis and thermic effect of protein are several factors that can aid in weight loss on KD [10]. Ghrelin, a neuropeptide hormone that stimulates feeding behavior, is released in response to dieting. Sumithran et al studied 39 patients where the expected release of ghrelin and increased appetite were alleviated when the subjects were in ketosis [100].
Very low-calorie KDs (VLCKDs) may promote quicker hepatic fat mobilization than other compartments, and this effect is likely due to the ketogenic state rather than calorie restriction alone, making it a potential treatment option for conditions like non-alcoholic fatty liver disease [63]. Moreover, patients with obesity and mild renal failure over 3 months noted that 27.7% of patients with mild renal impairment experienced a return to normal glomerular filtrate after the VLCKD dietary intervention [64]. Choi et al evaluated ketogenic nutrition drinks with different ketogenic ratios and concluded that the production of ketone bodies was induced and maintained through the consumption of a ketogenic nutrition drink with a more moderate ketogenic ratio (1.7:1) than the typical ratio of 4:1 [101]. Overweight or obese adult females with abnormal glucose control factors may benefit from implementing a low-calorie KD (LCKD) combined with weight loss interventions and insulin resistance avoidance. However, they can present unique challenges as research subjects due to hormonal influences; further investigations are necessary to assess the long-term safety and effectiveness of the proposed dietary strategy [102]. Additionally, long-term adherence to KD was found to be challenging, so periodic KDs could be beneficial for newly diagnosed overweight or obese patients with type 2 diabetes mellitus by helping manage their blood glucose and lipid levels, as well as promoting weight loss (Table 6) [8, 63-65, 101-104].
 Click to view | Table 6. Role of KD in Obesity |
Role of IF in obesity
IF, which could be done for 1 day (IF1) or 2 days straight (IF2) per week, is frequently utilized to achieve the best possible body weight loss results. In a randomized control trial, IF2-P resulted in significantly more significant body weight and waist circumference reductions than IF1-P. It also showed a strong tendency for more significant reductions in fat mass, glucose, hunger levels, and hormone responses [60]. From pre- to post-intervention, the time-restricted eating (TRE)/time-restricted feeding (TRF) group experienced considerably more significant losses of total body mass and fat mass than the standard eating group. In conclusion, the use of TRE and concurrent exercise training as a short-term dietary therapy for persons who are overweight or obese to reduce fat mass and increase lean mass [61]. Overweight older men and women (aged 65 - 74) with visceral fat were evaluated to see how well a 6-week TRE intervention reduced body weight, fat loss, and visceral fat. While both men and women significantly lost body weight after the 6-week TRE intervention, waist circumference and visceral fat mass were significantly decreased in men [62]. Combining IF with concurrent exercise programs was proven to be more effective results of weight loss, improved biomarkers compared to diet alone therapy [67, 105]. Compared to regular calorie restriction, patients on the IF 5:2 program (30% of energy requirements on fast days and 70% on non-fast days) were more successful in losing weight (Table 7) [60-62, 66, 67, 68, 105-107].
 Click to view | Table 7. Role of IF in Obesity |
Evidence-based recommendations
KD is a low-carbohydrate diet (20 - 50 g/day) that seems effective and safe to consume under guidance [108]. Gradually reducing carbohydrates in the diet before introducing KD can improve tolerance. Transition to KD can be sudden or over a week, and it should be done under the physician’s supervision. Patients should be advised about common side effects like constipation, headache, bad breath, muscle cramps, diarrhea, general weakness, and rash [108]. Other unfavorable changes are gradual increments in total cholesterol and low-density lipoprotein (LDL) cholesterol (LDL-C) levels but favorable changes in triglyceride and probably high-density lipoprotein (HDL) cholesterol (HDL-C) values [109]. Before starting KD, it is essential to get a baseline lipid profile, hemogram, thyroid profile, and renal function tests [110] and monitor lipid profile, thyroid profile, and renal function during the diet as few patients may show worsening parameters. So regular watch on lipid profile becomes necessary though no strict timeline is studied. A tool kit to measure ketone bodies is available in the market to measure to evaluate the optimal level. Long-term use of a KD may progressively reduce bone-mineral content [109, 111] and result in other nutritional deficiencies, so regular check-ups or replacement options should be evaluated.
Consulting a physician if concurrent disorders like type 1 diabetes mellitus, renal impairment, and thyroid are present is strongly advised. KD can be short-term or long-term, but withdrawal must be gradual after achieving the goal, and adding 10 g of carbohydrates per day for the first week is recommended. Healthy carbohydrates and more protein sources on the plate are suggested; adding more fiber (Ispaghula, i.e., Psyllium) to avoid constipation is advised. While absolute contraindications for KD include disorders of fat metabolism, porphyria, and pyruvate carboxylase deficiency, relative contraindications for KD are propofol concurrent use, parent or caregiver noncompliance, and inability to maintain adequate nutrition [28]. Although there is no current recommendation for the amount of ketosis to be achieved for beneficial effects in cancer, very low carbohydrate KD seems to be beneficial in patients starting KD in cancer [34]. Research on KD for epilepsy shows that blood β-hydroxybutyrate greater than 4 mmol/L necessary to deliver promising clinical outcomes, which is superior to the urine ketone dipstick test [112].
It is important to note that the concentration of ketone bodies has to be more than 4 mmol/L to be utilized by the brain as an energy source [113], and the ideal to measure ketosis is after dinner or early morning [114].
| Conclusions | ▴Top |
The mechanism underlying the beneficial effects of KD and IF needs to be well studied. However, the neuroprotective effect is more likely to have mitigated excitotoxicity, oxidative stress, and apoptosis events and enhanced mitochondrial energy metabolism to counteract neural inflammation. Through all these mechanisms, both are reported as effective therapeutic interventions in treating various common neurological disorders with different clinical presentations. These findings suggest that the KD holds promise as a potential adjunctive therapy in various neurological disorders, offering new avenues for treatment and neuroprotection. While KD is commonly used for weight loss, there is limited literature about its effectiveness and safety in neurological disorders in humans. IF has shown potential in slowing the progression of neurodegenerative diseases such as Alzheimer’s and Parkinson’s by promoting antioxidant defense and suppressing inflammation. Different IF regimens offer flexibility in implementation and may have varying effects on biomarkers. Thus, further prospective human studies are warranted to evaluate more details about the potential therapeutic mechanism, effectiveness, and safety of both KD and IF.
KD and IF show promise in cancer therapy by targeting altered cancer cell metabolism. Some of the most robust reports of keto’s possible benefits have come from glioblastoma, a very aggressive brain cancer. However, it does not work on other kinds of brain cancer. There has been minimal evidence that a high-fat, low-carb diet may help suppress solid prostate, breast, stomach, and liver cancers. Nevertheless, researchers have not ruled out the possibility that KD may worsen cancer by promoting tumor growth; also, very low-fat diets have been shown to lower the chances that certain types of breast cancer may come back.
Additionally, KD may enhance the effects of standard therapies like chemotherapy and radiotherapy. More research is needed to understand cancer treatment’s therapeutic potential and safety fully. These dietary interventions offer exciting possibilities as complementary measures for cancer management.
Acknowledgments
None to declare.
Financial Disclosure
The study had no internal or external funding source.
Conflict of Interest
Authors declare no conflict of interest.
Informed Consent
The data used in this study is identified from previously published studies so informed consent is not required.
Author Contributions
Conceptualization: Nikhila Chelikam, Sai Anusha Akella, Urvish Patel. Methodology: Nikhila Chelikam, Sai Anusha Akella. Acquisition of data: Nikhila Chelikam, Manisha Lakhanpal, Simmy Lahori, Sai Anusha Akella, Ruslan Ilyassov, Sana Zafar, Rahul Gujarathi, Divesh Manjani, Avinash Adiga. Formal analysis and/or investigation: Nikhila Chelikam, Aryak Singh, Lokesh Manjani, Sai Anusha Akella, Sana Zafar, Abdirazak Ibrahim Ali, Roghayeh Marandi, Avinash Adiga, Urvish Patel. Writing - original draft preparation: Nikhila Chelikam, Manisha Lakhanpal, Simmy Lahori, Sai Anusha Akella, Prashanth Gumpu Shivashankar, Avinash Adiga. Writing - review, critical feedback, and editing: Nikhila Chelikam, Aryak Singh, Lokesh Manjani, Sana Zafar, Abdirazak Ibrahim Ali, Roghayeh Marandi, Rahul Gujarathi, Divesh Manjani, Urvish Patel. Funding acquisition: None. Resources: Nikhila Chelikam, Lokesh Manjani, Rahul Gujarathi, Divesh Manjani. Supervision: Nikhila Chelikam, Lokesh Manjani, Urvish Patel.
Data Availability
The data are collected from the studies published online, publicly available.
| References | ▴Top |
- Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: A summary report and call to action. Ann Neurol. 2017;81(4):479-484.
doi pubmed - Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011-1020.
doi pubmed - U.S. Cancer Statistics Data Visualizations Tool | CDC. Accessed: April 7, 2024. [Online]. Available: https://www.cdc.gov/cancer/uscs/dataviz/index.htm.
- Woolf EC, Syed N, Scheck AC. Tumor metabolism, the ketogenic diet and beta-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122.
doi pubmed pmc - Ma DC, Anderson CM, Rodman SN, Buranasudja V, McCormick ML, Davis A, Loth E, et al. Ketogenic diet with concurrent chemoradiation in head and neck squamous cell carcinoma: preclinical and phase 1 trial results. Radiat Res. 2021;196(2):213-224.
doi pubmed pmc - Voss M, Wenger KJ, von Mettenheim N, Bojunga J, Vetter M, Diehl B, Franz K, et al. Short-term fasting in glioma patients: analysis of diet diaries and metabolic parameters of the ERGO2 trial. Eur J Nutr. 2022;61(1):477-487.
doi pubmed pmc - Luo W, Zhang J, Xu D, Zhou Y, Qu Z, Yang Q, Lv Q. Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: A meta-analysis of randomized controlled trials. Front Nutr. 2022;9:1092031.
doi pubmed pmc - Perissiou M, Borkoles E, Kobayashi K, Polman R. The effect of an 8 week prescribed exercise and low-carbohydrate diet on cardiorespiratory fitness, body composition and cardiometabolic risk factors in obese individuals: a randomised controlled trial. Nutrients. 2020;12(2):482.
doi pubmed pmc - Verrotti A, Iapadre G, Pisano S, Coppola G. Ketogenic diet and childhood neurological disorders other than epilepsy: an overview. Expert Rev Neurother. 2017;17(5):461-473.
doi pubmed - Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789-796.
doi pubmed pmc - Cicero AF, Benelli M, Brancaleoni M, Dainelli G, Merlini D, Negri R. Middle and long-term impact of a very low-carbohydrate ketogenic diet on cardiometabolic factors: a multi-center, cross-sectional, clinical study. High Blood Press Cardiovasc Prev. 2015;22(4):389-394.
doi pubmed pmc - Grandl G, Straub L, Rudigier C, Arnold M, Wueest S, Konrad D, Wolfrum C. Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet. J Physiol. 2018;596(19):4597-4609.
doi pubmed pmc - Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31.
doi pubmed pmc - McKenzie AL, Hallberg SJ, Creighton BC, Volk BM, Link TM, Abner MK, Glon RM, et al. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes. 2017;2(1):e5.
doi pubmed pmc - Husain AM, Yancy WS, Jr., Carwile ST, Miller PP, Westman EC. Diet therapy for narcolepsy. Neurology. 2004;62(12):2300-2302.
doi pubmed - Kossoff EH, Dorward JL, Turner Z, Pyzik PL. Prospective study of the modified atkins diet in combination with a ketogenic liquid supplement during the initial month. J Child Neurol. 2011;26(2):147-151.
doi pubmed - Mahdi GS. The Atkin's diet controversy. Ann Saudi Med. 2006;26(3):244-245.
doi pubmed pmc - Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40(2):265-274.
doi pubmed - Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O'Connor HM, Knopman DS, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis. 2012;32(2):329-339.
doi pubmed pmc - Challa HJ, Ameer MA, Uppaluri KR. DASH diet to stop hypertension. In: StatPearls. Treasure Island (FL) ineligible companies. 2024.
pubmed - Kowalski LM, Bujko J. [Evaluation of biological and clinical potential of paleolithic diet]. Rocz Panstw Zakl Hig. 2012;63(1):9-15.
pubmed - T. R. A. C. of general Practitioners. Cutting through the Paleo hype: The evidence for the Palaeolithic diet. Australian Family Physician. Accessed: April 7, 2024. [Online]. Available: https://www.racgp.org.au/afp/2016/january-february/cutting-through-the-paleo-hype-the-evidence-for-th.
- Devathasan G, Koh C. Wernicke's encephalopathy in prolonged fasting. Lancet. 1982;2(8307):1108-1109.
doi pubmed - Genuth SM. Insulin secretion in obesity and diabetes: an illustrative case. Ann Intern Med. 1977;87(6):714-716.
doi pubmed - Greenfield M, Kolterman O, Olefsky JM, Reaven GM. The effect of ten days of fasting on various aspects of carbohydrate metabolism in obese diabetic subjects with significant fasting hyperglycemia. Metabolism. 1978;27(12 Suppl 2):1839-1852.
doi pubmed - Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. West J Med. 1982;137(5):379-399.
pubmed pmc - Mount SJ. Death during therapeutic starvation. Lancet. 1968;2(7558):44.
doi pubmed - Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421-424.
doi pubmed - Sharma S, Jain P, Gulati S, Sankhyan N, Agarwala A. Use of the modified Atkins diet in Lennox Gastaut syndrome. J Child Neurol. 2015;30(5):576-579.
doi pubmed - Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: a randomized controlled trial. Epilepsia. 2013;54(3):481-486.
doi pubmed - Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, 3rd, Leeuwenburgh C, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring). 2018;26(2):254-268.
doi pubmed pmc - Finnell JS, Saul BC, Goldhamer AC, Myers TR. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement Altern Med. 2018;18(1):67.
doi pubmed pmc - Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48(12):978-981.
doi pubmed - Cervenka MC, Henry-Barron BJ, Kossoff EH. Is there a role for diet monotherapy in adult epilepsy? Epilepsy Behav Case Rep. 2017;7:6-9.
doi pubmed pmc - Marsh EB, Freeman JM, Kossoff EH, Vining EP, Rubenstein JE, Pyzik PL, Hemingway C. The outcome of children with intractable seizures: a 3- to 6-year follow-up of 67 children who remained on the ketogenic diet less than one year. Epilepsia. 2006;47(2):425-430.
doi pubmed - Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102(6):1358-1363.
doi pubmed - Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108(4):898-905.
doi pubmed - Steriade C, Andrade DM, Faghfoury H, Tarnopolsky MA, Tai P. Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) may respond to adjunctive ketogenic diet. Pediatr Neurol. 2014;50(5):498-502.
doi pubmed - Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311-314.
doi pubmed - Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
doi pubmed - Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alzheimers Dement (N Y). 2018;4:28-36.
doi pubmed pmc - Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33(2):425.e419-427.
doi pubmed pmc - Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Sirianni G, Rossi P, Di Lorenzo G, et al. A ketogenic diet normalizes interictal cortical but not subcortical responsivity in migraineurs. BMC Neurol. 2019;19(1):136.
doi pubmed pmc - Di Lorenzo C, Coppola G, Di Lenola D, Evangelista M, Sirianni G, Rossi P, Di Lorenzo G, et al. Efficacy of modified Atkins ketogenic diet in chronic cluster headache: an open-label, single-arm, clinical trial. Front Neurol. 2018;9:64.
doi pubmed pmc - Di Lorenzo C, Pinto A, Ienca R, Coppola G, Sirianni G, Di Lorenzo G, Parisi V, et al. A randomized double-blind, cross-over trial of very low-calorie diet in overweight migraine patients: a possible role for ketones? Nutrients. 2019;11(8):1742.
doi pubmed pmc - Di Lorenzo C, Coppola G, Sirianni G, Di Lorenzo G, Bracaglia M, Di Lenola D, Siracusano A, et al. Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur J Neurol. 2015;22(1):170-177.
doi pubmed - Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64(4):728-730.
doi pubmed - Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: A pilot randomized controlled trial. Mov Disord. 2018;33(8):1306-1314.
doi pubmed pmc - Veldink JH, Kalmijn S, Groeneveld GJ, Wunderink W, Koster A, de Vries JH, van der Luyt J, et al. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2007;78(4):367-371.
doi pubmed pmc - Okamoto K, Kihira T, Kobashi G, Washio M, Sasaki S, Yokoyama T, Miyake Y, et al. Fruit and vegetable intake and risk of amyotrophic lateral sclerosis in Japan. Neuroepidemiology. 2009;32(4):251-256.
doi pubmed - Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62(10):1545-1548.
doi pubmed - Klement RJ, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: V. Final results of the KETOCOMP study for head and neck cancer patients. Strahlenther Onkol. 2022;198(11):981-993.
doi pubmed pmc - Klement RJ, Koebrunner PS, Meyer D, Kanzler S, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: IV. Final results of the KETOCOMP study for rectal cancer patients. Clin Nutr. 2021;40(7):4674-4684.
doi pubmed - Porper K, Shpatz Y, Plotkin L, Pechthold RG, Talianski A, Champ CE, Furman O, et al. A Phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J Neurooncol. 2021;153(3):487-496.
doi pubmed - Klement RJ, Weigel MM, Sweeney RA. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr. 2021;40(6):4267-4274.
doi pubmed - Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH. Effects of Ketogenic metabolic therapy on patients with breast cancer: A randomized controlled clinical trial. Clin Nutr. 2021;40(3):751-758.
doi pubmed - Khodabakhshi A, Akbari ME, Mirzaei HR, Mehrad-Majd H, Kalamian M, Davoodi SH. Feasibility, safety, and beneficial effects of MCT-based ketogenic diet for breast cancer treatment: a randomized controlled trial study. Nutr Cancer. 2020;72(4):627-634.
doi pubmed - Cohen CW, Fontaine KR, Arend RC, Gower BA. A ketogenic diet is acceptable in women with ovarian and endometrial cancer and has no adverse effects on blood lipids: a randomized, controlled trial. Nutr Cancer. 2020;72(4):584-594.
doi pubmed - Augustus E, Granderson I, Rocke KD. The impact of a ketogenic dietary intervention on the quality of life of stage II and III cancer patients: a randomized controlled trial in the Caribbean. Nutr Cancer. 2021;73(9):1590-1600.
doi pubmed - Arciero PJ, Arciero KM, Poe M, Mohr AE, Ives SJ, Arciero A, Boyce M, et al. Intermittent fasting two days versus one day per week, matched for total energy intake and expenditure, increases weight loss in overweight/obese men and women. Nutr J. 2022;21(1):36.
doi pubmed pmc - Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, Hackney KJ. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep. 2021;9(10):e14868.
doi pubmed pmc - Domaszewski P, Konieczny M, Dybek T, Lukaniszyn-Domaszewska K, Anton S, Sadowska-Krepa E, Skorupska E. Comparison of the effects of six-week time-restricted eating on weight loss, body composition, and visceral fat in overweight older men and women. Exp Gerontol. 2023;174:112116.
doi pubmed - Cunha GM, Guzman G, Correa De Mello LL, Trein B, Spina L, Bussade I, Marques Prata J, et al. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front Endocrinol (Lausanne). 2020;11:607.
doi pubmed pmc - Bruci A, Tuccinardi D, Tozzi R, Balena A, Santucci S, Frontani R, Mariani S, et al. Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. 2020;12(2):333.
doi pubmed pmc - Li S, Lin G, Chen J, Chen Z, Xu F, Zhu F, Zhang J, et al. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr Disord. 2022;22(1):34.
doi pubmed pmc - Kang J, Shi X, Fu J, Li H, Ma E, Chen W. Effects of an intermittent fasting 5:2 plus program on body weight in Chinese adults with overweight or obesity: a pilot study. Nutrients. 2022;14(22):4734.
doi pubmed pmc - Maaloul R, Marzougui H, Ben Dhia I, Ghroubi S, Tagougui S, Kallel C, Driss T, et al. Effectiveness of Ramadan diurnal intermittent fasting and concurrent training in the management of obesity: is the combination worth the weight? Nutr Metab Cardiovasc Dis. 2023;33(3):659-666.
doi pubmed - Witjaksono F, Prafiantini E, Rahmawati A. Effect of intermittent fasting 5:2 on body composition and nutritional intake among employees with obesity in Jakarta: a randomized clinical trial. BMC Res Notes. 2022;15(1):323.
doi pubmed pmc - Elia M, Klepper J, Leiendecker B, Hartmann H. Ketogenic diets in the treatment of epilepsy. Curr Pharm Des. 2017;23(37):5691-5701.
doi pubmed - Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17(5-6):431-439.
doi pubmed pmc - Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, Heales SJR, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17(1):84-93.
doi pubmed - Roehl K, Sewak SL. Practice Paper of the Academy of Nutrition and Dietetics: Classic and Modified Ketogenic Diets for Treatment of Epilepsy. J Acad Nutr Diet. 2017;117(8):1279-1292.
doi pubmed - Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077.
doi pubmed - Picot MC, Baldy-Moulinier M, Daures JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia. 2008;49(7):1230-1238.
doi pubmed - Barborka C J. Ketogenic diet treatment of epilepsy in adults. JAMA. 1928;91(2):73.
doi - Kverneland M, Selmer KK, Nakken KO, Iversen PO, Tauboll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197-201.
doi pubmed - Liu H, Yang Y, Wang Y, Tang H, Zhang F, Zhang Y, Zhao Y. Ketogenic diet for treatment of intractable epilepsy in adults: A meta-analysis of observational studies. Epilepsia Open. 2018;3(1):9-17.
doi pubmed pmc - Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500-506.
doi pubmed - Agarwal N, Arkilo D, Farooq O, Gillogly C, Kavak KS, Weinstock A. Ketogenic diet: Predictors of seizure control. SAGE Open Med. 2017;5:2050312117712887.
doi pubmed pmc - Koppel SJ, Swerdlow RH. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem Int. 2018;117:114-125.
doi pubmed pmc - Nei M, Ngo L, Sirven JI, Sperling MR. Ketogenic diet in adolescents and adults with epilepsy. Seizure. 2014;23(6):439-442.
doi pubmed - Seo JH, Lee YM, Lee JS, Kang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios—comparison of 3:1 with 4:1 diet. Epilepsia. 2007;48(4):801-805.
doi pubmed - Ketogenic diet provides neuroprotective effects against ischemic stroke neuronal damages. Semantic Scholar. Accessed: April 9, 2024. [Online]. Available: https://www.semanticscholar.org/paper/Ketogenic-Diet-Provides-Neuroprotective-Effects-Shaafi-Mahmoudi/e39e0c90e8708a1276012e9cd5548a54cd96e3b1.
- Shaafi S, Sharifi-Bonab M, Ghaemian N, Mokhtarkhani M, Akbari H. Early motor-behavioral outcome of ischemic stroke with ketogenic diet preconditioning: interventional animal study. J Stroke Cerebrovasc Dis. 2019;28(4):1032-1039.
doi pubmed - Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145(1):256-264.
doi pubmed pmc - Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7(5):63.
doi pubmed pmc - Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6(6):1307-1315.
doi pubmed pmc - McDougall A, Bayley M, Munce SE. The ketogenic diet as a treatment for traumatic brain injury: a scoping review. Brain Inj. 2018;32(4):416-422.
doi pubmed - Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci. 2012;35(8):507-520.
doi pubmed - Barbanti P, Fofi L, Aurilia C, Egeo G, Caprio M. Ketogenic diet in migraine: rationale, findings and perspectives. Neurol Sci. 2017;38(Suppl 1):111-115.
doi pubmed - Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S, et al. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47(1-2):119-128.
doi pubmed - Tefera TW, Tan KN, McDonald TS, Borges K. Alternative fuels in epilepsy and amyotrophic lateral sclerosis. Neurochem Res. 2017;42(6):1610-1620.
doi pubmed - Napoli E, Duenas N, Giulivi C. Potential therapeutic use of the ketogenic diet in autism spectrum disorders. Front Pediatr. 2014;2:69.
doi pubmed pmc - Michalsen A, Li C. Fasting therapy for treating and preventing disease - current state of evidence. Forsch Komplementmed. 2013;20(6):444-453.
doi pubmed - Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58.
doi pubmed pmc - Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11:85.
doi pubmed pmc - Wang X, Zhou Y, Tang D, Zhu Z, Li Y, Huang T, Muller R, et al. ACC1 (Acetyl Coenzyme A Carboxylase 1) is a potential immune modulatory target of cerebral ischemic stroke. Stroke. 2019;50(7):1869-1878.
doi pubmed - Malinowski B, Zalewska K, Wesierska A, Sokolowska MM, Socha M, Liczner G, Pawlak-Osinska K, et al. Intermittent fasting in cardiovascular disorders-an overview. Nutrients. 2019;11(3):673.
doi pubmed pmc - Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017;37:371-393.
doi pubmed - Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur J Clin Nutr. 2013;67(7):759-764.
doi pubmed - Choi HR, Kim J, Lim H, Park YK. Two-week exclusive supplementation of modified ketogenic nutrition drink reserves lean body mass and improves blood lipid profile in obese adults: a randomized clinical trial. Nutrients. 2018;10(12):1895.
doi pubmed pmc - Michalczyk MM, Klonek G, Maszczyk A, Zajac A. The effects of a low calorie ketogenic diet on glycaemic control variables in hyperinsulinemic overweight/obese females. Nutrients. 2020;12(6):1854.
doi pubmed pmc - D'Abbondanza M, Ministrini S, Pucci G, Nulli Migliola E, Martorelli EE, Gandolfo V, Siepi D, et al. Very low-carbohydrate ketogenic diet for the treatment of severe obesity and associated non-alcoholic fatty liver disease: the role of sex differences. Nutrients. 2020;12(9):2748.
doi pubmed pmc - Di Rosa C, Lattanzi G, Spiezia C, Imperia E, Piccirilli S, Beato I, Gaspa G, et al. Mediterranean diet versus very low-calorie ketogenic diet: effects of reaching 5% body weight loss on body composition in subjects with overweight and with obesity-a cohort study. Int J Environ Res Public Health. 2022;19(20):13040.
doi pubmed pmc - Salis S, Shefa S, Sharma N, Vora N, Anjana RM, Mohan V, Ranjani H. Effects of intermittent fasting on weight loss in Asian Indian adults with obesity. J Assoc Physicians India. 2022;70(9):11-12.
doi pubmed - Headland ML, Clifton PM, Keogh JB. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int J Obes (Lond). 2019;43(10):2028-2036.
doi pubmed - de Oliveira Maranhao Pureza IR, da Silva Junior AE, Silva Praxedes DR, Lessa Vasconcelos LG, de Lima Macena M, Vieira de Melo IS, de Menezes Toledo Florencio TM, et al. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin Nutr. 2021;40(3):759-766.
doi pubmed - Summaries for patients. Effectiveness and safety of low-carbohydrate diets. Ann Intern Med. 2004;140(10):I10.
doi pubmed - Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr., Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285-293.
doi pubmed - Guisado Rosa JP. Importance of a blood test before starting a protein ketogenic diet. OCTOA, 2015;2(2):1-2.
doi - Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88(6):1678-1684.
doi pubmed - Gilbert DL, Pyzik PL, Freeman JM. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J Child Neurol. 2000;15(12):787-790.
doi pubmed - Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6:27.
doi pubmed pmc - Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutr Metab (Lond). 2016;13:77.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
