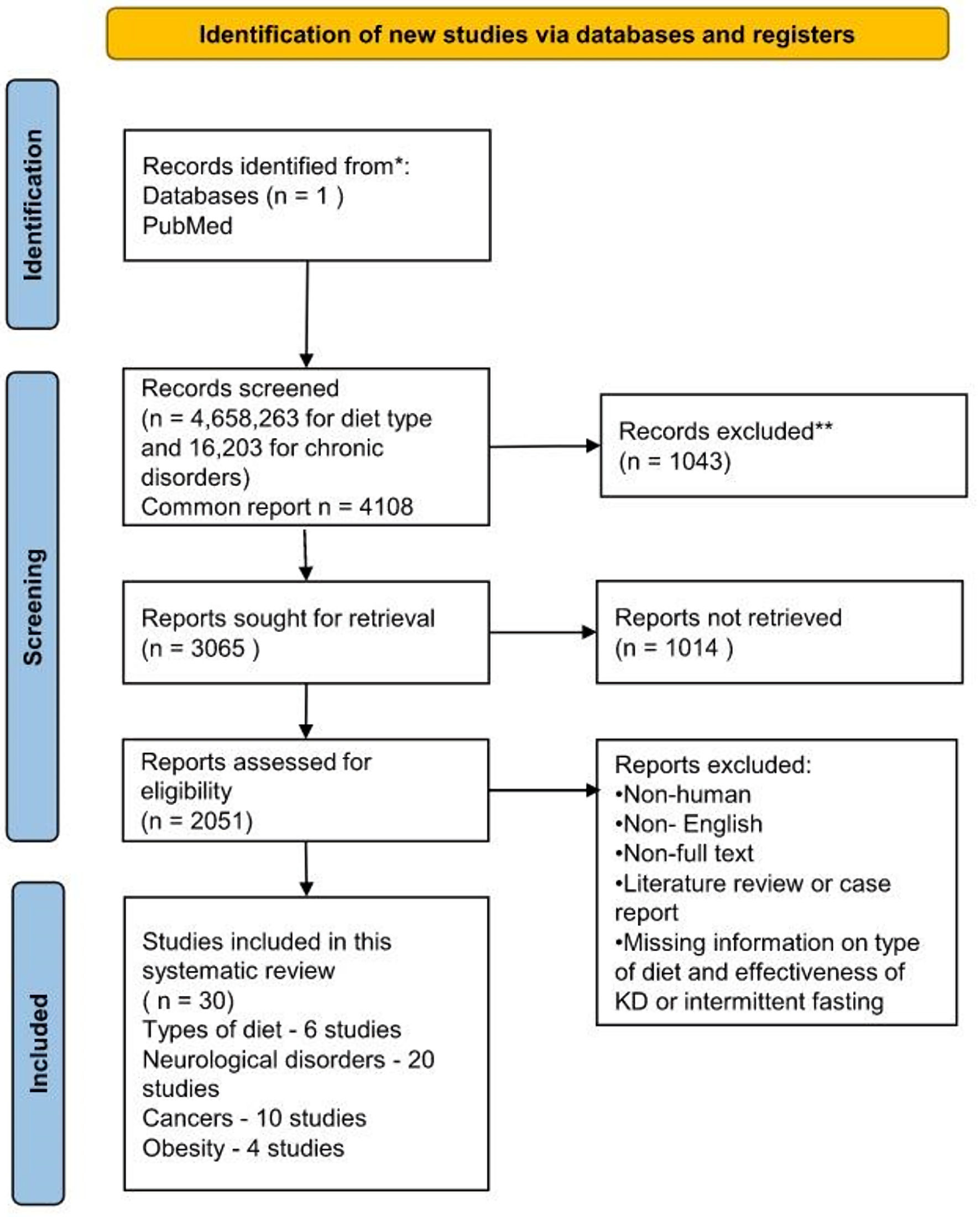
Figure 1. Flow diagram of the study selection process. KD: ketogenic diet.
| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 14, Number 3, June 2024, pages 103-127
Role of Ketogenic Diets and Intermittent Fasting in Neurologic Diseases, Cancers, and Obesity: A Systematic Review of Human Studies
Figure

Tables
| Type of diet | Description | Pros | Cons |
|---|---|---|---|
| LDL: low-density lipoprotein; HDL: high-density lipoprotein. | |||
| Atkins [15-19] | Initially, carbohydrate intake must be restricted to 20 g/day, with the allowance to consume as much protein and fat as desired. | No limitation on the amount of protein and fat consumed | Side effects associated with ketosis: nausea, dizziness, constipation, headache, fatigue, smelly breath |
| Weight loss and seizure reduction in epilepsy patients | Metabolic dehydration | ||
| Risk of dementia or mild cognitive impairment (high carbohydrate levels) | Risk of hyperuricemia (leading to joint pain and gout), hypercalciuria (leading to kidney stones, hypocalcemia, and osteoporosis | ||
| Modest improvement in day-time sleepiness for narcolepsy patients | Risk of permanent loss of kidney function in anyone with reduced kidney function | ||
| Modified Atkins diet [16, 28, 29, 30] | Carbohydrate intake is restricted to 10 - 15 g/day for children and 15 - 20 g/day for adolescents/adults with encouragement of high-fat foods (about 65% of calories from fat sources) | Less restrictive than the ketogenic diet | Approximate 25 - 50 mg/dL increase in total cholesterol in both pediatric and adult studies |
| Decreased risk of growth impairment, kidney stones, dyslipidemia, gastroesophageal reflux | Increase in blood urea nitrogen (BUN) levels | ||
| Seizure frequency reduction with about 45% of patients with epilepsy responding with greater than 50% seizure reduction | |||
| DASH [20] | Promotes consumption of vegetables and fruits, lean meat, and dairy products and the inclusion of micronutrients in the diet and advocates in the reduction of sodium in the diet to about 1,500 mg/day | Lowers blood pressure | Not designed for weight loss |
| Lowers risk of adverse cardiac events and stroke | |||
| Lowers blood glucose levels, triglycerides, LDL cholesterol, and insulin resistance | |||
| Improvements in control of type 2 diabetes and reduction in the incidence of colorectal cancer (mainly in the White population) | |||
| Paleo [21, 22] | Dietary plan is based on foods similar to foods that might have been eaten in the Paleolithic era, which dates to approximately 2.5 million to 10,000 years ago. Diet includes lean meats, fish, fruits, vegetables, nuts, and seeds. | Reduce the risk of cardiovascular disease, metabolic syndrome, type 2 diabetes, cancer, acne vulgaris, and myopia. | Low calcium intake (risk for individuals at risk for osteoporosis) |
| Favorable changes in risk factors, such as weight, waist circumference, glucose tolerance, insulin secretion, insulin sensitivity, and lipid profiles. | |||
| Ketogenic diet [11-14] | High-fat, low carbohydrate diet (20 - 50 g/day) in which carbohydrates are nearly eliminated, thus enabling fatty acids to become the required obligatory source of cellular energy production by peripheral tissues and the brain. | Increased weight loss during the first 3 - 6 months compared with those who follow more balanced diets. | Muscle cramps, bad breath, changes in bowel habits, keto-flu, and energy loss |
| Reversal/control of type 2 diabetes for primary and secondary prevention of cerebrovascular and cardiovascular disorders. | Long-term low-carbohydrate diets with increased fat consumption could stimulate inflammatory pathways, oxidative stress and promote biological aging, induction of hepatic insulin, micronutrient deficiencies and cardiovascular safety. | ||
| Reduction of serum triglycerides and improvement of lipid profiles. | |||
| Increase in the level of HDL cholesterol in obese patients. | |||
| Improvement of lipid disorders that are characteristic of atherogenic dyslipidemia. | |||
| Beneficial effects on neurological disorders include epilepsy and Alzheimer’s disease. | |||
| Fasting [23-27] | 24-h-fast, intermittent daily fasting 16:8 (restriction: intake) or 18:6, skipping meals, one-meal-a-day fast, water or egg fasting | Control of type 2 diabetes to mitigate cardiovascular and cerebrovascular disorders | Nausea and vomiting |
| Improvement in glucose tolerance | Edema | ||
| Improvement of insulin sensitivity and glucose tolerance in people with diabetes immediately following a fast weight loss | Alopecia and motor neuropathy | ||
| Hyperuricemia and urate nephropathy | |||
| Irregular menses | |||
| Abnormal liver function tests and decreased bone density | |||
| Thiamine deficiency and Wernicke’s encephalopathy | |||
| Mild metabolic acidosis | |||
| Possible death (due to lactic acidosis, small bowel obstruction, renal failure, and cardiac arrhythmias) | |||
| Type of fasting | Description |
|---|---|
| 24-h fast | Fast in which no food is consumed for 24 h. Individuals can consume water, and in most cases, black coffee and/or green tea is allowed. |
| Intermittent daily fasting 16:8 [31] | Restricting food intake/fasting for 16 h a day and consuming food for 8 h a day |
| Skipping meals | Occasionally skipping meals such as breakfast, lunch, and/or dinner according to the individual’s level of hunger or time restraints. |
| One-meal-a-day (OMAD) fast | Type of intermittent fasting is referred to as 23:1, in which an individual spends 23 h fasting and leaves 1 h a day to consume calories by eating and drinking. |
| Water fasting [32] | Fasting in which a person does not eat and drink anything other than pure water; a “zero calorie diet” |
| Eggs fasting | Fast in which eggs are prepared without butter/oil and beverages such as water and zero-calorie beverages are permitted (use of artificial sugar is not recommended) |
| Clinical conditions | Study author, country (year) | Sample size/timeline | Methods | Findings |
|---|---|---|---|---|
| MELAS: mitochondrial encephalopathy with lactic acidosis and stroke-like episodes; ALS: amyotrophic lateral sclerosis; ASD: anti-seizure drug; CDR: Clinical Dementia Rating; MAD: modified Atkins diet; KD: ketogenic diet; IF: intermittent fasting; UPDRS: Unified Parkinson’s Disease Rating Scale; VLCKD: very low-calorie KD; VLCnKD: very low-calorie non-KD; AD: Alzheimer’s disease; HR: hazard ratio; CI: confidence interval; ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive; MCT: medium-chain triglyceride; APOE4: apolipoprotein E4; PUFA: polyunsaturated fatty acid; SD: standard deviation; WHR: waist-to-hip ratio. | ||||
| Epilepsy [33] | Groesbeck et al, USA (2006) | Retrospective chart review of children treated with the KD for more than 6 years at the Johns Hopkins Hospital | 24 children experienced more than a 90% reduction in seizures over prolonged periods on KD, and 3 achieved complete freedom from seizures. | |
| Epilepsy [34] | Cervenka et al, USA (2017) | Ten adults were treated with KD monotherapy for epilepsy (4 patients were naive to ASDs, and six previously tried and stopped ASDs) | Adults (age ≥ 18 years) evaluated in the Johns Hopkins Adult Epilepsy Diet Center (AEDC) from August 2010 to August 2016 were followed, and descriptive statistics were used to represent patient characteristics and outcomes. | 50% of treatment-naive participants were free from disabling seizures on the MAD monotherapy for > 1 year. |
| 67% (4 out of 6) of patients who previously tried ASDs became seizure-free on diet monotherapy. Two patients experienced > 50% seizure reduction. | ||||
| Epilepsy [36] | Freeman et al, USA (1998) | 150 consecutive children, ages 1 to 16 years, all of whom continued to have more than two seizures per week despite therapy with at least two anticonvulsant medications | Children were treated with the KD and followed for at least 1 year. | The children (mean age: 5.3 years) averaged 410 seizures per month before the diet. |
| Seizure frequency was tabulated from patients’ daily seizure calendars. | Three months after diet initiation, 83% remained on the diet, and 34% had a > 90% decrease in seizures. | |||
| Furthermore, seizure reduction was calculated as a percentage of baseline frequency. | At 6 months, 71% still remained on the diet, and 32% had a > 90% decrease in seizures. | |||
| At 1 year, 55% remained on the diet and 27% had a > 90% decrease in seizure frequency. | ||||
| Epilepsy [37] | Hemingway et al, USA (2001) | 150 consecutive children entered prospectively into a study of the KDs efficacy and tolerability | 3 to 6 years after diet initiation, all 150 families were sent a survey inquiring about their child’s health status, seizure frequency, and anticonvulsant medications. | Of the original 150-patient cohort, 20 (13%) were seizure-free, and 21 (14%) had a 90-99% decrease in their seizures. |
| 29 were free of medications, and 28 were on only one; 15 remained on the diet. | ||||
| Epilepsy [35] | Marsh et al, USA (2006) | 150 children with epilepsy, refractory to at least two medications, who initiated the KD between 1994 and 1996 | 3 to 6 years after diet initiation, all the families were contacted by telephone or questionnaire to assess their child's current seizure status, medications, and therapies. | Almost half of the children who discontinued the diet during the first year had fewer seizures when assessed 3 - 6 years later. 22% of these had become seizure-free without surgery. |
| Stroke/MELAS [38] | Steriade et al, USA (2014) | A 22-year-old woman with multiple episodes of generalized and focal status epilepticus and migratory cortical stroke-like lesions who underwent muscle biopsy for mitochondrial genome sequencing | Clinical, electrophysiologic, and radiologic data of the patient were analyzed. | KD improves mitochondrial dysfunction in MELAS, which may promote better seizure control and less frequent stroke-like episodes. |
| AD [39] | Reger et al, USA (2004) | 20 subjects with AD or mild cognitive impairment | Subjects consumed a drink containing emulsified MCTs or placebo, and cognitive tests were administered, and levels of the ketone body β-hydroxybutyrate were observed through blood draws. | MCT treatment facilitated performance on the ADAS-cog for APOE4 (-) subjects but not for APOE4 (+) subjects. |
| Higher ketone values were associated with greater improvement in paragraph recall with MCT treatment relative to placebo across all subjects. | ||||
| AD [13] | Henderson et al, USA (2009) | 152 subjects diagnosed with mild to moderate AD | Daily administration of AC-1202, an oral ketogenic compound, was evaluated in subjects in a US-based, 90-day, randomized, double-blinded, placebo-controlled, parallel-group study. | AC-1202 rapidly elevated serum ketone bodies in AD patients, resulting in significant differences in ADAS-Cog scores compared to the placebo. Effects were most notable in APOE4 (-) dosage-compliant subjects. |
| Participants received one dose of the agent during the first 7 days of the study, followed by two doses (20 g of MCT) administered at breakfast from day 8 to day 90. | ||||
| AD [40] | Luchsinger et al, USA (2002) | 80 elderly individuals free of dementia were followed for four years. | Daily intake of calories, carbohydrates, fats, and protein was recalled using a semiquantitative food frequency questionnaire between baseline and first follow-up visits. | Individuals with the highest calorie intake compared to the lowest quartile had an increased risk of AD (HR: 1.5; 95% CI: 1.0 - 2.2). |
| For individuals with the APOE4 allele, the HR of AD for the highest quartiles of calorie and fat intake were 2.3 (95% CI: 1.1 - 4.7) and 2.3 (95% CI: 1.1 - 4.9), respectively, compared with the lowest quartiles. | ||||
| AD [41] | Taylor et al, USA (2017) | 7: CDR 0.5, 4: CDR 1, and 4: CDR 2 participants (a total of 15 patients with AD) were enrolled in the KD retention and feasibility trial | 3-month, medium-chain triglyceride-supplemented KD followed by 1-month washout participants. Administered the ADAS-Cog subscale and Mini-Mental State Examination before the KD and following the intervention and washout. | In achieving ketosis, the mean of the ADAS-Cog subscale score improved significantly during the diet and reverted to baseline after the washout. |
| AD [42] | Krikorian et al, USA (2012) | 23 older adults with mild cognitive impairment | Patients were randomly assigned either a high carbohydrate or very low carbohydrate diet in a 6-week intervention. | Improved verbal memory performance for the subjects on the low-carbohydrate diet was noted. The levels of ketone bodies were positively correlated with memory performance. |
| Migraine [43] | Di Lorenzo et al, Italy (2019) | 18 migraine patients without aura before and after a 1-month of KD | To prove if the KD-related cortical excitability changes are primarily due to cerebral cortex activity or are modulated by the brainstem, the study concurrently recorded the interictally nociceptive blink reflex (nBR) and the pain-related evoked potentials (PREP). | Following 1-month on KD, the mean number of attacks and headache duration reduced significantly. KD significantly normalized the interictal PREP habituation, while the nBR habituation deficit did not change. |
| Migraine [46] | Di Lorenzo et al, Italy (2014) | 96 overweight female migraineurs patients (45: KD and 51: standard diet) for 3 months | Mean monthly attack frequency, number of days with headaches, and tablet intake were assessed before and 1, 2, 3, and 6 months after diet initiation. | Drastic improvement in attack frequency, days, and medication use during the 1-month (P < 0.0001), followed by a worsening during the transitional diet and subsequent standard diet period. According to the authors, KD efficacy’s underlying mechanisms could be related to its ability to enhance mitochondrial energy metabolism and counteract neural inflammation. |
| Migraine [45] | Di Lorenzo et al, Italy (2019) | Randomized double-blind, cross-over trial of 35 overweight obese migraines | To determine the therapeutic effect of a very low-calorie diet in overweight episodic migraine patients during a weight-loss intervention in which subjects alternated randomly between a VLCKD and a VLCnKD each for 1 month. The primary outcome was reducing migraine days each month compared to a 1-month pre-diet baseline. Secondary outcome measures were a 50% responder rate for migraine days, reduction of monthly migraine attacks, abortive drug intake, and body mass index (BMI) change. | VLCKD patients experienced fewer migraine days with respect to VLCnKD (P < 0.0001). |
| The 50% responder rate for migraine days was 74.28% (26/35 patients) during the VLCKD period, but only 8.57% (3/35 patients) during VLCnKD. Migraine attacks decreased during VLCKD with respect to VLCnKD (P < 0.00001). | ||||
| The two diets showed no differences in acute anti-migraine drug consumption (P = 0.112) and BMI (P = 0.354) between the two diets. | ||||
| VLCKD has a preventive effect in overweight episodic migraine patients that appears within 1 month, suggesting that ketogenesis may be a useful therapeutic strategy for migraines. VLCKD is effective for rapid, short-term improvement of migraines in overweight patients, while VLCnKD is not. | ||||
| Whether this dietary strategy should be applied to all overweight migraine patients and for how long remains to be determined in future studies. | ||||
| Chronic cluster headache [44] | Di Lorenzo et al, Italy (2018) | 18 drug-resistant chronic cluster headache (CCH) patients | Patients underwent a 12-week KD (MAD), and the clinical response was evaluated in terms of response (≥ 50% attack reduction). | 3-month KD ameliorated clinical features of chronic cluster headache |
| Parkinson’s disease [47] | Vanitaille et al, USA (2005) | 7 patients with Parkinson’s Disease | Patients prepared a “hyperketogenic” diet at home for 28 days. Used the UPDRS to measure effects. | A hyperketogenic diet at home and adherence for 28 days resulted in high ketogenic bodies, which improved the UPDRS scores. |
| Parkinson’s disease [48] | Phillips et al, USA (2018) | 47 patients with Parkinson's disease (38 individuals completed the study). | This study assessed the effect of a low-fat versus KD in patients. Diets were followed for 8 weeks. | Both diet groups showed significantly improved motor and non-motor symptoms; however, the ketogenic group showed greater improvements in non-motor symptoms. |
| ALS [49] | Veldink et al, Netherlands (2007) | A case-control study (132 patients and 220 healthy controls) between 2001–2002. | Patients’ dietary intake for the nutrients of fatty acids, cholesterol, glutamate, or antioxidants was assessed using a food-frequency questionnaire to evaluate their link with the risk of developing ALS. | A high intake of PUFAs and vitamin E is associated with a 50-60% decreased risk of developing ALS, and these nutrients appear synergistically. |
| ALS [50] | Okamoto et al, Japan (2009) | The study comprised 153 patients and 306 gender- and age-matched controls randomly selected from the general population. | A self-administered food frequency questionnaire was used to estimate pre-illness intakes of food groups and nutrients. | The high intakes of carbohydrates and low intakes of fat and some kinds of fatty acids may, when combined, increase the risk of ALS. |
| Behavioral Disease [51] | Jagust et al, USA, 2005 | 112 Latino individuals aged 60 years and above were selected from an ongoing larger cohort of 1789 individuals. | Baseline anthropomorphic measures (WHR) and measurements of fasting blood glucose, cholesterol, insulin levels, and blood pressure were obtained. Baseline anthropomorphic measures (WHR) and measurements of fasting blood glucose, cholesterol, insulin levels, and blood pressure were obtained. | The WHR and age were positively related to white matter hyperintensities (P = 0.02 and P = 0.001, respectively). A 1-SD increase in WHR was associated with a 0.2-SD decrease in hippocampal volume and a 27% increase in white matter hyperintensities. |
| A larger WHR may be related to neurodegenerative, vascular, or metabolic processes that affect brain structures underlying cognitive decline and dementia. | ||||
| Type of cancer (n) | References/studies included | Type of diet | Total patients/intervention | Role of KD |
|---|---|---|---|---|
| KD: ketogenic diet; IF: intermittent fasting; SD: standard diet; IL: interleukin; TNF: tumor necrosis factor; NA: not available. | ||||
| Head and neck cancer [52] | Klement et al, Germany (2022) | KD (7) + SD (21) | 28/with radiotherapy and chemotherapy | KD may partially counteract the detrimental effects of both radio and chemotherapy on body composition in head and neck cancer patients. |
| Brain tumors [6] | Voss et al, Germany (2022) | KD + IF | 20/with irradiation | The short diet schedule led to significant metabolic changes, with low glucose emerging as a marker of better prognosis. |
| Non-metastasized rectal cancer [55] | Klement et al, Germany (2021) | KD (18) + SD (23) | 41/during radiotherapy | This study demonstrated a trend for KDs contributing synergistically to pathological tumor response. |
| High-grade glioma - new, recurrent [54] | Porper et al, Israel (2021) | KD + KD and metformin | 13/with radiotherapy | Higher serum ketone levels were associated with both dietary intake and metformin use. |
| Head and neck squamous cell carcinoma [5] | Ma et al, USA (2021) | KD | 12/8 patients with concurrent radiation and platinum-chemotherapy | This study demonstrated difficulty with diet compliance when combined with standard-of-care radiation therapy and cisplatin chemotherapy. |
| Breast cancer [55] | Klement et al, Germany (2021) | KD (29) + SD (30) | 59/during radiotherapy | It supports that consuming a KD during radiotherapy is safe for women with breast cancer and has the potential to improve quality of life and metabolic health. |
| Locally advanced and metastatic breast cancer [56] | Khodabakhshi et al, Iran (2020) | KD + SD | 80/with chemotherapy | KD in breast cancer patients might exert beneficial effects by decreasing TNF-α and insulin and increasing IL-10. KD may result in a better response through reductions in tumor size and downstaging in patients with locally advanced disease. |
| Locally advanced and metastatic breast cancer [57] | Khodabakhshi et al, Iran (2021) | KD + SD | 60/with chemotherapy | Results suggested that a combination of chemotherapy and KDs could improve the biochemical parameters, body composition, and overall survival with no substantial side effects in breast cancer patients. |
| Ovarian and endometrial cancer [58] | Cohen et al, USA (2020) | KD + SD | 57/with usual care | The findings suggest that KD may be a safe and achievable component of treatment for some cancer patients. |
| Stage II and III cancer patients [59] | Augustus et al, West Indies (2020) | KD | NA | KD was suitable for stage II and III cancer patients in improving their quality of life and nutritional, functional, and psychosocial statuses. |
| Aspect | Findings |
|---|---|
| KD: ketogenic diet; IF: intermittent fasting. | |
| Weight loss mechanisms [10, 60-62] | KD induces weight loss through appetite suppression, increased satiety effect of proteins, modification in hormone levels, increased lipolysis, and metabolic rate. IF results in significant reductions in body weight, fat mass, and glucose levels. |
| Very low-calorie KDs (VLCKDs) [63, 64] | VLCKDs promote hepatic fat mobilization and may reverse mild renal impairment. |
| Challenges and considerations [65-68] | Long-term adherence to KD can be challenging, prompting the consideration of periodic KDs for managing conditions like type 2 diabetes mellitus. IF variations like IF1 and IF2 demonstrate significant reductions in body weight and fat mass. Combining IF with exercise programs yields more favorable outcomes compared to diet alone therapy. |
| Time-restricted eating (TRE) [61, 62] | TRE combined with exercise training has shown promise in reducing fat mass and visceral fat, particularly in overweight and older populations. |
| Disease | Study, country (year) | Sample size/timeline | Methods | Findings |
|---|---|---|---|---|
| KD: ketogenic diet; VLCKDs: very low-calorie ketogenic diets; LCKD: low-calorie ketogenic diet; LC: low-calorie; BMI: body mass index; NAFLD: non-alcoholic fatty liver disease; DM: diabetes mellitus; VAT: visceral adipose tissue; LDL: low-density lipoprotein; HDL: high-density lipoprotein; GGT: gamma-glutamyl transferase; EOSS: Edmonton Obesity Staging System; MD: Mediterranean diet; CRP: C-reactive protein; FBG: fasting blood glucose; GFR: glomerular filtration rate; HbA1c: hemoglobin A1c; TG: triglycerides. | ||||
| Obesity, visceral fat, and liver fat accumulation [63] | Cunha et al, Brazil (2020) | 39 patients (20 VLCKD, 19 LC) for 2 months | Prospective study to determine the efficacy of VLCKD compared to LCD in reducing visceral and liver fat accumulation in patients with obesity. | At 2 months, the VLCKD group had a relative weight reduction of 9.59±2.87%, while the LC group had a relative weight loss of 1.87±2.4% (P < 0.001). The average VAT reductions were 32 cm2 for the VLCKD group and 12 cm2 for the LC group (P < 0.05). The VLCKD group experienced reductions in the liver fat fraction that were noticeably more severe than those in the LC group (4.77 vs. 0.79%; P < 0.005). |
| Obesity and mild kidney failure [64] | Bruci et al, Rome (2020) | 92 individuals (38 mild renal disease, 54 no renal disease), for 3 months | A prospective observational study where participants underwent VLCKD for 3 months. Anthropometric parameters, bioelectrical impedance, and biochemistry were gathered before and after dietary intervention. | Notable decrease in fat mass, average weight loss close to 20% of initial weight, improvement in metabolic markers, and GFR returned to normal at 27.7% after intervention in the group with mild renal disease. |
| Severe obesity and NAFLD [103] | D’Abbondanza et al, Italy (2020) | 100 subjects: 72 severely obese women, 28 severely obese men (BMI ≥ 40, BMI ≥ 35 with obesity-related comorbidities, age between 18 and 65 years, followed for 25 days | All subjects were evaluated at enrolment and 25 days after following VLCKD. Statistical analysis determined the difference in primary endpoints (excess of body weight loss (EBWL), reduction in GGT). Secondary endpoints (variations of obesity grade according to EOSS, degree of liver steatosis). | Significant weight loss, fat mass, and degree of steatosis were observed in all groups. Males experienced significantly larger EBWL, and greater GGT reduction, and higher waist circumference, insulin resistance, and HbA1c reduction than females. |
| Obesity or overweight with newly diagnosed type 2 DM [65] | Li et al, China (2022) | 60 patients with overweight or obesity newly diagnosed with type 2 DM, 30 on KD, 30 on standard diabetes diet for 12 weeks. | Variables such as uric acid, insulin, blood lipids, body weight, and blood glucose were measured before and after intervention. | A significant decrease in rates of weight, BMI, waist circumference, TG, cholesterol, LDL, HDL, FBG, fasting insulin, and HbA1C was noted in the KD group compared to the control group. |
| Obesity and overweight [104] | Rosa et al, Italy (2022) | 268 obese patients randomly assigned to MD or VLCKD, maximum of 3 months, or lose 5 % body weight | Population stratified according to gender, BMI, and age. | Both groups lost 5 %body weight but required different periods (VLCKD: 1 month, MD group: 3 months) |
| Follow-up visits were done monthly until 5% body weight loss, anthropometric parameters, and body composition were obtained at the end of the study. | Higher waist circumference and fat mass percentage reduction in the MD group compared to the VLCKD group. | |||
| Obesity- cardiorespiratory fitness, body composition, cardiometabolic risk factors [8] | Perissiou et al, Australia (2020) | 64 obese men and women were randomly assigned to experimental (structured exercise + low carbohydrate meals) and control (structured exercise + standard dietary advice) for 8 weeks | Blocked randomization stratification applied by gender, anthropometric parameters, blood biomarkers, and cardiorespiratory fitness obtained at the study’s beginning and end, data were statistically analyzed between the experimental and control groups. | A more significant increase in cardiorespiratory fitness (measured by delta VO2 peak: mean diff -3.4), a greater reduction in fat mass index, lean muscle mass, fasting blood glucose, triglycerides, and CRP was noted in the experimental group than the control group. Reaching a ketogenic status was associated with a significant decrease in total body fat, VAT, fat mass index, and lean muscle mass. |
| Hyperinsulinemic overweight/obese [102] | Michalczyk et al, Poland (2020) | 100 females who reported to the clinic were randomly assigned to LCKD and control group followed for 12 weeks, 4 in LCKD, 5 in control resigned | A tailor-made hypocaloric diet was prescribed for each subject, where daily caloric consumption was 20% less than total daily energy expenditure. Blood biochemical analysis, body mass measurement, and circumference measurement were measured at the beginning and end of the study. | Compared to baseline, there was a decrease in glucose, insulin, HbA1c, TG, insulin resistance, body mass, waist circumference, hip circumference, thigh circumference, increase in HDL-C among the LCKD group after intervention. These changes are not observed in the control group. The LCKD group had lower glucose, insulin, HbA1c, insulin resistance, body mass, waist circumference, hip and thigh circumference, and an increase in HDL-C compared to the control group after the intervention. |
| Obesity [101] | Choi et al, Korea (2018) | 46 subjects between 19 - 49 years, BMI > 25, intervention for 2 weeks | Subjects were randomly assigned to 3 groups with equal gender distribution: 1) Ketogenic nutrition drink (fat: carb: 4:1); 2) Modified ketogenic nutrition drink (1.7:1); 3) Balanced nutrition drink. Measurements like anthropometric measurements, body composition analysis, blood lipid profile, and ketone bodies were performed before the intervention, during (after 1 week), and after (2 weeks) intervention. Changes in physical activity and body symptoms were surveyed through questionnaires. | Saturated fat intake was high among KD 4:1 compared to KD 1.7:1. All groups showed a decrease in body water and minerals from 0 weeks to 1 week, with no significant change from 1 to 2 weeks. Protein and skeletal muscle mass decreased significantly in KD 4:1, BD groups. All groups showed decreased BMI, body fat mass, and weight. A decrease in total cholesterol and LDL-C was seen in KD 1.7:1 and BD groups, with no significant changes in KD 4:1 group. Ketone bodies significantly increased in KD 4:1 and KD 1.7:1 from 0 to 1 week, with no change from 1 to 2 weeks. Nausea, decreased appetite significantly increased from 0 to 1 week in KD 4:1, KD 1.7:1. Constipation significantly increased in KD 4:1 group. |
| Study, country, (year) | Sample size/timeline | Methods | Findings | |
|---|---|---|---|---|
| IF: intermittent fasting; RCT: randomized controlled trial; BMI: body mass index; ITT: insulin tolerance test; LDL-C: low-density lipoprotein cholesterol; HP: hypertension; HDL: high-density lipoprotein. | ||||
| IF + protein pacing (P) [60] | Arciero et al, USA (2022) | 42 participants, 21 to intervention (10 assigned to IF1-P, 10 to IF2-P), 5 weeks of intervention. | RCT where 42 were eligible, 20 IF-P matched for weight, BMI, randomly assigned to either 1) IF diet for 1 day/week (36 h), protein pacing diet for remaining 6 days/week; or 2) Intermittent diet for 2 consecutive days (60 h) and protein pacing for remaining 5 days/week. All lab testing procedures were performed at baseline control (week 0), and in week 5, ITT analysis was conducted. | Dietary energy and macronutrient intake decreased; specifically, total energy intake decreased significantly (40%) during weight loss, with no difference in groups. IF2-P group had greater body weight and waist circumference than IF1-P. Fat-free mass increased by 2%. No significant hormone changes in both groups from baseline were noted. Both groups had significant reductions in BP, fasting total cholesterol, LDL-C, and triglycerides from baseline, with no differences between groups. Significant reduction in desire to eat and quantity of food to eat, the tendency of hunger ratings lower in IF2-P compared to IF1-P. |
| Intermittent vs. continuous energy restriction [106] | Headland et al, Australia (2019) | 332 overweight and obese adults between 18 - 72 years, randomly assigned to 1 of 3 groups: 1) Continuous energy restriction; 2) week on week off restriction; 3) 5:2 restriction for a total of 12 months. | Randomized, parallel trial design to compare three different dietary patterns: 146 completed study, all three groups visited the clinic every 3 months, a total of eight visits. Participants were grouped based on gender, BMI, and age, randomized 1:1 to intervention groups. | ITT analysis showed a significant effect of interventions on weight loss at 12 months, although there were no differences between the three groups. Fat and lean mass decreased significantly over time, with no differences between the dietary groups. HDL increased, and triglycerides decreased by a similar degree in all groups. |
| Only 56%of participants finished the trial. The primary outcome was weight loss. Secondary outcomes were changes in body composition, weight loss, and glucose. | ||||
| IF and concurrent exercise [61] | Kotarsky et al, USA (2021) | 21 participants with a BMI of 25 - 34.9 were randomly assigned to normal eating (NE) or time-restricted eating (TRE), resistance training, and aerobic exercise standardized for both groups for 8 weeks. | RCT. Body composition, muscle performance, energy intake, macronutrient intake, physical activity, and physiological variables were assessed between both groups. | Mild energy restriction was observed in TRE and NE. Loss of total body mass was more significant for the TRE group compared to both the NE group and pre-intervention. Lean mass increased during intervention for both TRE and NE with no differences. |
| IF on body weight, composition, vital signs in low-income women with obesity [107] | Puerza et al, Brazil (2020) | 58 women were randomized to hypoenergetic diet and time-restricted diet for 12 months. 31 lost to follow-up. | RCT. Body fat and waist circumference were measured at baseline and after 4,6, and 12 months of intervention. Systolic and diastolic blood pressure, heart rate, and axillary temperature were measured at baseline and 12 months of intervention. | ITT analysis showed no significant changes in body weight after 12 months. An increase in axillary temperature, a reduction in percentage body fat, and waist circumference were observed in the interventional group compared to the control group. |
| IF: overweight older men and women [62] | Domaszewski et al, Poland (2023) | 116 healthy, non-smoking participants assigned to time-restricted eating (TRE) or educational control participants for 6 weeks | TRE group was advised not to consume calories for 16 h/day, control group to continue the previous diet. Changes in body weight and composition were compared. | TRE group had a decrease in body weight in both men and women. A significant reduction in visceral fat mass and waist circumference was observed in men. No changes in visceral fat or waist circumference were seen in women. |
| IF vs. IF with concurrent training [67] | Maaloul et al, Tunisia (2023) | 20 obese men regularly performing Ramadan diurnal intermittent fasting (RDIF) were randomized into two groups: 1) RDIF with concurrent training (RDIF-CT); 2) RDIF without training (RDIF-NCT) for 4 weeks. | RCT. Body composition, blood glucose, lipid profile, and inflammation were assessed before and after the 4-week RDIF. | Both groups had decreased weight, fat mass, fat percentage, and waist circumference and improved blood glucose, lipid profile, and inflammation. Fat-free mass decreased significantly in RDIF-NCT compared to the RDIF-CT group. RDIF-CT group showed more remarkable improvement in body composition (weight, fat mass, fat percentage, waist circumference) and a more significant decrease in lipid biomarkers, inflammation, and liver damage compared to RDIF-NCT group pre- and post-intervention. |
| IF, weight loss [105] | Salis et al, India (2022) | 32 overweight/obese adults were assigned consecutively to an IF plan and followed up for 3 months | Demographic, anthropometric, and dietary assessments were done pre- and post-intervention. Qualitative interviews were done at the end of the study to record the participants’ overall well-being, experience, and sustainability of IF. | Significant reductions in mean body weight, waist circumference, BMI, daily calories, carbohydrate intake, and increased protein intake were noted. Participants reported positive experiences of practicing IF, such as improved fitness, sleep cycle, and adoption of healthy eating habits. |
| IF 5:2 plus program [66] | Kang et al, China (2022) | 131 participants in three groups: 1) IF group (n = 42); 2) Daily calorie restriction group (CR) (n = 1); 3) Daily calorie restriction with high protein meal replacement group (HP) (n = 48); 12-week weight loss data analyzed | In this retrospective cohort study, participants were divided into two groups: 1) IF 5:2 plus groups: 30% of energy requirement on fast days, 70% on rest; 2) Daily calories restriction group: 70% of daily energy requirement given. Clinical data such as age, sex, weight, and body composition at 0 and 12 weeks, data on adverse events were also collected. | A mean weight loss of 7.8 after 12 weeks was noted. Weight change from baseline is higher in IF and HP groups compared to the CR group. BMI, fat mass, and total mass of all three groups were significantly decreased at 12 weeks compared to baseline. No serious adverse events were reported in the three groups. |
| IF 5:2 [68] | Witjaksono et al, Indonesia (2022) | 50 participants, 25 allocated to the fasting group, and 25 to the control group for 8 weeks | Non-blinded 1:1 two-arm RCT, the fasting group fasted twice a week (5:2) while the control group did not fast. Interviews were conducted before, during, and after an intervention to gather data on education, income, knowledge, physical activity, and food intake history. Per protocol, analyses were done. | Significant differences in total calories, carbohydrate, protein, and fat intake between intervention and control groups after intervention. No significant difference in fat mass, skeletal muscle, and visceral fat rating before and after the study in intervention and control groups. Fat-free mass before and after showed a significant difference. |