| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 14, Number 3, June 2024, pages 89-102
Pharmacological Treatment of Diabetes Mellitus: An Overview of New Sodium-Glucose Cotransporter 2 Inhibitors for the Treatment of Diabetes Mellitus
Mateusz Nieczyporuka, b , Pawel Tyrnaa
, Tomasz Cadera
, Aleksandra Sikoraa
, Szymon Stanetaa
aDepartment of Medicine, Medical University of Warsaw, Warsaw, Poland
bCorresponding Author: Mateusz Nieczyporuk, Department of Medicine, Medical University of Warsaw, Warsaw, Poland
Manuscript submitted February 20, 2024, accepted April 25, 2024, published online June 29, 2024
Short title: SGLT2 Inhibitors for the Treatment of DM
doi: https://doi.org/10.14740/jem942
- Abstract
- Introduction
- Mechanisms of action of SGLT2 inhibitors
- Methods and Materials
- Results
- Conclusions
- References
| Abstract | ▴Top |
Diabetes mellitus is a metabolic disease characterized by chronic hyperglycemia, which leads to irreversible damage to the vascular endothelium and causes many complications. Type 2 diabetes accounts for the vast majority of cases and is characterized by a deficit of insulin action on tissues. In recent years, several new oral drugs have emerged to treat the disease, including sodium-glucose cotransporter 2 (SGLT2) inhibitors (flozins) that prevent glucose reabsorption in the kidneys. In this review, we analyzed seven different SGLT2 inhibitors, including several novel ones, based on 38 selected papers. Flozins display high efficacy in reducing hemoglobin A1c (HbA1c), body weight, and systolic blood pressure. Therefore, flozins should be considered one of first-line treatment options in type 2 diabetes not only for patients with heart failure or kidney disease, but also for overweight and hypertensive patients.
Keywords: Diabetes; SGLT2 inhibitor; Novel drugs; HbA1c; Body weight; Systolic blood pressure
| Introduction | ▴Top |
Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from a defect in insulin secretion or its action on tissues. The vast majority of individuals with diabetes have type 2 diabetes mellitus (T2DM), which is related to insulin resistance. The development of T2DM is largely influenced by modifiable environmental factors. These include inappropriate caloric balance leading to overweight or obesity, excessive intake of monosaccharides, and lack of physical activity. In recent decades, there has been a significant increase in the incidence of T2DM [1-3]. At the same time, new groups of drugs are emerging that not only control glycemic levels but also other risk factors, such as body weight.
Flozins, or sodium-glucose cotransporter 2 (SGLT2) inhibitors, are a relatively new and promising group of oral antidiabetic agents. They have been recognized in diabetology for their antihyperglycemic effect and have also been used in cardiology and nephrology to treat heart failure and chronic kidney disease [4-8]. In our review, we will focus on presenting the long-used flozins, as well as some promising new drugs in this group, and compare their effectiveness in the management of diabetes.
Among the drugs discussed in this article: canagliflozin, empagliflozin, and dapagliflozin have been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of T2DM. Bexagliflozin was approved by the FDA in 2020 and is currently under registration process in Europe. However, enavogliflozin, janagliflozin and remogliflozin have recently been registered in China, Korea and India, respectively, but have not yet received approval from the FDA or EMA.
| Mechanisms of action of SGLT2 inhibitors | ▴Top |
SGLT2 cotransporters belong to a large family of symporters responsible for facilitated transport of different solutes, aided by a positive sodium gradient [9, 10]. Two cotransporters can be distinguished in this group: SGLT1 and SGLT2. SGLT2 is responsible for the reabsorption of glucose from the proximal tubule of the nephron and is found almost exclusively in renal tissue. SGLT2 is capable of removing up to 97% of glucose from the primary urine [11-13]. The SGLT1 cotransporter, located further down the proximal tubule, reabsorbs any remaining glucose. Therefore, in healthy individuals all glucose is reabsorbed from the filtered primary urine because glucose excretion by the kidneys results in a loss of valuable calories for the body.
SGLT2 has a high capacity but low affinity for glucose, while SGLT1 has low capacity and high affinity.
The level of reabsorption is directly proportional to the concentration of glucose in the primary urine, but it is not unlimited. The maximum capacity of SGLT2 is reached at a glucose filtration rate of approximately 350 mg/min/1.73 m2 [14, 15]. This is achieved at a glycemia of approximately 180 mg/dL. Above this threshold, the amount of filtered glucose exceeds the capacity of SGLT2, leading to glucosuria. To prevent chronic hyperglycemia from causing this process, the expression of SGLT2 cotransporter in the proximal tubule increases. The overexpression of SGLT2 is accompanied by an increase in the expression of the sodium-hydrogen exchanger 3 (NHE3) transporter, which is responsible for reabsorption of two-thirds of sodium filtered by the kidney [16-18]. It should be noted that SGLT2 itself not only transports glucose but also sodium at a 1:1 ratio.
Excessive reabsorption of sodium in the proximal tubule leads to a significant reduction in its concentration further down the loop of Henle and the distal tubule, which is misinterpreted by the macula densa as hypovolemia [19]. As a result, the secretion of adenosine by the macula densa is decreased. Adenosine typically induces vasodilation through A2 receptors, including those in efferent arterioles, but in afferent arterioles it causes vasoconstriction through A1 receptors [20]. Consequently, the decreased adenosine concentration causes afferent arteriole dilation and efferent arteriole constriction, both of which generate a high intraglomerular pressure. This leads to glomerular damage. Additionally, the juxtaglomerular cells activate the renin-angiotensin-aldosterone system (RAAS), contributing to hypervolemia and hypertension [21, 22].
Moreover, heightened filtration and reabsorption increases ATP and oxygen consumption. This can lead to local hypoxia, which results in the release of proinflammatory cytokines that can induce fibrosis and loss of glomerular function [23-25]. This chain of events can be prevented by SGLT2 inhibitors, thereby demonstrating their nephroprotective effect in diabetes (Fig. 1).
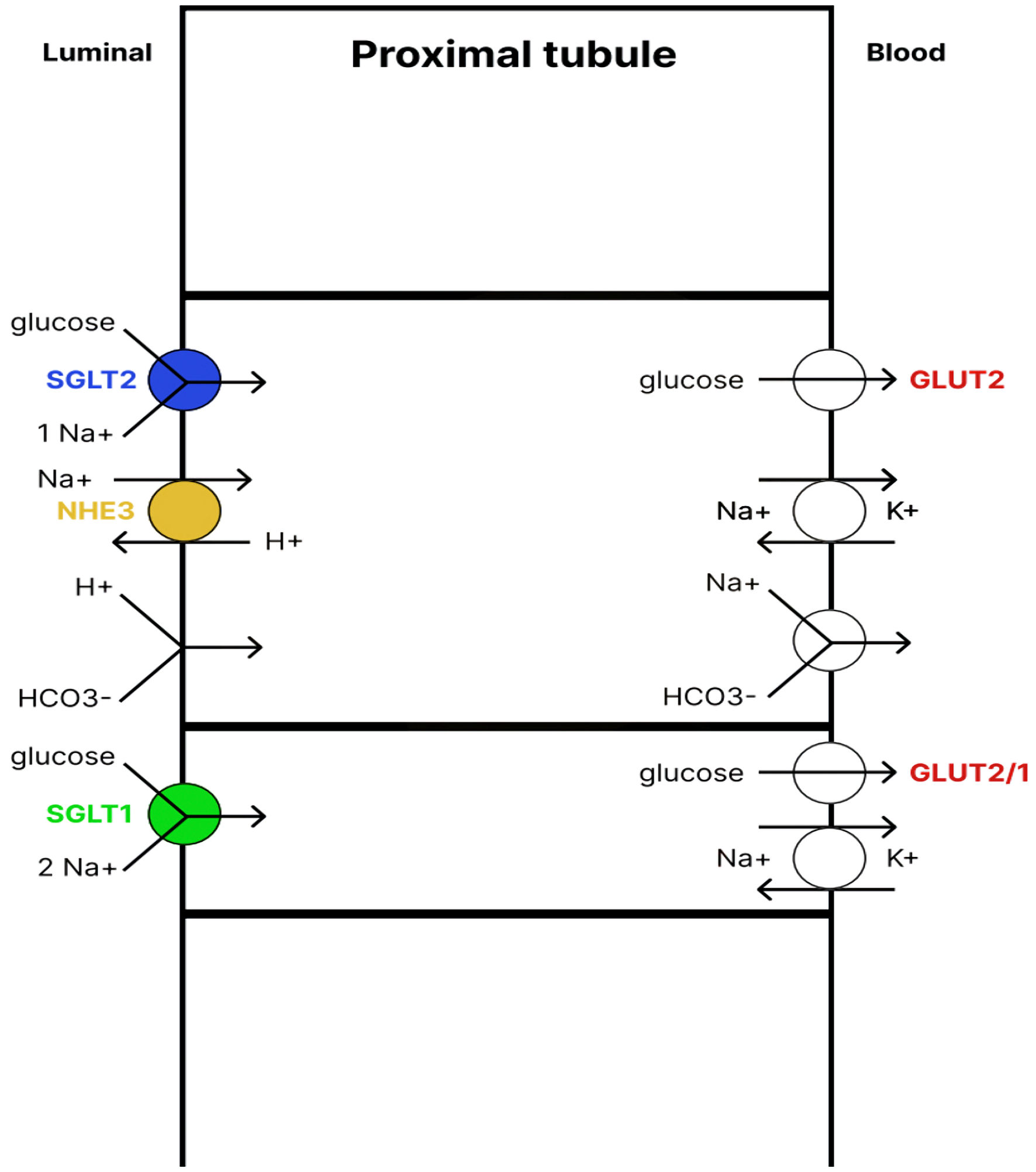 Click for large image | Figure 1. Renal glucose reabsorption in the proximal tubule. SGLT2 cotransporter, located in the apical membrane of the early proximal tubule, absorbs up to 97% of glucose. The glucose is then transported back into the blood with the help of the GLUT2 transporter. The remaining glucose is absorbed by the SGLT1 cotransporter, located in the apical membrane of the late proximal tubule, and then enters the blood with the help of GLUT2/1 transporters. Therefore, under physiological conditions, almost 100% of glucose from the primary urine is reabsorbed. SGLT1/2: sodium-glucose cotransporter1/2; NHE3: sodium-hydrogen exchanger 3; GLUT1/2: glucose transporter1/2. |
Theoretically, inhibiting SGLT2 should significantly reduce renal reabsorption capacity, causing glucosuria of more than 90% of daily glucose filtration. The level of glucosuria is directly proportional to the dose of flozin, but even at the maximum dose, the level of glucose excretion reaches at most about 50%. The paradox arises because inhibition of the SGLT2 cotransporter leads to the SGLT1 cotransporter taking over its role. Physiologically, the SGLT1 cotransporter is responsible for reabsorbing only several grams of glucose flowing through the proximal tubule, but its daily capacity can reach 120 - 140 g of glucose. This lowers the renal threshold to about 140 mg/dL [15, 26].
T-1095 was the first oral inhibitor of the SGLT2 cotransporter. However, due to its lack of selectivity, it also inhibited the SGLT1 cotransporter, resulting in gastrointestinal side effects [27-30]. At present, numerous selective new drugs in this category have been developed, with many more currently in the trial phase. Their application is not restricted to diabetes alone. Clinical trials of dapagliflozin [31-34] and empagliflozin [35-37] have shown significant benefits in patients at high cardiovascular risk. These include the treatment of heart failure, slowing down the progression of albuminuria in diabetic and non-diabetic kidney disease, and slowing down the overall progression of chronic kidney disease [38, 39] (Fig. 2).
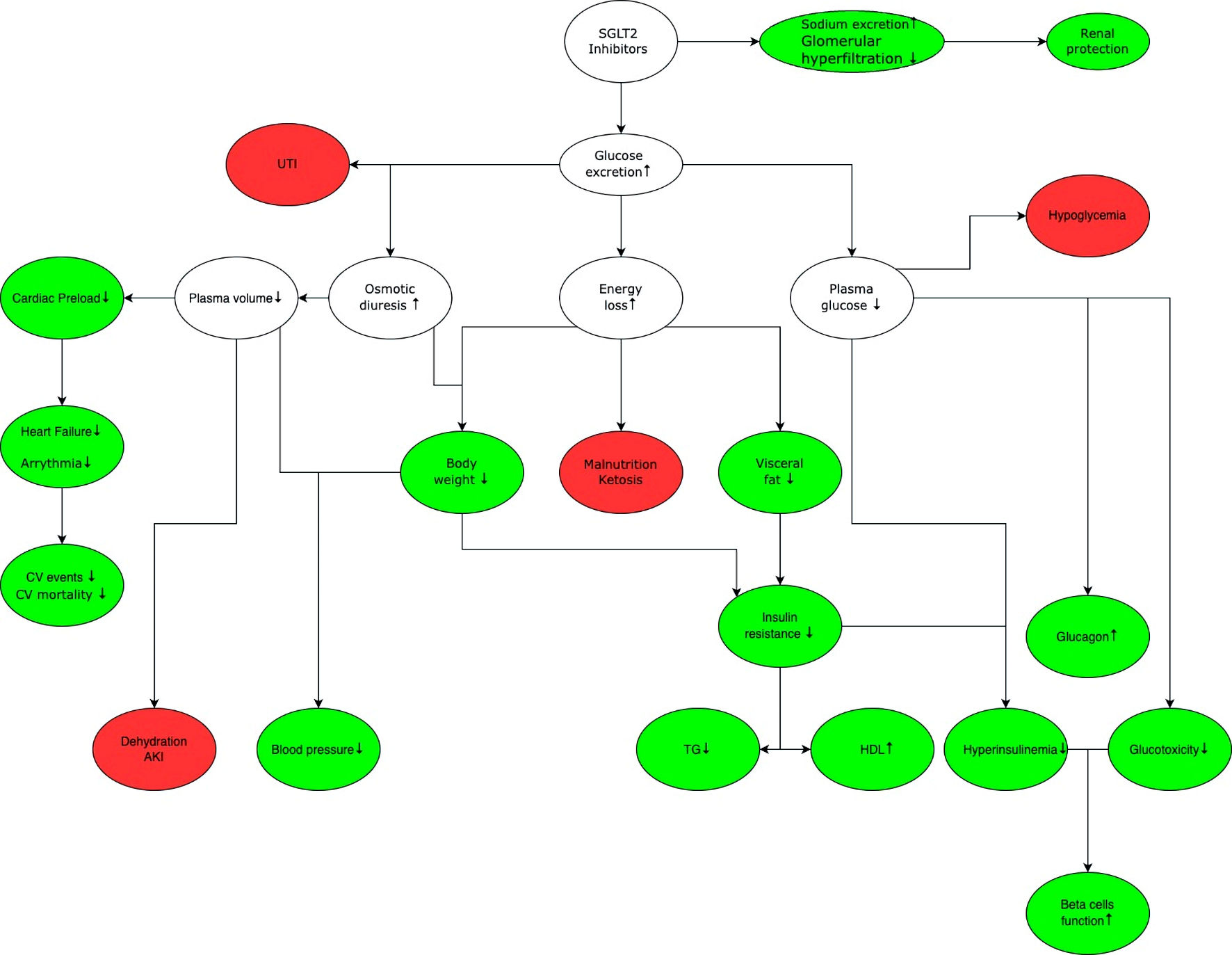 Click for large image | Figure 2. General mechanism of action of SGLT2 inhibitors. The green color marks benefits associated with the use of SGLT2 inhibitors, while white indicates the effects that can lead to benefits and/or adverse effects depending on the clinical situation, red shows potential adverse effects associated with the use of SGLT2 inhibitors. SGLT2 inhibition increases glucose and sodium excretion. Enhanced sodium excretion and decreased renal hyperfiltration delay the progression of chronic kidney disease. Increased glucose excretion leads to osmotic diuresis, which increases the risk of urinary tract infection, and sepsis. Increased diuresis reduces cardiac preload, thereby reducing the risk of cardiovascular events and mortality. In addition, there is a decrease in blood glucose levels, which reduces the need for insulin and glucotoxicity on the vascular endothelium, improving function of pancreatic beta cells. Glucose excretion leads to energy loss and weight loss, which lowers blood pressure and increases insulin sensitivity. It is important to note that while SGLT2 inhibitors do not cause hypoglycemia in monotherapy, they may increase the risk of occurrence of hypoglycemia when used in combination with other drugs that have hypoglycemic effect (e.g., insulin, sulfonylurea derivatives). SGLT2: sodium-glucose cotransporter 2; UTI: urinary tract infection; CV: cardiovascular; AKI: acute kidney injury; TG: triglycerides; HDL: high-density lipoprotein. |
Endothelial dysfunction plays a pivotal role in the pathogenesis of atherosclerosis and is frequently accompanied by elevated oxidative stress and inflammatory response [40]. Correction of hyperglycemia by SGLT2 inhibitors, which results in increased urinary glucose excretion, leads to a reduction in glucotoxicity by decreasing advanced glycation end products (AGE) formation. This alleviates the oxidative stress and inflammatory response [41]. An in vitro study showed that SGLT2 inhibitors provide protection against high glucose-induced mitochondrial dysfunction. In particular, empagliflozin was observed to restore nitrite levels in cultured human umbilical vein endothelial cells during hyperglycemia. Furthermore, SGLT2 inhibitors, as well as antioxidant gene induction with sulforaphane, prevented high-glucose-induced endothelial dysfunction in mouse aortic tissue maintained in hyperglycemic medium [42].
Heart failure pathophysiology in T2DM arises through multiple mechanisms which affect cardiac contractility, relaxation and compliance. Diabetes accelerates the development of coronary artery disease, which may lead to myocardial infarction and structural changes [43]. Hyperactivation of the renin-angiotensin-aldosterone system (RAAS), as described above, contributes to hypertension, myocardial hypertrophy, inflammation, increased cardiac preload and afterload, and fibrosis. These factors inevitably lead to a decline in diastolic function [44]. Chronic hyperglycemia plays a key role in the development of heart failure in T2DM [45]. It results in the formation of non-enzymatic AGE, which negatively affect contractility and relaxation [46]. In physiological conditions, a healthy heart requires a significant amount of energy from a variety of substrates, including glucose and free fatty acids [47]. In hyperglycemic conditions, the ability of the myocardium to obtain energy from glucose is compromised, leading to a switch to fatty acid metabolism. This is less efficient, especially in the presence of increased oxygen demand, and is associated with elevated reactive oxygen species formation. Additionally, fatty acid metabolism affects calcium absorption, which in turn impairs diastolic function [48]. Ketone bodies may be a good alternative as a substrate for energy production. They have been observed to improve the metabolic efficiency of the heart, preventing the formation of reactive oxygen species and reversing ventricular remodeling [49]. Additionally, ketones exhibit anti-inflammatory properties by suppressing inflammasome subunit NLRP3 [50]. This suggests another potential therapeutic mechanism for SGLT2 inhibitors in the treatment and prevention of heart failure, which has been shown to induce euglycemic ketoacidosis.
Adverse effects of SGLT2 inhibitors
Despite the tremendous benefits of SGLT2 inhibitors, these drugs are not without side effects. The direct effects of SGLT2 inhibitors are osmotic diuresis and plasma volume reduction, which are the main causes of adverse effects. These especially affect patients predisposed to genital or urinary tract infections, in most cases mild to moderate [51-55]. A very rare, but potentially fatal adverse effect is Fournier’s gangrene [56]. Euglycemic ketoacidosis has been observed in some cases [57-60]; however, this observation was not reported in the CREDENCE and DAPA-CKD trials.
| Methods and Materials | ▴Top |
The PubMed database was used for the analysis, and the criteria used depended on the number of publications available for a given flozin, resulting in a total of 395 publications that were further reviewed. For enavogliflozin and janagliflozin, the only primary criterion was the presence of the flozin name in the title. This resulted in nine and eight results, respectively. The publications were verified by rejecting off-topic papers, those lacking sufficient data for analysis, and studies conducted on non-human models. Finally, five papers on enavogliflozin and three on janagliflozin were included in this publication.
We applied the same criteria to papers published within the last 5 years to select relevant research on remogliflozin. Initially, 36 results were obtained, but after further verification, only three papers were used for analysis. For bexagliflozin, the criteria were expanded to include publications from the last 5 years and those with “diabetes” in the title. This resulted in 15 potential publications, eight out of which were eventually used in the analysis.
For canagliflozin, empagliflozin, and dapagliflozin, the primary criteria were expanded to include papers from the last 5 years that contain “diabetes” in the title and “HbA1c” in any field. This resulted in 47, 115, and 165 results, respectively. For canagliflozin, we further expanded the criteria to include “body weight” in any field, resulting in 19 papers. After analysis, we were able to include seven of these papers. In the case of empagliflozin and dapagliflozin, the criteria were expanded to include “body weight” and “systolic” content in all fields, resulting in 17 and 22 papers, respectively. After subsequent analysis, six papers were used in both cases. In the end, 38 papers were used to analyze the reduction of hemoglobin A1c (HbA1c), body weight and/or systolic blood pressure by each flozin (Fig. 3).
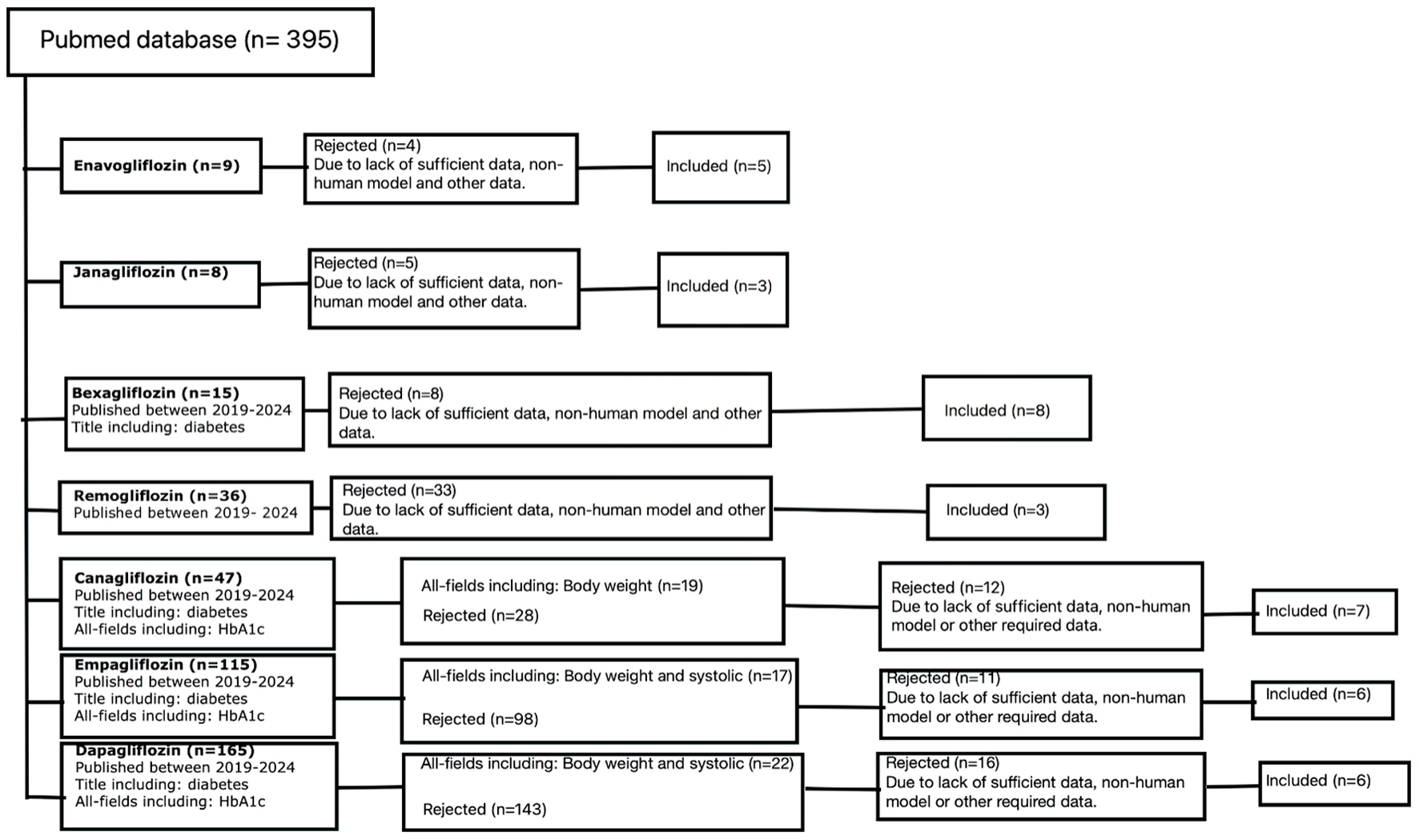 Click for large image | Figure 3. Out of the 395 initially obtained results, 38 were eventually included in this review. The selection process involved scaling up with additional criteria based on the available pool of results for a given flozin, as well as a content analysis that rejected papers which were off topic, did not contain sufficient data, or involved non-human models. N: number of papers. |
| Results | ▴Top |
Enavogliflozin
Enavogliflozin is a selective SGLT2 inhibitor. It is currently in clinical trials in Korea. It has more than 667-fold greater affinity for SGLT2 than for SGLT1 and a linear dose-effect relationship with both oral and intravenous administration [61]. To date, a few studies have been conducted to evaluate the safety and efficacy of enavogliflozin in the treatment of diabetes with good results. Enavogliflozin, like other drugs in this class, reduces HbA1c, body weight and blood pressure in patients (Table 1) [62-66]. Studies comparing HbA1c reductions induced by enavogliflozin and dapagliflozin showed a slight advantage of the former. This makes enavogliflozin likely to be another promising new drug in this group for a wider group of patients [62-66].
 Click to view | Table 1. Effectiveness of Enavogliflozin in Reducing HbA1c, Body Weight, and Systolic Blood Pressure |
Janagliflozin
Janagliflozin is an oral selective SGLT2 inhibitor, chemically developed and patented in China [67], which has demonstrated good efficacy and safety [68-72], including in patients with coexisting cirrhosis and chronic kidney disease [73, 74]. Three studies have been conducted to determine the efficacy of janagliflozin in reducing HbA1c. The results showed concentration decrease after 24 weeks of therapy: -0.58% for the 25 mg and 50 mg dose [70], -0.8% (95% confidence interval (CI): -0.98% to -0.62%) for the 25 mg dose, and -0.88% (95% CI: -1.06% to -0.7%) for the 50 mg dose [71], and -0.78% for the 25 mg dose and -0.93% for the 50 mg dose [72], respectively. Statistically significant reductions were observed in fasting plasma glucose, body weight, and systolic blood pressure in all of these studies. Additionally, increases in high-density lipoprotein (HDL) and insulin sensitivity were observed compared to placebo. The trends in improvement of these variables were sustained during the 28-week extension period.
Bexagliflozin
Bexagliflozin is a novel and highly potent SGLT2 inhibitor with more than 2,435-fold selectivity for SGLT2 over SGLT1 [75]. The FDA granted the first approval of bexagliflozin on January 20, 2023, for usage as an adjunctive treatment alongside lifestyle changes and exercise in T2DM. Studies have shown that bexagliflozin can be used effectively (Table 2) [76-83] and safely, including patients with stage 3a and 3b chronic kidney disease [76].
 Click to view | Table 2. Effectiveness of Bexagliflozin in Reducing HbA1c, Body Weight, and Systolic Blood Pressure |
Remogliflozin
Remogliflozin is a SGLT2 inhibitor that was introduced in India in 2019 as a antidiabetic agent [84]. Additionally, it has been found to be useful in treating non-alcoholic steatohepatitis (NASH). A meta-analysis (Dutta et al [85]) of 535 subjects from three randomized clinical trials demonstrated that remogliflozin is as effective as pioglitazone and dapagliflozin (Table 3) [85-87], with similar rates of adverse effects. In contrast, the use of remogliflozin was associated with a greater reduction in weight, with a mean difference of -2.79kg (95% CI: -2.51 to -3.07 kg). Two studies were conducted to investigate the safety and tolerability of remogliflozin at doses of 500 mg and 750 mg twice a day (bis in die (BID)), which is higher than the typical dose of 100 mg BID. Both studies also included metformin at a daily dose of 2,000 mg. The results confirmed the safety and efficacy of this regimen [88, 89].
 Click to view | Table 3. The Effectiveness of Remogliflozin in Reducing HbA1c, Body Weight, and/or Systolic Blood Pressure |
However, another study suggested a potential link between remogliflozin therapy and cases of acute kidney injury [90].
Canagliflozin
Canagliflozin is the first approved SGLT2 inhibitor in the USA [91, 92]. It is indicated for the treatment of T2DM in combination with exercise and diet. It is known to reduce the risk of major cardiac events, end-stage renal disease and hospitalization for heart failure in patients with T2DM and chronic kidney disease. The CREDENCE trial aimed to assess the impact of canagliflozin on renal outcomes, including nephropathy in T2DM. The primary outcome was to evaluate the risk of end-stage renal disease, doubling of serum creatinine, or death from renal or cardiovascular causes. The group treated with 100 mg canagliflozin daily showed a significant risk reduction compared to the placebo group (hazard ratio (HR): 0.70; 95% CI: 0.59 to 0.82; P < 0.00001). The study also found a 32% risk reduction of end-stage renal disease (HR: 0.68; 95% CI: 0.54 to 0.86; P = 0.002). Similar observations were made for the risk of death from cardiovascular causes, myocardial infarction or stroke (HR: 0.80; 95% CI: 0.67 to 0.95; P = 0.01) and hospitalization for heart failure (HR: 0.61; 95% CI: 0.47 to 0.80; P < 0.001) [93].
The CANVAS trial also demonstrated favorable results for canagliflozin, with a reduction in the combined risk of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke by 14% (HR: 0.86; 95% CI: 0.75 to 0.97; P < 0.001 for noninferiority; P = 0.02 for superiority) in the canagliflozin group compared to the placebo group. Furthermore, canagliflozin caused HbA1c change by -0.58% (95% CI: -0.61% to -0.56%), body weight by -1.60 kg (95% CI: -1.70 kg to -1.51 kg) and systolic blood pressure by -3.93 mm Hg (95% CI: -4.30 to -3.56 mm Hg) [94].
The following table summarizes the reductions in HbA1c, weight, and/or systolic blood pressure obtained in other canagliflozin trials (Table 4) [93, 95-100].
 Click to view | Table 4. Effectiveness of Canagliflozin in Reducing HbA1c, Body Weight and/or Systolic Blood Pressure |
Empagliflozin
Empagliflozin is an oral SGLT2 inhibitor that gained approval in the USA in 2014. It has the highest selectivity to SGLT2 among flozins, more than 2,500-fold over SGLT1 [101]. Numerous studies have demonstrated the high efficacy of empagliflozin in reducing HbA1c, body weight, and systolic blood pressure (Table 5) [102-107]. Like canagliflozin, empagliflozin is also used to treat heart failure, in addition to treating T2DM.
 Click to view | Table 5. The Effectiveness of Empagliflozin in Reducing HbA1c, Body Weight and SBP |
The EMPA-REG OUTCOME trial confirmed the efficacy of empagliflozin in treating heart failure. The primary outcome of the study was to evaluate the composite risk of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke in a group of patients treated with empagliflozin at doses of 10 mg or 25 mg compared with placebo; a 14% risk reduction was observed in the empagliflozin-treated groups (HR: 0.86; 95% CI: 0.74 to 0.99; P = 0.04 for superiority) [108].
Dapagliflozin
Dapagliflozin was approved by the EMA in 2012, making it the first SGLT2 inhibitor to receive regulatory approval anywhere. Its primary indication is the treatment of T2DM alongside with diet and exercise. Dapagliflozin was proven to reduce the risk of hospitalization for heart failure and chronic kidney disease [33, 109, 110].
The DAPA-CKD trial involved 2,152 participants and its primary outcome was defined as a sustained decline in glomerular filtration rate (GFR) by at least 50%, end-stage renal disease, or death from renal or cardiovascular causes. The results showed a significant risk reduction by 44% (HR: 0.56; 95% CI: 0.45 to 0.68; P < 0.001). The study also revealed reduced risk of death from cardiovascular causes or hospitalization for heart failure (HR: 0.71; 95% CI: 0.55 to 0.92; P = 0.009) [111]. Furthermore, other studies also support the effectiveness of dapagliflozin in reducing HbA1c levels, body weight, and systolic blood pressure (Table 6) [63, 112-116].
 Click to view | Table 6. The Effectiveness of Dapagliflozin in Reducing HbA1c, Body Weight, and Systolic Blood Pressure |
| Conclusions | ▴Top |
All the studies presented in this review demonstrate the high efficacy of SGLT2 inhibitors in decreasing not only HbA1c, but also body weight and systolic blood pressure. These SGLT 2 inhibitors have beneficial effects on reducing cardiovascular risk and progression of chronic kidney disease. This outcome is not limited to relieving glucotoxicity on the vascular endothelium by reducing glycemia. Flozins also prevent glomerular hyperfiltration and damage, thus exerting their nephroprotective effect. Indirect inhibition of the renin-angiotensin-aldosterone system explains the cardioprotective activity of this group of drugs.
The most common adverse effect of SGLT2 inhibitors is an increased risk of urogenital infections due to glucosuria and osmotic diuresis. Euglycemic ketoacidosis or acute kidney injury are much less common and have only been reported in a few cases, such as with remogliflozin. However, numerous publications have demonstrated the safety of SGLT2 inhibitors.
Type 2 diabetes is often associated with weight gain and hypertension, therefore SGLT2 inhibitors should be one of the first-line agents in the treatment of this disease, especially in patients with comorbidities such as heart failure or kidney disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Mateusz Nieczyporuk: conceptualization, design, methodology, analysis, writing, investigation and project. Pawel Tyrna: writing, editing, revision, graphics. Tomasz Cader, Aleksandra Sikora and Szymon Staneta: supervision, review, writing and editing. Each author read and approved the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6(6):850-867.
doi pubmed pmc - Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur J Prev Cardiol. 2019;26(2_suppl):7-14.
doi pubmed - Singer ME, Dorrance KA, Oxenreiter MM, Yan KR, Close KL. The type 2 diabetes 'modern preventable pandemic' and replicable lessons from the COVID-19 crisis. Prev Med Rep. 2022;25:101636.
doi pubmed pmc - Saisho Y. SGLT2 inhibitors: the star in the treatment of type 2 diabetes? Diseases. 2020;8(2):14.
doi pubmed pmc - Evans M, Morgan AR, Whyte MB, Hanif W, Bain SC, Kalra PA, Davies S, et al. New therapeutic horizons in chronic kidney disease: the role of SGLT2 inhibitors in clinical practice. Drugs. 2022;82(2):97-108.
doi pubmed - Talha KM, Anker SD, Butler J. SGLT-2 inhibitors in heart failure: a review of current evidence. Int J Heart Fail. 2023;5(2):82-90.
doi pubmed pmc - Skrabic R, Kumric M, Vrdoljak J, Rusic D, Skrabic I, Vilovic M, Martinovic D, et al. SGLT2 inhibitors in chronic kidney disease: from mechanisms to clinical practice. Biomedicines. 2022;10(10):2458.
doi pubmed pmc - Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255-270.
doi pubmed - Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010;5(1):133-141.
doi pubmed - Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12(2):78-89.
doi pubmed pmc - Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, Feder JN. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010;1(2):57-92.
doi pubmed pmc - Perry RJ, Shulman GI. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem. 2020;295(42):14379-14390.
doi pubmed pmc - Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75(12):1272-1277.
doi pubmed - Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355-366.
doi pubmed pmc - Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62(10):3324-3328.
doi pubmed pmc - Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, et al. MAP17 is a necessary activator of renal Na+/Glucose cotransporter SGLT2. J Am Soc Nephrol. 2017;28(1):85-93.
doi pubmed pmc - DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17(5):319-334.
doi pubmed - Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16(6):317-336.
doi pubmed pmc - Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304(2):F156-167.
doi pubmed pmc - Ren Y, Arima S, Carretero OA, Ito S. Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int. 2002;61(1):169-176.
doi pubmed - Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol. 2012;74:351-375.
doi pubmed pmc - Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol. 2009;111(3):p30-38.
doi pubmed pmc - Evans RG, Harrop GK, Ngo JP, Ow CP, O'Connor PM. Basal renal O2 consumption and the efficiency of O2 utilization for Na+ reabsorption. Am J Physiol Renal Physiol. 2014;306(5):F551-560.
doi pubmed - Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887-899.
doi pubmed - Takiyama Y, Haneda M. Hypoxia in diabetic kidneys. Biomed Res Int. 2014;2014:837421.
doi pubmed pmc - Osaki A, Okada S, Saito T, Yamada E, Ono K, Niijima Y, Hoshi H, et al. Renal threshold for glucose reabsorption predicts diabetes improvement by sodium-glucose cotransporter 2 inhibitor therapy. J Diabetes Investig. 2016;7(5):751-754.
doi pubmed pmc - Ueta K, Yoneda H, Oku A, Nishiyama S, Saito A, Arakawa K. Reduction of renal transport maximum for glucose by inhibition of NA(+)-glucose cotransporter suppresses blood glucose elevation in dogs. Biol Pharm Bull. 2006;29(1):114-118.
doi pubmed - Oku A, Ueta K, Arakawa K, Kano-Ishihara T, Matsumoto M, Adachi T, Yasuda K, et al. Antihyperglycemic effect of T-1095 via inhibition of renal Na+-glucose cotransporters in streptozotocin-induced diabetic rats. Biol Pharm Bull. 2000;23(12):1434-1437.
doi pubmed - Oku A, Ueta K, Arakawa K, Ishihara T, Nawano M, Kuronuma Y, Matsumoto M, et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes. 1999;48(9):1794-1800.
doi pubmed - Ueta K, Ishihara T, Matsumoto Y, Oku A, Nawano M, Fujita T, Saito A, et al. Long-term treatment with the Na+-glucose cotransporter inhibitor T-1095 causes sustained improvement in hyperglycemia and prevents diabetic neuropathy in Goto-Kakizaki Rats. Life Sci. 2005;76(23):2655-2668.
doi pubmed - Saleem F. Dapagliflozin: cardiovascular safety and benefits in type 2 diabetes Mellitus. Cureus. 2017;9(10):e1751.
doi pubmed pmc - Nazer R, Albratty M, Aldhahi MI, Alqurashy M, Halawi MA, Albarrati A. Effect of dapagliflozin on exercise capacity and cardiovascular risk in patients with heart failure. Healthcare (Basel). 2022;10(11):2133.
doi pubmed pmc - Ali AE, Mazroua MS, ElSaban M, Najam N, Kothari AS, Mansoor T, Amal T, et al. Effect of dapagliflozin in patients with heart failure: a systematic review and meta-analysis. Glob Heart. 2023;18(1):45.
doi pubmed pmc - Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
doi pubmed - Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461.
doi pubmed - Frampton JE. Empagliflozin: a review in symptomatic chronic heart failure. Drugs. 2022;82(16):1591-1602.
doi pubmed - Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568-574.
doi pubmed pmc - Herrington WG, Baigent C, Haynes R. Empagliflozin in patients with chronic kidney disease. Reply. N Engl J Med. 2023;388(24):2301-2302.
doi pubmed - Colbert GB, Madariaga HM, Gaddy A, Elrggal ME, Lerma EV. Empagliflozin in adults with chronic kidney disease (CKD): current evidence and place in therapy. Ther Clin Risk Manag. 2023;19:133-142.
doi pubmed pmc - Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115-126.
doi pubmed - Lastra G, Manrique C. Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm Mol Biol Clin Investig. 2015;22(1):19-26.
doi pubmed - El-Daly M, Pulakazhi Venu VK, Saifeddine M, Mihara K, Kang S, Fedak PWM, Alston LA, et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul Pharmacol. 2018;109:56-71.
doi pubmed - Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294-e324.
doi pubmed - Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J Diabetes. 2015;6(7):943-960.
doi pubmed pmc - Tate M, Deo M, Cao AH, Hood SG, Huynh K, Kiriazis H, Du XJ, et al. Insulin replacement limits progression of diabetic cardiomyopathy in the low-dose streptozotocin-induced diabetic rat. Diab Vasc Dis Res. 2017;14(5):423-433.
doi pubmed - Qin CX, Sleaby R, Davidoff AJ, Bell JR, De Blasio MJ, Delbridge LM, Chatham JC, et al. Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications. Pharmacol Res. 2017;116:45-56.
doi pubmed - Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133(8):698-705.
doi pubmed pmc - Sowton AP, Griffin JL, Murray AJ. Metabolic profiling of the diabetic heart: toward a richer picture. Front Physiol. 2019;10:639.
doi pubmed pmc - Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation. 2019;139(18):2129-2141.
doi pubmed pmc - Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263-269.
doi pubmed pmc - Yang T, Zhou Y, Cui Y. Urinary tract infections and genital mycotic infections associated with SGLT-2 inhibitors: an analysis of the FDA Adverse Event Reporting System. Expert Opin Drug Saf. 2023:1-6.
doi pubmed - Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503-514.
doi pubmed - Katsuhara Y, Ogawa T. Acute renal failure, ketoacidosis, and urogenital tract infections with SGLT2 inhibitors: signal detection using a japanese spontaneous reporting database. Clin Drug Investig. 2020;40(7):645-652.
doi pubmed - Lega IC, Bronskill SE, Campitelli MA, Guan J, Stall NM, Lam K, McCarthy LM, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21(11):2394-2404.
doi pubmed - Tada K, Gosho M. Increased risk of urinary tract infection and pyelonephritis under concomitant use of sodium-dependent glucose cotransporter 2 inhibitors with antidiabetic, antidyslipidemic, and antihypertensive drugs: An observational study. Fundam Clin Pharmacol. 2022;36(6):1106-1114.
doi pubmed - Tran BA, Updike WH, Bullers K, Serag-Bolos E. Sodium-glucose cotransporter 2 inhibitor use associated with fournier's gangrene: a review of case reports and spontaneous post-marketing cases. Clin Diabetes. 2022;40(1):78-86.
doi pubmed pmc - Kawahara J, Kaku B, Yagi K, Kitagawa N, Yokoyama M, Wakabayashi Y, Senda S, et al. Life-threatening coronary vasospasm in patients with type 2 diabetes with SGLT2 inhibitor-induced euglycemic ketoacidosis: a report of two consecutive cases. Diabetol Int. 2024;15(1):135-140.
doi pubmed pmc - Chow E, Clement S, Garg R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diabetes Res Care. 2023;11(5):e003666.
doi pubmed pmc - Klinkner G, Steingraber-Pharr M. Euglycemic diabetic ketoacidosis associated with SGLT2 inhibitor therapy: a case report. AACN Adv Crit Care. 2023;34(1):27-32.
doi pubmed - Torre A, Bisogno N, Botta C, Caiazza A, D'Angelo F, Del Giudice L, Fiorentini P, et al. Treatment of a severe form of euglycemic ketoacidosis in a patient treated with SGLT-2 inhibitors with the aid of somatostatin. G Ital Nefrol. 2023;40(4):2023-vol4.
pubmed - Pang M, Jeon SY, Choi MK, Jeon JH, Ji HY, Choi JS, Song IS. Pharmacokinetics and Tissue Distribution of Enavogliflozin in Mice and Rats. Pharmaceutics. 2022;14(6):1210.
doi pubmed pmc - Dutta D, Harish BG, Anne B, Nagendra L. Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: a systematic review and meta-analysis. Diabetes Metab Syndr. 2023;17(8):102816.
doi pubmed - Han KA, Kim YH, Kim DM, Lee BW, Chon S, Sohn TS, Jeong IK, et al. Efficacy and safety of enavogliflozin versus dapagliflozin as add-on to metformin in patients with type 2 diabetes mellitus: a 24-week, double-blind, randomized trial. Diabetes Metab J. 2023;47(6):796-807.
doi pubmed pmc - Kim KS, Han KA, Kim TN, Park CY, Park JH, Kim SY, Kim YH, et al. Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study. Diabetes Metab. 2023;49(4):101440.
doi pubmed - Kwak SH, Han KA, Kim KS, Yu JM, Kim E, Won JC, Kang JG, et al. Efficacy and safety of enavogliflozin, a novel SGLT2 inhibitor, in Korean people with type 2 diabetes: A 24-week, multicentre, randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes Metab. 2023;25(7):1865-1873.
doi pubmed - Yang YS, Min KW, Park SO, Kim KS, Yu JM, Hong EG, Cho SR, et al. Efficacy and safety of monotherapy with enavogliflozin in Korean patients with type 2 diabetes mellitus: Results of a 12-week, multicentre, randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Obes Metab. 2023;25(8):2096-2104.
doi pubmed - Song L, Liu Y, Yao X, Liu H, Chen B, Ma X, Zhou H, et al. Development of an HPLC-MS/MS method to determine janagliflozin in human plasma and urine: application in clinical study. Bioanalysis. 2018;10(17):1439-1454.
doi pubmed - Li X, Zhu X, Liu J, Li Q, Zhang H, Li C, Wu M, et al. Pharmacokinetics, pharmacodynamics and tolerability of single and multiple doses of janagliflozin, a sodium-glucose co-transporter-2 inhibitor, in Chinese people with type 2 diabetes mellitus. Diabetes Obes Metab. 2020;22(12):2316-2324.
doi pubmed - Song L, Yao X, Liu Y, Zhong W, Jiang J, Liu H, Zhou H, et al. Translational prediction of first-in-human pharmacokinetics and pharmacodynamics of janagliflozin, a selective SGLT2 inhibitor, using allometric scaling, dedrick and PK/PD modeling methods. Eur J Pharm Sci. 2020;147:105281.
doi pubmed - Gao L, Cheng Z, Su B, Su X, Song W, Guo Y, Liao L, et al. Efficacy and safety of janagliflozin as add-on therapy to metformin in Chinese patients with type 2 diabetes inadequately controlled with metformin alone: A multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Obes Metab. 2023;25(3):785-795.
doi pubmed - Ji L, Jiang X, Hao Q, Cheng Z, Wang K, Pang S, Liu M, et al. Efficacy and safety of janagliflozin monotherapy in Chinese patients with type 2 diabetes mellitus inadequately controlled on diet and exercise: A multicentre, randomized, double-blind, placebo-controlled, Phase 3 trial. Diabetes Obes Metab. 2023;25(5):1229-1240.
doi pubmed - Song L, Wang X, Sun J, Hu X, Li H, Hu P, Liu D. A model-informed approach to accelerate the clinical development of janagliflozin, an innovative SGLT2 inhibitor. Clin Pharmacokinet. 2023;62(3):505-518.
doi pubmed - Zhao H, Wei Y, He K, Zhao X, Mu H, Wen Q. Prediction of janagliflozin pharmacokinetics in type 2 diabetes mellitus patients with liver cirrhosis or renal impairment using a physiologically based pharmacokinetic model. Eur J Pharm Sci. 2022;179:106298.
doi pubmed - Zhao H, Zhao Z, He K, Mi N, Lou K, Dong X, Zhang W, et al. Pharmacokinetics, pharmacodynamics and safety of janagliflozin in Chinese type 2 diabetes mellitus patients with renal impairment. Clin Pharmacokinet. 2023;62(8):1093-1103.
doi pubmed - McMurray JJV, Solomon SD, Lock JP, Massaro JM, Zhu F, Zhou W, Skali H, et al. Meta-analysis of risk of major adverse cardiovascular events in adults with type 2 diabetes treated with bexagliflozin. Diabetes Obes Metab. 2024;26(3):971-979.
doi pubmed - Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, Trescoli C, et al. Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am J Kidney Dis. 2019;74(3):328-337.
doi pubmed pmc - Halvorsen YC, Walford GA, Massaro J, Aftring RP, Freeman MW. A 96-week, multinational, randomized, double-blind, parallel-group, clinical trial evaluating the safety and effectiveness of bexagliflozin as a monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2496-2504.
doi pubmed - Halvorsen YD, Lock JP, Zhou W, Zhu F, Freeman MW. A 24-week, randomized, double-blind, active-controlled clinical trial comparing bexagliflozin with sitagliptin as an adjunct to metformin for the treatment of type 2 diabetes in adults. Diabetes Obes Metab. 2019;21(10):2248-2256.
doi pubmed - Halvorsen YD, Walford G, Thurber T, Russell H, Massaro M, Freeman MW. A 12-week, randomized, double-blind, placebo-controlled, four-arm dose-finding phase 2 study evaluating bexagliflozin as monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. 2020;22(4):566-573.
doi pubmed - Dholariya S, Dutta S, Singh R, Parchwani D, Sonagra A, Kaliya M. Bexagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, for improvement of glycemia in type 2 diabetes mellitus: a systematic review and meta-analysis. Expert Opin Pharmacother. 2023;24(18):2187-2198.
doi pubmed - Halvorsen YD, Conery AL, Lock JP, Zhou W, Freeman MW. Bexagliflozin as an adjunct to metformin for the treatment of type 2 diabetes in adults: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2023;25(10):2954-2962.
doi pubmed - Halvorsen YD, Lock JP, Frias JP, Tinahones FJ, Dahl D, Conery AL, Freeman MW. A 96-week, double-blind, randomized controlled trial comparing bexagliflozin to glimepiride as an adjunct to metformin for the treatment of type 2 diabetes in adults. Diabetes Obes Metab. 2023;25(1):293-301.
doi pubmed - Pasqualotto E, Figueiredo Watanabe JM, Gewehr DM, da Silva Maintinguer R, van de Sande-Lee S, de Araujo GN, Leal FS, et al. Efficacy and safety of bexagliflozin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes Metab. 2023;25(7):1794-1802.
doi pubmed - Markham A. Remogliflozin Etabonate: First Global Approval. Drugs. 2019;79(10):1157-1161.
doi pubmed - Dutta D, Jindal R, Mehta D, Khandelwal D, Sharma M. Efficacy and safety of novel sodium glucose cotransporter-2 inhibitor remogliflozin in the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15(6):102315.
doi pubmed - Dharmalingam M, Aravind SR, Thacker H, Paramesh S, Mohan B, Chawla M, Asirvatham A, et al. Efficacy and safety of remogliflozin etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: a 24-week, randomized, double-blind, active-controlled trial. Drugs. 2020;80(6):587-600.
doi pubmed pmc - Khaladkar K, Mohan B, Khaladkar K, Suryawanshi S, Barkatestrong/Strong H. Efficacy and safety of a fixed dose combination of remogliflozin etabonate and vildagliptin in patients with type-2 diabetes mellitus: a randomized, active-controlled, double-blind, phase III study. J Assoc Physicians India. 2022;70(4):11-12.
pubmed - Sykes AP, O'Connor-Semmes R, Dobbins R, Dorey DJ, Lorimer JD, Walker S, Wilkison WO, et al. Randomized trial showing efficacy and safety of twice-daily remogliflozin etabonate for the treatment of type 2 diabetes. Diabetes Obes Metab. 2015;17(1):94-97.
doi pubmed - Dobbins R, Hussey EK, O'Connor-Semmes R, Andrews S, Tao W, Wilkison WO, Cheatham B, et al. Assessment of safety and tolerability of remogliflozin etabonate (GSK189075) when administered with total daily dose of 2000 mg of metformin. BMC Pharmacol Toxicol. 2021;22(1):34.
doi pubmed pmc - Jain R, Bhavatharini N, Saravanan T, Seshiah V, Jain N, 3rd. Use of sodium-glucose transport protein 2 (SGLT2) inhibitor remogliflozin and possibility of acute kidney injury in type-2 diabetes. Cureus. 2022;14(12):e32573.
doi pubmed pmc - Kaushal S, Singh H, Thangaraju P, Singh J. Canagliflozin: A novel SGLT2 inhibitor for type 2 diabetes mellitus. N Am J Med Sci. 2014;6(3):107-113.
doi pubmed pmc - Elkinson S, Scott LJ. Canagliflozin: first global approval. Drugs. 2013;73(9):979-988.
doi pubmed - Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
doi pubmed - Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
doi pubmed - Bhosle D, Quazi Z, Chavan S, Shaikh H. Efficacy and safety of canagliflozin in patients with type II diabetes mellitus inadequately controlled on triple drug therapy. J Assoc Physicians India. 2019;67(10):36-38.
pubmed - Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, Farrell K, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21(4):812-821.
doi pubmed - Woo V, Bell A, Clement M, Noronha L, Tsoukas MA, Camacho F, Traina S, et al. CANadian CAnagliflozin REgistry: Effectiveness and safety of canagliflozin in the treatment of type 2 diabetes mellitus in Canadian clinical practice. Diabetes Obes Metab. 2019;21(3):691-699.
doi pubmed pmc - Ali AM, Martinez R, Al-Jobori H, Adams J, Triplitt C, DeFronzo R, Cersosimo E, et al. Combination therapy with canagliflozin plus liraglutide exerts additive effect on weight loss, but not on HbA(1c), in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1234-1241.
doi pubmed pmc - Kadowaki T, Inagaki N, Watada H, Sasaki K, Mori-Anai K, Iwasaki T, Teranishi T. Real-world evidence of treatment with teneligliptin/canagliflozin combination tablets for type 2 diabetes mellitus: a post-marketing surveillance in Japan. Adv Ther. 2022;39(4):1642-1658.
doi pubmed pmc - Gorgojo-Martinez JJ, Ferreira-Ocampo PJ, Galdon Sanz-Pastor A, Cardenas-Salas J, Anton-Bravo T, Brito-Sanfiel M, Almodovar-Ruiz F. Effectiveness and tolerability of the intensification of canagliflozin dose from 100 mg to 300 mg daily in patients with type 2 diabetes in real life: the INTENSIFY study. J Clin Med. 2023;12(13):4248.
doi pubmed pmc - Anker SD, Butler J. Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle. ESC Heart Fail. 2018;5(4):549-551.
doi pubmed pmc - de Boer RA, Nunez J, Kozlovski P, Wang Y, Proot P, Keefe D. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin vs placebo or empagliflozin in patients with type 2 diabetes and heart failure. Br J Clin Pharmacol. 2020;86(7):1346-1356.
doi pubmed pmc - Gupta A, Malhotra P, Jamwal V, Khalse M. A retrospective analysis of fixed combination of empagliflozin and linagliptin in addition to the existing treatment for its clinical effectiveness in adults with type 2 diabetes: a real-world clinical experience. J Assoc Physicians India. 2021;69(7):11-12.
pubmed - Inzucchi SE, Davies MJ, Khunti K, Trivedi P, George JT, Zwiener I, Johansen OE, et al. Empagliflozin treatment effects across categories of baseline HbA1c, body weight and blood pressure as an add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2021;23(2):425-433.
doi pubmed pmc - Liu SC, Lee CC, Chuang SM, Sun FJ, Zeng YH. Comparison of efficacy and safety of empagliflozin vs linagliptin added to premixed insulin in patients with uncontrolled type 2 diabetes: A randomized, open-label study. Diabetes Metab. 2021;47(3):101184.
doi pubmed - Althobaiti FM, Alsanosi SM, Falemban AH, Alzahrani AR, Fataha SA, Salih SO, Alrumaih AM, et al. Efficacy and safety of empagliflozin in type 2 diabetes mellitus saudi patients as add-on to antidiabetic therapy: a prospective, open-label, observational study. J Clin Med. 2022;11(16):4769.
doi pubmed pmc - Khan A, Khan IA, Abidi H, Ahmed M. Comparison of empagliflozin and vildagliptin for efficacy and safety in type 2 diabetes mellitus in the Pakistani population. Front Endocrinol (Lausanne). 2022;13:926633.
doi pubmed pmc - Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
doi pubmed - McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
doi pubmed - Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089-1098.
doi pubmed - Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446.
doi pubmed - McGurnaghan SJ, Brierley L, Caparrotta TM, McKeigue PM, Blackbourn LAK, Wild SH, Leese GP, et al. The effect of dapagliflozin on glycaemic control and other cardiovascular disease risk factors in type 2 diabetes mellitus: a real-world observational study. Diabetologia. 2019;62(4):621-632.
doi pubmed - Wilding JPH, Rigney U, Blak BT, Nolan ST, Fenici P, Medina J. Glycaemic, weight, and blood pressure changes associated with early versus later treatment intensification with dapagliflozin in United Kingdom primary care patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;155:107791.
doi pubmed - Chen JF, Peng YS, Chen CS, Tseng CH, Chen PC, Lee TI, Lu YC, et al. Use and effectiveness of dapagliflozin in patients with type 2 diabetes mellitus: a multicenter retrospective study in Taiwan. PeerJ. 2020;8:e9998.
doi pubmed pmc - Frias JP, Gonzalez-Galvez G, Johnsson E, Maaske J, Testa MA, Simonson DC, Dronamraju N, et al. Efficacy and safety of dual add-on therapy with dapagliflozin plus saxagliptin versus glimepiride in patients with poorly controlled type 2 diabetes on a stable dose of metformin: Results from a 52-week, randomized, active-controlled trial. Diabetes Obes Metab. 2020;22(7):1083-1093.
doi pubmed pmc - Sethi B, Sahay R, Tiwaskar M, Negalur V, Dhediya R, Gaurav K, Rathod R, et al. Effectiveness of dapagliflozin as add-on to metformin with or without other oral antidiabetic drugs in type 2 diabetes mellitus: a multicentre, retrospective, real-world database study. Drugs Real World Outcomes. 2024;11(1):81-90.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
