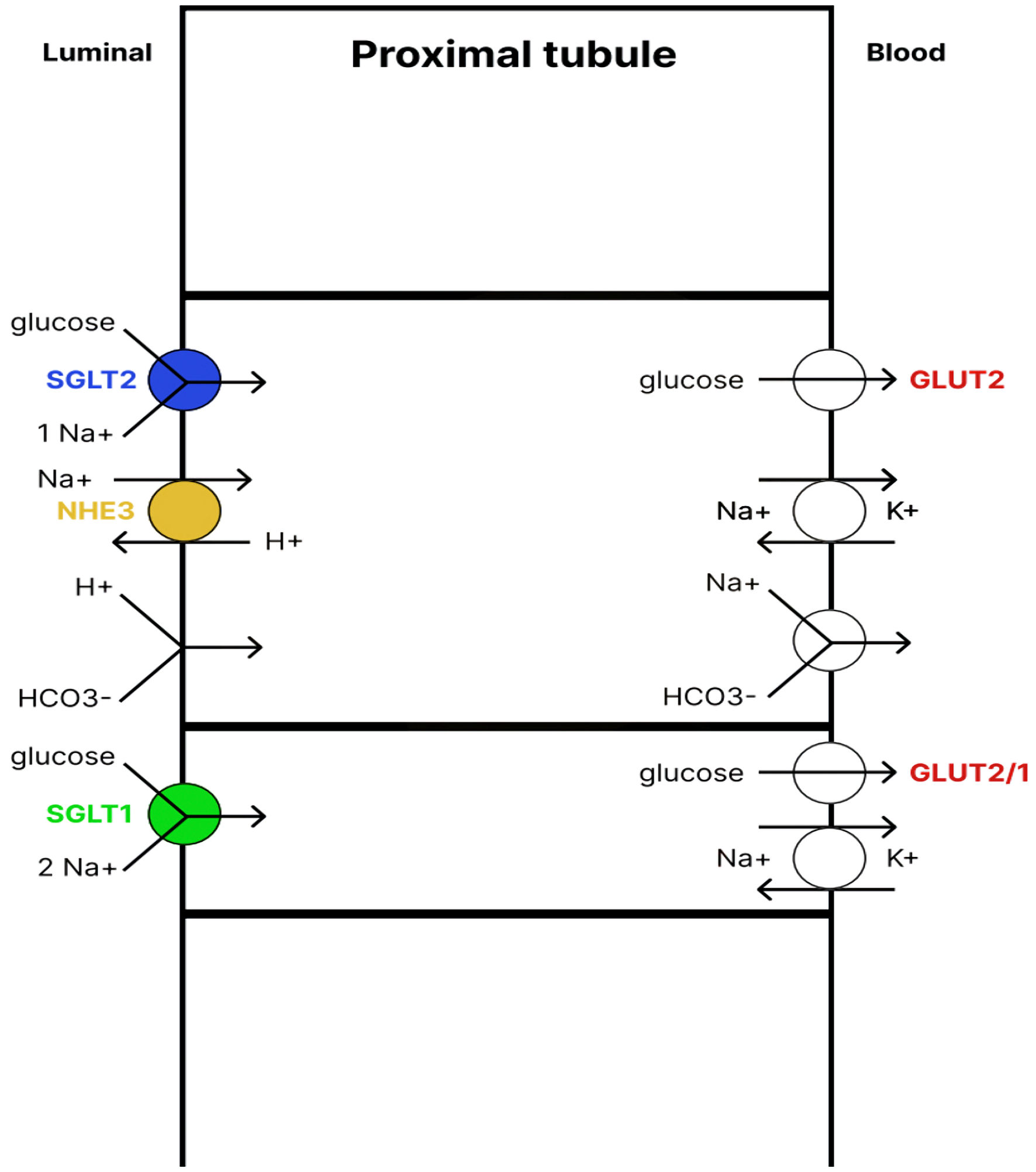
Figure 1. Renal glucose reabsorption in the proximal tubule. SGLT2 cotransporter, located in the apical membrane of the early proximal tubule, absorbs up to 97% of glucose. The glucose is then transported back into the blood with the help of the GLUT2 transporter. The remaining glucose is absorbed by the SGLT1 cotransporter, located in the apical membrane of the late proximal tubule, and then enters the blood with the help of GLUT2/1 transporters. Therefore, under physiological conditions, almost 100% of glucose from the primary urine is reabsorbed. SGLT1/2: sodium-glucose cotransporter1/2; NHE3: sodium-hydrogen exchanger 3; GLUT1/2: glucose transporter1/2.
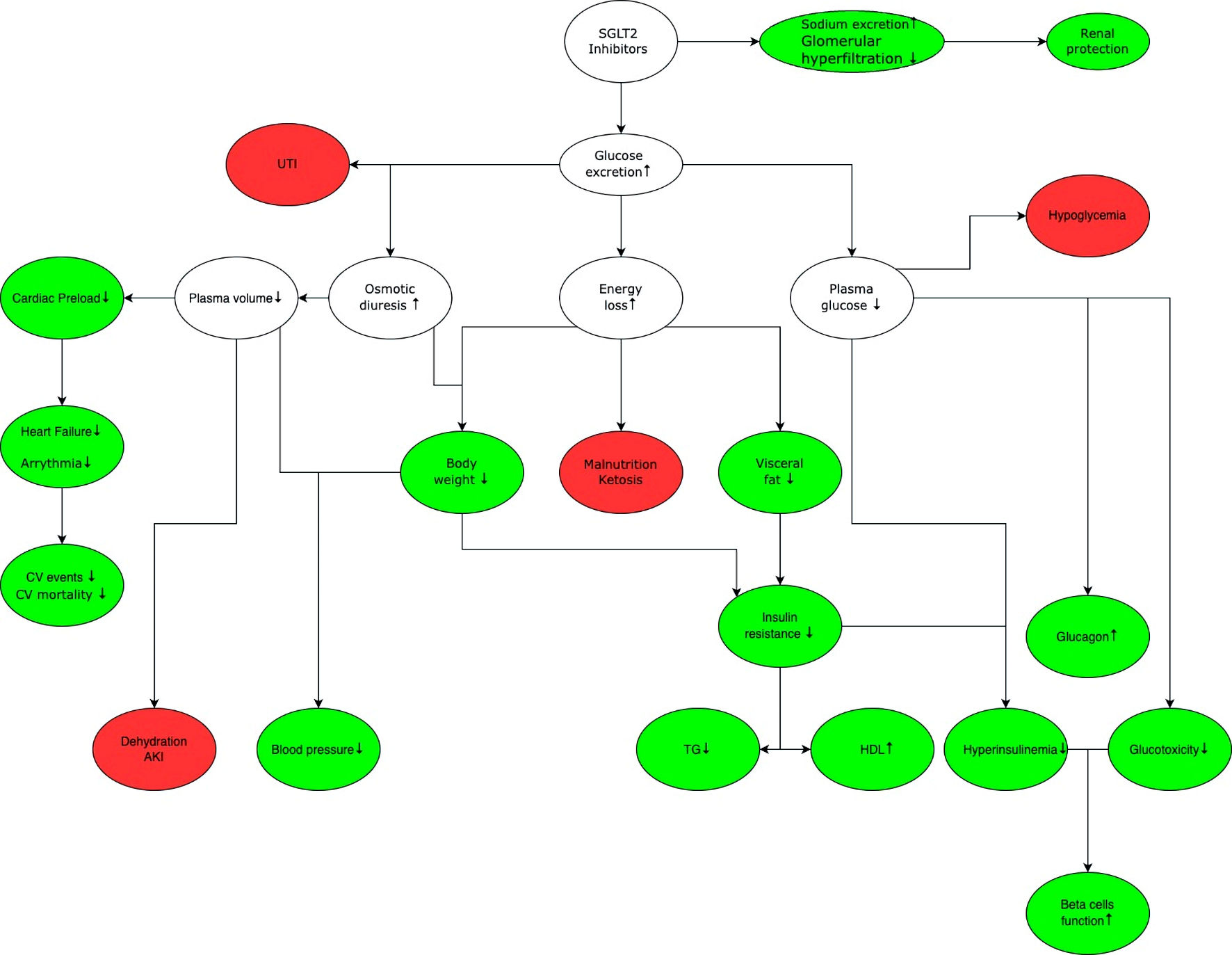
Figure 2. General mechanism of action of SGLT2 inhibitors. The green color marks benefits associated with the use of SGLT2 inhibitors, while white indicates the effects that can lead to benefits and/or adverse effects depending on the clinical situation, red shows potential adverse effects associated with the use of SGLT2 inhibitors. SGLT2 inhibition increases glucose and sodium excretion. Enhanced sodium excretion and decreased renal hyperfiltration delay the progression of chronic kidney disease. Increased glucose excretion leads to osmotic diuresis, which increases the risk of urinary tract infection, and sepsis. Increased diuresis reduces cardiac preload, thereby reducing the risk of cardiovascular events and mortality. In addition, there is a decrease in blood glucose levels, which reduces the need for insulin and glucotoxicity on the vascular endothelium, improving function of pancreatic beta cells. Glucose excretion leads to energy loss and weight loss, which lowers blood pressure and increases insulin sensitivity. It is important to note that while SGLT2 inhibitors do not cause hypoglycemia in monotherapy, they may increase the risk of occurrence of hypoglycemia when used in combination with other drugs that have hypoglycemic effect (e.g., insulin, sulfonylurea derivatives). SGLT2: sodium-glucose cotransporter 2; UTI: urinary tract infection; CV: cardiovascular; AKI: acute kidney injury; TG: triglycerides; HDL: high-density lipoprotein.
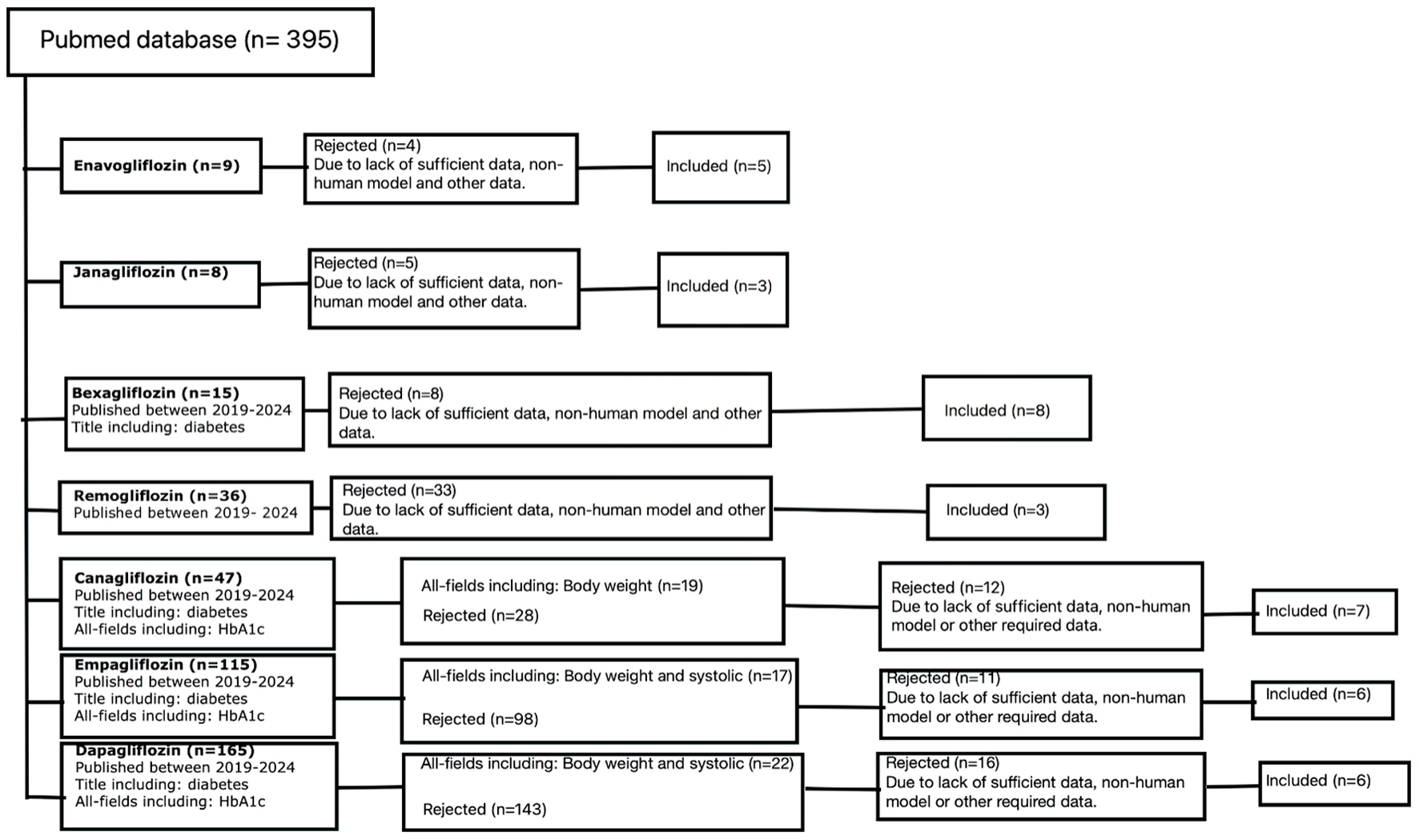
Figure 3. Out of the 395 initially obtained results, 38 were eventually included in this review. The selection process involved scaling up with additional criteria based on the available pool of results for a given flozin, as well as a content analysis that rejected papers which were off topic, did not contain sufficient data, or involved non-human models. N: number of papers.


