| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 14, Number 3, June 2024, pages 149-157
Diagnostic Performance of Anthropometric Weight and Height Markers Associated With Insulin Resistance Diagnosis
Victor Juan Vera-Poncea, g , Jenny Raquel Torres-Malcab
, Andrea P. Ramirez-Ortegab
, Rosa Angelica Garcia Larab, Joan A. Loayza-Castrob
, Fiorella E. Zuzunaga-Montoyac
, Mario J. Valladares-Garridod, e
, Eder Jesus Orihuela Manriquef
, Cori Raquel Iturregui Paucarf
, Jhony A. De La Cruz-Vargasb
aUniversidad Tecnologica del Peru, Lima, Peru
bInstituto de Investigacion en Ciencias Biomedicas de la Universidad Ricardo Palma, Lima, Peru
cUniversidad Cientifica del Sur, Lima, Peru
dUniversidad Continental Lima, Peru
eOficina de Epidemiologia, Hospital Regional Lambayeque, Chiclayo, Peru
fUniversidad Privada del Norte, Lima, Peru
gCorresponding Author: Victor Juan Vera-Ponce, Universidad Tecnologica del Peru, Lima, Peru
Manuscript submitted August 31, 2023, accepted October 13, 2023, published online June 29, 2024
Short title: Anthropometry and Insulin Resistance in Peru
doi: https://doi.org/10.14740/jem891
| Abstract | ▴Top |
Background: The detection of insulin resistance (IR) is crucial to avoid long-term complications. Given that the classic methods for its measurement are challenging to implement, simpler methods are sought for its detection. The aim of the study is to determine the association and diagnostic performance of four anthropometric markers based on weight and height for IR in a sample of Peruvians.
Methods: This study is a secondary analysis of the data. The variables were body mass index (BMI), the triponderal index (TPI), the new BMI (NBMI), and the University of Navarra Clinic-Body Fat Estimator index (CUN-BAE index). IR was measured using the homeostatic model assessment of insulin resistance (HOMA-IR). The association was evaluated using the odds ratio (OR), while for diagnostic performance, the receiver operating characteristic (ROC) curve and the corresponding area under it (AUC) were applied.
Results: The prevalence of IR was 17.11%. The adjusted multivariate analysis found that the association with IR significantly increased with the increase of their levels, especially in the third tertile in BMI (adjusted odds ratio (aOR): 18.2; 95% confidence interval (CI): 8.73 - 44.6), TPI (aOR: 17.2; 95% CI: 8.34 - 40.6), NBMI (aOR: 16.5; 95% CI: 8.12 - 38.3) and CUN-BAE index (aOR: 20.8; 95% CI: 10.6 - 47.1). In addition, BMI had the highest AUC = 0.854 (0.824 - 0.884), cutoff = 27.44, sensitivity = 85.03 (78.70 - 90.07) and specificity = 73.42 (70.23 - 76.44).
Conclusions: Based on the markers that only use weight and height, BMI showed the best association and diagnostic performance for detecting IR. It is advisable to conduct prospective studies to verify these findings. If such results are corroborated, BMI could become a valuable predictor for identifying IR in different populations.
Keywords: Insulin resistance; Body mass index; Adult; Anthropometry; Body weights and measures
| Introduction | ▴Top |
Insulin resistance (IR) refers to a state where the cells in the body are less responsive to the insulin hormone, leading to challenges in maintaining stable blood sugar levels [1]. This condition is crucial from a public health perspective because it serves as a key factor in the onset of multiple chronic and metabolic illnesses. These include metabolic syndrome, type 2 diabetes, obesity, high blood pressure, liver-related issues, and different kinds of cancer, among other conditions [2, 3].
IR underpins the onset of numerous chronic and metabolic diseases. Recent global data indicate a rising prevalence of IR, underscoring its significance as a pressing public health concern both globally and regionally [1]. As such, the early detection of IR, even among at-risk populations without overt symptoms, is paramount. While the euglycemic hyperinsulinemic clamp (a method maintaining euglycemia during insulin administration) is heralded as the gold standard for detecting IR [4], its practicality is limited by its invasiveness and cost. Conversely, the homeostatic model assessment (HOMA-IR) is more commonly deployed in the identification of IR [5]. Yet, literature highlights its limited accessibility across all populations, especially in resource-constrained settings [5].
It has been found that biochemical markers, in symptomless individuals, such as glucose, triglyceride levels, among others, are also valuable indicators to diagnose this entity [6]. Given the pressing need for practical, cost-effective, and noninvasive methods to detect IR, it is vital to assess whether simpler markers, like formulas based on weight and height, could be efficacious. Among them are the body mass index (BMI) [6], the triponderal index (TPI) [7, 8], the new body mass index (NBMI) [9], and the University of Navarra Clinic-Body Fat Estimator index (CUN-BAE index) [10]. Due to the need to understand the behavior of these, the aim of this study is to determine the diagnostic performance of four anthropometric markers based on weight and height for IR in a sample of Peruvians.
| Materials and Methods | ▴Top |
This is an analytical and diagnostic test cross-sectional study. We utilized the secondary database from the PERU MIGRANT study, facilitated by the CRONICAS Center. The core objective of the original study was to discern differences in cardiovascular risk factors across urban, rural, and urban-rural migrant demographics. This initiative was driven by the evolving epidemiological landscape in these populations and the need for targeted public health interventions. Detailed insights into the selection criteria, variables employed, sample size, and participation rates have been articulated in prior publications [11].
The primary study included groups of subjects over 30 years of age who had no history of mental illness or pregnancy. Only those subjects who had variables of interest were considered in this study.
The response variable was IR. This was obtained with the HOMA-IR index, which was calculated using the formula = (glucose (mmol/L) × insulin (µU/mL))/22.5 [12]. The results were categorized as “insulin resistant” if HOMA-IR ≥ 2.8 [13], and “not insulin resistant” if HOMA-IR < 2.8.
The anthropometric markers that were tested were as follows:
BMI = weight (kg)/height (m)2
TPI = weight (kg)/height (m)3 [14]
NBMI = 1.3 × (weight (kg)/height (m)2.5) [15]
CUN-BAE index = -44.988 + (0.503 × age) + (10.689 × sex) + (3.172 × BMI) - (0.026 × BMI2) + (0.181 × BMI × sex) - (0.02 × BMI × age) - (0.005 × BMI2 × sex) + (0.00021 × BMI2 × age) [16]
Height was gauged with an accuracy of up to 0.1 cm using a height-measuring instrument, while weight was recorded with the individual in lightweight attire to a precision of 0.05 kg on an SECA 940 digital scale.
Additional parameters scrutinized included age brackets, gender (either male or female), demographic group (urban, rural, or transient), current tobacco use, alcohol intake, and exercise habits. Definitions for excessive drinking were categorized based on low or high alcohol consumption levels according to the amount of alcohol reported consuming. Smoker classification was split into current smokers and non-smokers. Activity levels were determined using the guidelines set by the International Physical Activity Questionnaire (IPAQ), categorizing individuals into high, medium, or low activity levels based on days of physical activity and the metabolic equivalent measured in minutes per week.
Analysis procedures and statistical methods
All data crunching was carried out via R software, version 4.0.5. Estimates were made for the frequency and mean values of sociodemographic traits and the metrics under study. The Chi-square test was employed to identify disparities among categorized variables, while the Student’s t-test was utilized for variables on a continuous scale. Multifaceted logistic regression analysis was applied to investigate the relationships between each segmented marker and IR, as evaluated by the odds ratio (OR). A P value of 0.05 was set as the benchmark for statistical significance.
Diagnostic efficacy was gauged using the receiver operating characteristic (ROC) curve, a graphical representation showcasing the diagnostic ability of a binary classifier system, and the area underneath it (AUC). Sensitivity, specificity, positive and negative predictive values, as well as likelihood ratios, were meticulously calculated. The Youden index, a metric that balances sensitivity and specificity to derive the optimal threshold, was harnessed to ascertain the best cut-off point.
Ethical aspects
Study protocol was approved by the Institutional Review Board (IRB) at Institute for Research in Biomedical Sciences of the Ricardo Palma University. The survey information is freely accessible [11], with no personal identifiers and no contact with human subjects. Therefore, it was not considered necessary to undergo a review by an ethics committee.
| Results | ▴Top |
The prevalence of IR was 17.11%; the female sex, 52.87%, and the percentage of older adults, 14.86%. High physical activity constituted 44.52%. With respect to harmful habits, high alcohol consumption was 8.91% and cigarette consumption, 11.27% (Table 1).
 Click to view | Table 1. Demographic and Anthropometric Characteristics of Participants |
The variables that found an association between IR and the covariates were sex (P < 0.001), age group (P = 0.018), group (P < 0.001), physical activity (P < 0.001), alcohol volume (P = 0.041) and all anthropometric markers (P < 0.001) (Table 2).
 Click to view | Table 2. Bivariate Analysis of the Characteristics Associated With Insulin Resistance |
Finally, according to the adjusted multivariate analysis, it was found that the association with IR significantly increased with the rise of its levels, especially in the upper tertile in BMI (adjusted odds ratio (aOR): 18.2; 95% confidence interval (CI): 8.73 - 44.6), TPI (aOR: 17.2; 95% CI: 8.34 - 40.6), NBMI (aOR: 16.5; 95% CI: 8.12 - 38.3) and CUN-BAE index (aOR: 20.8; 95% CI: 10.6 - 47.1) (Table 3).
 Click to view | Table 3. Simple and Adjusted Multivariate Regression Analysis of the BMI, TPI, NBMI and CUN-BAE Quartiles for IR |
Regarding the ROC analysis, all showed an AUC greater than 0.80, except for the CUN-BAE index. The BMI had the highest AUC = 0.854 (0.824, 0.884), cutoff = 27.44, Se = 85.03 (78.70, 90.07), and Sp = 73.42 (70.23, 76.44). In women, the CUN-BAE index has the highest AUC with 0.013, both the BMI and the NBMI have AUCs slightly higher than 0.80, suggesting good diagnostic performance. In men, the CUN-BAE index showed an AUC of 0.878, while the BMI and TMI have AUCs of 0.872 and 0.849, respectively.
The rest of the indicators are found in Table 4, and the ROC curves graph is in Figure 1.
 Click to view | Table 4. Diagnostic Values for Insulin Resistance |
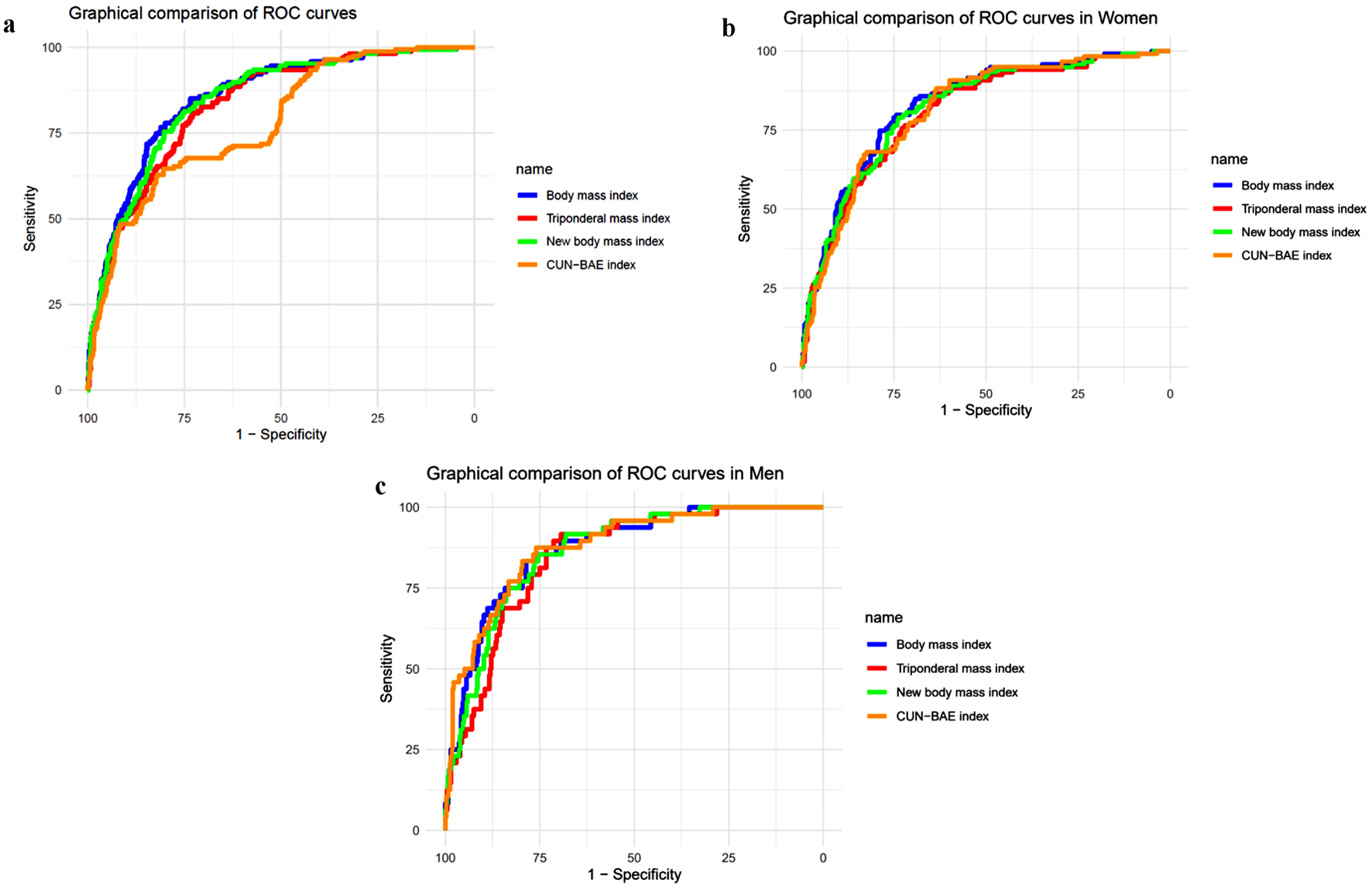 Click for large image | Figure 1. ROC curves graph. (a) General. (b) In women. (c) In men. ROC: receiver operating characteristic; CUN-BAE index: the University of Navarra Clinic-Body Fat Estimator index. |
| Discussion | ▴Top |
Main findings
With the purpose of determining the optimal anthropometric index of weight and height to predict IR, this study suggests that BMI was the best predictor among all other indicators analyzed. According to the available information, this is the first investigation in Peru related to this issue.
On the other hand, despite the variations observed between genders, the indices maintain significant accuracy across both groups. It is noteworthy that the BMI, TMI, and the NBMI boast AUCs above 0.80. While the CUN-BAE index generally has a lower AUC, it exhibits outstanding performance in specific gender populations. These findings lead us to conclude that all evaluated indices are valuable tools for identifying IR, especially considering they are based on simple anthropometric markers such as weight and height. Given these conclusions, the discussion will be approached globally, as differences between sexes do not seem to be decisive in this context.
Prevalence of IR
The prevalence of IR in our study for Peru is 17.11%. When compared to other countries, we find significant variations. For example, the study undertaken in Chennai, India discovered that the general occurrence of IR syndrome was approximately 11.2% [17]. A comprehensive analysis of population-level investigations exploring the epidemiology of IR among youth discovered that reported occurrence rates diverged between 3.1% and 44% across studies. This variation was partly due to different definitions used for IR [18]. Such a difference could be attributed to genetic, environmental, dietary factors, among others. While Peru’s prevalence may not be the absolute highest, the continued existence of this issue demands intervention, as it remains a problematic situation worthy of attention and resolution.
Comparison with other studies
This analysis revealed that BMI would be the most effective weight and height indicator in identifying IR, while the associative level was high in the higher tertile. Although it is a widely used and suggested anthropometric measurement [19], it has also faced criticism and obstacles, and its limitation is especially relevant in distinguishing between body fat mass and lean mass [20]. Likewise, the BMI cut-off value in Pakistani subjects is 27.44 kg/m2, while the World Health Organization (WHO) BMI cut-off values are > 30 kg/m2 for Americans and Europeans [19] and > 25 kg/m2 for Asians [21].
These findings coincide with other studies. Research in men of different ethnicities indicated that BMI was the best predictor of IR. Thus, Nadeem et al [22], Wang et al [23], and Hadaegh et al [24] found that BMI is the best indicator of IR in Pakistani adults (AUC = 0.690), Chinese (AUC = 0.692), and Tehranians (AUC = 0.716).
In terms of the relationship between variables, Chen et al found that BMI had a standalone and positive link with HOMA-IR [25]. In research conducted by Ferrannini et al, it was observed that both fasting insulin levels and post-oral glucose insulin secretion had a roughly linear correlation with BMI in individuals without diabetes [26]. However, research of Liu et al revealed that although BMI had an independent association with IR, this connection was not statistically significant when it came to the early and late stages of insulin secretion [27].
Other works have also evaluated these two variables in different populations. In young people, Lim et al [28] conducted a study in Korean adolescents and found that obesity indices, including BMI, had a quite high association with IR. The research of Chang-Rueda et al [29], in which they evaluated patients from 5 to 19 years old, found that IR has a moderately significant correlation with increased BMI. Bhattacharya et al [30] found that BMI had a low AUC (AUC = 0.585) for patients with polycystic ovary syndrome, while Jamar et al [31] conducted a similar study in subjects with obesity without diabetes, where the overall discriminatory capacity was higher (AUC = 0.730).
Regarding the rest of the markers, the number of studies that have evaluated the diagnostic performance for IR is scarce. Peng et al [10] followed a group of patients for about 5 years and found that for each point that the CUN-BAE increased, so did the incidence of diabetes linearly. Neves et al [8] and Akcan et al [32] highlighted the role of TPI in the diagnosis of IR in children and young people.
Interpretation of results
IR and obesity are closely related through several interconnected pathophysiological mechanisms. Firstly, adipose tissue in obese individuals releases pro-inflammatory molecules, such as interleukin (IL)-1, IL-6, or tumor necrosis factor (TNF)-α, which interfere with insulin action in cells [33]. Additionally, the accumulation of lipids in non-adipose organs and the oxidative stress caused by excess body fat also negatively affect cell function and insulin signaling [34].
Excess weight, by altering the homeostasis of the body’s intrinsic biochemicals, can impact the secretion and function of leptin and adiponectin, which ordinarily assist in modulating craving, vitality usage, and sensitivity to insulin. These mechanisms, combined with the impaired blood vessel functioning tied to obesity, contribute to IR and illuminate the relationship between the two conditions [35].
Contribution to the field
Given their ease of determination through accessible and affordable means without risk or invasiveness, anthropometric markers such as weight and height take on outsized significance for assessment purposes owing to the simple fact that they can be so readily and inexpensively ascertained. In many settings, especially in regions with limited resources, advanced diagnostic tests for IR are neither readily accessible nor affordable. This is where the measurement of BMI and other weight- and height-based indicators showcase their true value. These metrics, being straightforward to compute, provide a preliminary tool to identify individuals at risk and take preventative actions.
Additionally, how these indicators are employed could prove determinative for wide-ranging public health initiatives. For instance, awareness and screening campaigns in communities could greatly benefit from these simple yet effective tools, enabling early interventions and potentially reducing the burden of RI-related diseases.
The escalating prevalence of IR and its role in the onset of metabolic and cardiovascular diseases underscore the urgency to intervene. This study, being one of the first in the Peruvian context, establishes a benchmark and underscores the need for national preventive and intervention strategies. In this backdrop, the significance of anthropometric markers is heightened, as they ease the early identification of at-risk populations. Coupled with community-based interventions, employing these markers can be a cost-effective strategy to counteract the complications associated with IR.
Study limitations
To begin, as this analysis looked at a single point in time, determining which event came first is impossible, raising the risk that an outcome seemed to prompt a factor when truly the opposite was true. Second, the hyperinsulinemic euglycemic clamp technique was not performed nor was the gold standard for assessing insulin sensitivity [36] considered; however, it has been proven that HOMA-IR, a substitute for IR, correlates adequately with the IR index derived from this technique [37]. Furthermore, carrying it out on enormous groups would be unworkable. Likewise, the examination solely happened in two places of the country, limiting comprehensive inferences; despite this, owing to the probabilistic nature of the sample, there exists some degree of representativeness.
Conclusions
Based on the markers that only use weight and height, BMI demonstrated to have an association and the best diagnostic performance in detecting IR. Interestingly, while other indices were evaluated, their performance was not clearly superior to that of BMI, with values being close. This suggests that there might not be a compelling need to transition away from using BMI in clinical practice for this purpose. It is advisable to carry out prospective research to verify these findings. If such results are corroborated, BMI could become a valuable predictor for identifying IR in different populations.
Acknowledgments
A special thanks to the members of Intituto de Investigaciones en Ciencias Biomedicas de la Universidad Ricardo Palma, who provided valuable comments during the preparation of this study.
Financial Disclosure
This study is self-financed.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The informed consent was obtained.
Author Contributions
Victor Juan Vera-Ponce, Jhony A. De La Cruz-Vargas and Jenny Raquel Torres-Malca participated in the genesis of the idea and project design. Fiorella E. Zuzunaga-Montoya, Andrea P. Ramirez-Ortega, Cori Raquel Iturregui Paucar, and Mario J. Valladares-Garrido oversaw the data collection, interpretation, and analysis of results. Joan A. Loayza-Castro, Eder Jesus Orihuela Manrique and Rosa Angelica Garcia Lara contributed to the preparation of the manuscript of this research paper.
Data Availability
The data supporting the findings of this study can be accessed by the original research paper in the webpage Figshare.
| References | ▴Top |
- Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne). 2023;14:1149239.
doi pubmed pmc - Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw (Online). 2016;70(0):1245-1258.
pubmed - Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. 2023;51(3):3000605231164548.
doi pubmed pmc - Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol Biol. 2009;560:221-238.
doi pubmed - Buchanan TA, Watanabe RM, Xiang AH. Limitations in surrogate measures of insulin resistance. J Clin Endocrinol Metab. 2010;95(11):4874-4876.
doi pubmed pmc - Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11(3):e0149731.
doi pubmed pmc - Matsuo AR, Lopes WA, Locatelli JC, Simoes CF, de Oliveira GH, Nardo N, Jr. Tri-ponderal mass index as a tool for insulin resistance prediction in overweight adolescents: A cross-sectional study. Nutrition. 2020;74:110744.
doi pubmed - Neves FS, Alvim RO, Zaniqueli D, Pani VO, Martins CR, Pecanha MAS, Barbosa MCR, et al. Tri-ponderal mass index is useful for screening children and adolescents with insulin resistance. Rev Paul Pediatr. 2020;38:e2019066.
doi pubmed pmc - Kali A, Gusmanov A, Aripov M, Chan MY. Proposing new body mass index and waist circumference cut-offs based on cardiometabolic risks for a Central Asia population: A feasibility study. Front Endocrinol (Lausanne). 2022;13:963352.
doi pubmed pmc - Peng Q, Feng Z, Cai Z, Liu D, Zhong J, Zhao H, Zhang X, et al. The relationship between the CUN-BAE body fatness index and incident diabetes: a longitudinal retrospective study. Lipids Health Dis. 2023;22(1):21.
doi pubmed pmc - PERU MIGRANT Study | Baseline dataset [Internet]. figshare; 2016 [citado el 14 de marzo de 2021].
doi - Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495.
doi pubmed - Carrillo-Larco RM, Miranda JJ, Gilman RH, Checkley W, Smeeth L, Bernabe-Ortiz A, Cronicas Cohort Study G. The HOMA-IR performance to identify new diabetes cases by degree of urbanization and altitude in Peru: the CRONICAS cohort study. J Diabetes Res. 2018;2018:7434918.
doi pubmed pmc - Peterson CM, Su H, Thomas DM, Heo M, Golnabi AH, Pietrobelli A, Heymsfield SB. Tri-ponderal mass index vs body mass index in estimating body fat during adolescence. JAMA Pediatr. 2017;171(7):629-636.
doi pubmed pmc - Van Haute M, Rondilla E, 2nd, Vitug JL, Batin KD, Abrugar RE, Quitoriano F, Dela Merced K, et al. Assessment of a proposed BMI formula in predicting body fat percentage among Filipino young adults. Sci Rep. 2020;10(1):21988.
doi pubmed pmc - Ares Blanco J, Valdes Hernandez S, Botas Cervero P, Sanchez-Ragnarsson C, Pujante Alarcon P, Menendez-Torre E, Delgado Alvarez E. Estimation of body fat mass using the CUN-BAE index and mortality risk by sex in the Asturias Study cohort. Endocrinol Diabetes Nutr (Engl Ed). 2019;66(8):487-494.
doi pubmed - Deepa R, Shanthirani CS, Premalatha G, Sastry NG, Mohan V. Prevalence of insulin resistance syndrome in a selected south Indian population—the Chennai urban population study-7 [CUPS-7]. Indian J Med Res. 2002;115:118-127.
pubmed - van der Aa MP, Fazeli Farsani S, Knibbe CA, de Boer A, van der Vorst MM. Population-Based Studies on the Epidemiology of Insulin Resistance in Children. J Diabetes Res. 2015;2015:362375.
doi pubmed pmc - Obesity and overweight [Internet]. [citado el 17 de septiembre de 2022]. Disponible en: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008;32(6):959-966.
doi pubmed pmc - World Health Organization (WHO) Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-163.
doi pubmed - Nadeem A, Naveed AK, Hussain MM, Raza SI. Cut-off values of anthropometric indices to determine insulin resistance in Pakistani adults. J Pak Med Assoc. 2013;63(10):1220-1225.
pubmed - Wang F, Wu S, Song Y, Tang X, Marshall R, Liang M, Wu Y, et al. Waist circumference, body mass index and waist to hip ratio for prediction of the metabolic syndrome in Chinese. Nutr Metab Cardiovasc Dis. 2009;19(8):542-547.
doi pubmed - Hadaegh F, Zabetian A, Harati H, Azizi F. Waist/height ratio as a better predictor of type 2 diabetes compared to body mass index in Tehranian adult men—a 3.6-year prospective study. Exp Clin Endocrinol Diabetes. 2006;114(6):310-315.
doi pubmed - Chen G, Liu C, Yao J, Jiang Q, Chen N, Huang H, Liang J, et al. Overweight, obesity, and their associations with insulin resistance and beta-cell function among Chinese: a cross-sectional study in China. Metabolism. 2010;59(12):1823-1832.
doi pubmed - Ferrannini E, Camastra S, Gastaldelli A, Maria Sironi A, Natali A, Muscelli E, Mingrone G, et al. beta-cell function in obesity: effects of weight loss. Diabetes. 2004;53(Suppl 3):S26-33.
doi pubmed - Liu MM, Liu QJ, Wen J, Wang M, Wu LY, Qu ML, Li M, et al. Waist-to-hip ratio is the most relevant obesity index at each phase of insulin secretion among obese patients. J Diabetes Complications. 2018;32(7):670-676.
doi pubmed - Lim SM, Choi DP, Rhee Y, Kim HC. Association between obesity indices and insulin resistance among healthy Korean adolescents: the JS high school study. PLoS One. 2015;10(5):e0125238.
doi pubmed pmc - Menegol NA, Montemezzo D, Francisco Gulonda ASG, Sonza A, Castro CG, Sanada LS. Canadian and Brazilian Percentile Ranks for the Alberta Infant Motor Scale. Phys Occup Ther Pediatr. 2022;42(6):635-644.
doi pubmed - Bhattacharya K, Sengupta P, Dutta S, Chaudhuri P, Das Mukhopadhyay L, Syamal AK. Waist-to-height ratio and BMI as predictive markers for insulin resistance in women with PCOS in Kolkata, India. Endocrine. 2021;72(1):86-95.
doi pubmed - Jamar G, Almeida FR, Gagliardi A, Sobral MR, Ping CT, Sperandio E, Romiti M, et al. Evaluation of waist-to-height ratio as a predictor of insulin resistance in non-diabetic obese individuals. A cross-sectional study. Sao Paulo Med J. 2017;135(5):462-468.
doi pubmed pmc - Akcan N, Bundak R. Accuracy of tri-ponderal mass index and body mass index in estimating insulin resistance, hyperlipidemia, impaired liver enzymes or thyroid hormone function and vitamin D levels in children and adolescents. J Clin Res Pediatr Endocrinol. 2019;11(4):366-373.
doi pubmed pmc - Guerrero-Romero F, Castellanos-Juarez FX, Salas-Pacheco JM, Morales-Gurrola FG, Salas-Leal AC, Simental-Mendia LE. Association between the expression of TLR4, TLR2, and MyD88 with low-grade chronic inflammation in individuals with metabolically healthy obesity. Mol Biol Rep. 2023;50(5):4723-4728.
doi pubmed - Swiatkiewicz I, Wroblewski M, Nuszkiewicz J, Sutkowy P, Wroblewska J, Wozniak A. The Role of Oxidative Stress Enhanced by Adiposity in Cardiometabolic Diseases. Int J Mol Sci. 2023;24(7):6382.
doi pubmed pmc - Guzman-Ruiz R, Tercero-Alcazar C, Lopez-Alcala J, Sanchez-Ceinos J, Malagon MM, Gordon A. The potential role of the adipokine HMGB1 in obesity and insulin resistance. Novel effects on adipose tissue biology. Mol Cell Endocrinol. 2021;536:111417.
doi pubmed - Tang Q, Li X, Song P, Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov Ther. 2015;9(6):380-385.
doi pubmed - Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57-63.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
