| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 13, Number 3, August 2023, pages 89-95
Chronic Calcific Pancreatitis and Its Association With Secondary Diabetes Mellitus
Gurusha Bahla, Md Sadique Hussaina, c, Dinesh K. Upadhyaya, Madhumati Varmab, Rajveer Singha, Subhankar Dasa
aSchool of Pharmaceutical Sciences, Jaipur National University, Jaipur 302017, Rajasthan, India
bDepartment of Medicine, Jaipur National University Institute for Medical Sciences and Research Center, Jaipur 302017, Rajasthan, India
cCorresponding Author: Md Sadique Hussain, School of Pharmaceutical Sciences, Jaipur National University, Jaipur 302017, Rajasthan, India
Manuscript submitted May 26, 2023, accepted July 11, 2023, published online August 9, 2023
Short title: CCP and Association With Secondary Diabetes
doi: https://doi.org/10.14740/jem884
- Abstract
- Introduction
- Diagnostic Criteria for T3cDM
- Biomarkers for T3cDM
- Management of T3cDM
- Case Report
- Discussion
- Conclusion
- References
| Abstract | ▴Top |
Chronic calcific pancreatitis (CCP) is an inflammatory illness that impacts the pancreas, causing calcifications and scarring inside the gland. Secondary diabetes, commonly referred to as type 3c diabetes mellitus (T3cDM), is one of the probable problems linked with CCP. T3cDM is a form of diabetes caused by an underlying illness or disorder of the endocrine systems. It is a rare cause of diabetes caused by pancreatic pathology. In the regions of Southeast Asian and India, it accounted for 15-20% of individuals with diabetes. This is a rare case report and review of T3cDM. The patient was admitted with a complaint of hyperglycemia, blood glucose of 405 mg/dL with a glycated hemoglobin (HbA1c) level of 13.7%. Computed tomography evidence revealed CCP with intraductal calculi and dilated pancreatic duct.
Keywords: Pancreatogenic diabetes mellitus; Diabetes mellitus type 3c; Chronic pancreatitis
| Introduction | ▴Top |
Chronic calcific pancreatitis (CCP) is an inflammatory illness that impacts the pancreas, causing calcifications and scarring inside the gland [1]. Secondary diabetes, commonly referred to as type 3c diabetes mellitus (T3DM/T3cDM), is one of the probable problems linked with CCP [2]. We will look at how CCP might manifest with secondary diabetes and the consequences for diagnosis and therapy in this case report. CCP causes the destruction of pancreatic tissue, including beta cells that produce insulin. As a result, the pancreas loses its ability to make enough insulin, resulting in poor glucose management and the development of diabetes. This type of diabetes is known as secondary diabetes because it develops as a result of an underlying illness [3, 4].
CCP is a rare cause of secondary diabetes. According to a study, 20.1% (118 out of 587) of patients with chronic pancreatitis (CP) developed diabetes type [5]. The prevalence of diabetes in CP is estimated to be between 5% and 10% in Western populations [6, 7]. Depending on the cohort studied and the duration of follow-up, diabetes has been observed in 26% to 80% of patients with CP. The risk of diabetes in patients with CP increases with longer disease duration, worsening pancreatic damage, especially early-onset pancreatic calcification, and prior distal pancreatectomy [8].
Diabetes mellitus resulting from CP is classified as T3cDM, also known as pancreatic diabetes. Approximately 80% of patients with pancreatogenic diabetes have CP [9]. Compared to patients with CP but no diabetes, those with post-pancreatitis diabetes mellitus (PPDM) were predominantly male, had a higher prevalence of alcoholic etiology, and showed increased rates of pancreatic calcifications and exocrine insufficiency [10].
DM is a collection of metabolic illnesses defined primarily by hyperglycemia, polyphagia, polydipsia, and polyuria caused by abnormalities in insulin production, effect, or perhaps both. Secondary diabetes can be caused by a variety of conditions, including pancreatitis, Cushing’s syndrome, cystic fibrosis (CF), and hormonal abnormalities such as acromegaly. Furthermore, several drugs, such as corticosteroids, antipsychotics, and organ transplant medicines, might cause secondary diabetes. Let us examine each of these factors more closely.
Pancreatitis: Pancreatitis, characterized by inflammation of the pancreas, can potentially contribute to the development of secondary diabetes. The pancreas plays a vital role in producing insulin, and when it becomes damaged or inflamed, it may hinder insulin production. The severity and duration of pancreatitis can influence the likelihood of developing secondary diabetes [11].
Cushing’s syndrome: Cushing’s syndrome is a hormonal disorder resulting from prolonged exposure to high levels of cortisol, a hormone produced by the adrenal glands [12, 13]. Excessive cortisol can disrupt insulin function and lead to insulin resistance, potentially leading to the development of diabetes. Individuals with Cushing’s syndrome face an elevated risk of developing secondary diabetes [14].
CF: CF, an inherited genetic disorder affecting multiple organs including the pancreas, can lead to pancreatic insufficiency. In pancreatic insufficiency, the pancreas fails to produce an adequate number of digestive enzymes, impairing nutrient absorption [15]. Over time, this insufficiency can damage the insulin-producing cells in the pancreas, resulting in secondary diabetes [16].
Hormonal abnormalities: Various hormonal imbalances can contribute to the development of secondary diabetes [17]. Conditions such as acromegaly (excessive growth hormone), hyperthyroidism (overactive thyroid gland), and pheochromocytoma (adrenal gland tumor) can disrupt insulin production or action, potentially leading to diabetes [18, 19].
Certain medications: Some medications can induce secondary diabetes by affecting insulin production or increasing insulin resistance. Glucocorticoids, commonly prescribed for treating inflammatory conditions, can elevate blood glucose levels and contribute to the development of diabetes [20, 21]. Similarly, specific antipsychotics and immunosuppressants have been associated with an increased risk of secondary diabetes [22, 23].
It is important to note that the development of secondary diabetes is influenced by multiple factors, and these conditions or factors may not always result in diabetes for every individual. However, they do significantly increase the risk, necessitating close monitoring for signs of diabetes and the provision of appropriate management and treatment to individuals with these conditions.
These variables can impair the production of insulin or impede the body’s capacity to use insulin properly, resulting in high blood sugar concentrations [24]. Symptoms of diabetes include blurred vision, dry mouth, frequent urination, frequent unexplained infections, increased thirst, numbness or tingling in the hands or feet, and slow-healing sores or cuts [25]. Pancreatogenic diabetes is classified as a form of type 3 DM by the American Diabetes Association and refers to diabetes secondary to an existing disease or condition of the exocrine pancreas [26, 27]. Despite other kinds of diabetes, which are caused by the failure of the pancreas’ insulin-producing beta cells, pancreatogenic diabetes is caused by anatomical and functional damage to the pancreas directly. A variety of illnesses and causes can contribute to the development of pancreatogenic diabetes. CP, pancreatic cancer, CF, pancreatic surgery, and pancreatic trauma are among them. These disorders can harm pancreatic tissue and decrease insulin generation and release, resulting in poor glucose management [28]. According to Ewald et al, about 78.5% of patients of T3cDM suffer from CP [29]. Pancreatogenic diabetes accounts for 15-20% of patients with diabetes in Indian as well as Southeast Asian regions where fibrocalcific pancreatitis is native [30]. As per Cui and Andersen, T3cDM affects approximately 5-10% of the western population with diabetes and fibrocalcific pancreatitis is estimated to be the etiology of diabetes for up to 50% among all young patients suffering from diabetes in India [31, 32]. Universally T3cDM has no established criteria for a diagnosis but Ewald and Bretzel developed the well-known criterion [33]. Identification can be achieved in individuals who fit three fundamental requirements: some who satisfy the clinical criteria for diabetes, people with an exocrine pancreatic illness, and people with diabetes due to their exocrine pancreatic condition [34]. This patient was diagnosed with pancreatic T3cDM.
| Diagnostic Criteria for T3cDM | ▴Top |
T3cDM refers to pancreatogenic diabetes, a form of diabetes that arises as a result of pancreatic diseases affecting both the exocrine and digestive functions of the pancreas. The primary cause of T3cDM is CP, which accounts for many cases. Unfortunately, there is a dearth of established criteria for accurately diagnosing T3cDM. This lack of standardized diagnostic criteria often results in its misidentification as type 2 diabetes mellitus (T2DM), leading to an underestimation of its true incidence [35-38].
The diagnostic criteria for T3cDM have been proposed, which include both major and minor criteria.
Major criteria (all must be met) [37, 39] include: 1) Presence of exocrine pancreatic insufficiency, confirmed by the monoclonal fecal elastase-1 test or direct function tests. 2) Pathological pancreatic imaging, assessed through endoscopic ultrasound, magnetic resonance imaging (MRI), or computed tomography (CT). 3) Absence of autoantibodies associated with type 1 diabetes mellitus (T1DM).
Minor criteria [37, 38] include: 1) Lack of pancreatic polypeptide (PP) response to mixed-nutrient ingestion. 2) Confirmation of T3cDM can be established by documenting the absence of PP response to mixed-nutrient ingestion, which helps differentiate the pathological islet response from that of T2DM.
| Biomarkers for T3cDM | ▴Top |
Recent studies have indicated that untargeted metabolomics could be a valuable tool in distinguishing between T3cDM and T2DM. Novel biomarkers specific to T3cDM have been identified [35].
| Management of T3cDM | ▴Top |
Managing patients with T3cDM poses a challenge for physicians due to the presence of multiple metabolic dysfunctions and poor nutritional status. Patients with T3cDM require pancreatic enzyme replacement therapy to optimize glycemic control and typically rely on insulin instead of oral anti-hyperglycemic agents. T3cDM patients often experience “brittle” diabetes, characterized by impaired production of both insulin and glucagon in the pancreas, leading to frequent episodes of both hypoglycemia and hyperglycemia [39].
Ewald and Bretzel have put forth major criteria for diagnosing T3cDM, which include the following [33]: 1) Exocrine pancreatic insufficiency: This can be confirmed by conducting tests such as monoclonal fecal elastase-1 or direct function tests. 2) Consistent pancreatic abnormalities on imaging: Detecting pancreatic abnormalities can be achieved through various methods such as endoscopic ultrasound, MRI, or CT scans. 3) Diabetes mellitus: Diagnosis of diabetes mellitus can be made using standard criteria.
For a definitive diagnosis of T3cDM, all three major criteria mentioned above must be present.
| Case Report | ▴Top |
A 36-year-old male patient with worsening glycemic control and with complaints of polyuria, polydipsia along with progressive and gradual abdominal pain presented to the male medicine ward in November 2022. Vital signs on presentation showed blood pressure of 137/96 mm Hg, respiratory rate of 18 breaths/min, heart rate of 90 bpm, and temperature of 98.5 °F. Patient’s glycated hemoglobin (HbA1c) value at the time of admission was 13.7% and plasma glucose random was found to be 405 mg/dL. Other laboratory investigations showed normal hemoglobin levels but slightly decreased total leukocyte count along with sodium and mild elevation in globulin was observed. A CT of the abdomen revealed CCP with intraductal calculi and dilated pancreatic duct as shown in Figure 1. An atrophic pancreas was seen which showed diffuse parenchymal calcification as shown in Figure 2 and calculus of size 8 mm was seen in the duct region of the body of the pancreas and another calculus of 11 mm was seen in the duct region of the head of the pancreas as shown in Figure 3. Ultrasonography of the abdomen was suggestive of mild hepatomegaly (enlarged size: 16.3 cm).
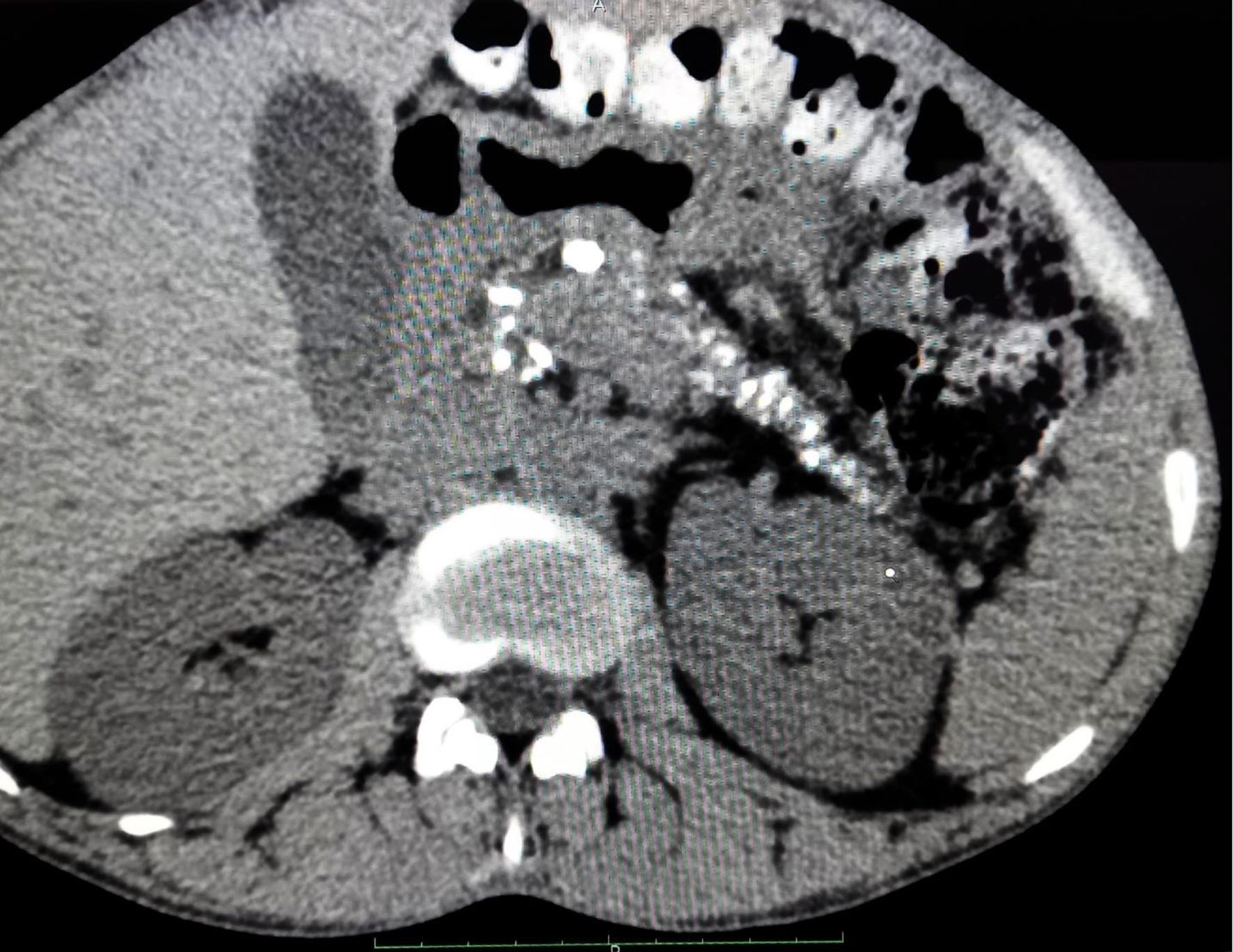 Click for large image | Figure 1. Contrast-enhanced computed tomography scan of abdomen showing diffuse parenchymal calcifications and dilated pancreatic duct. |
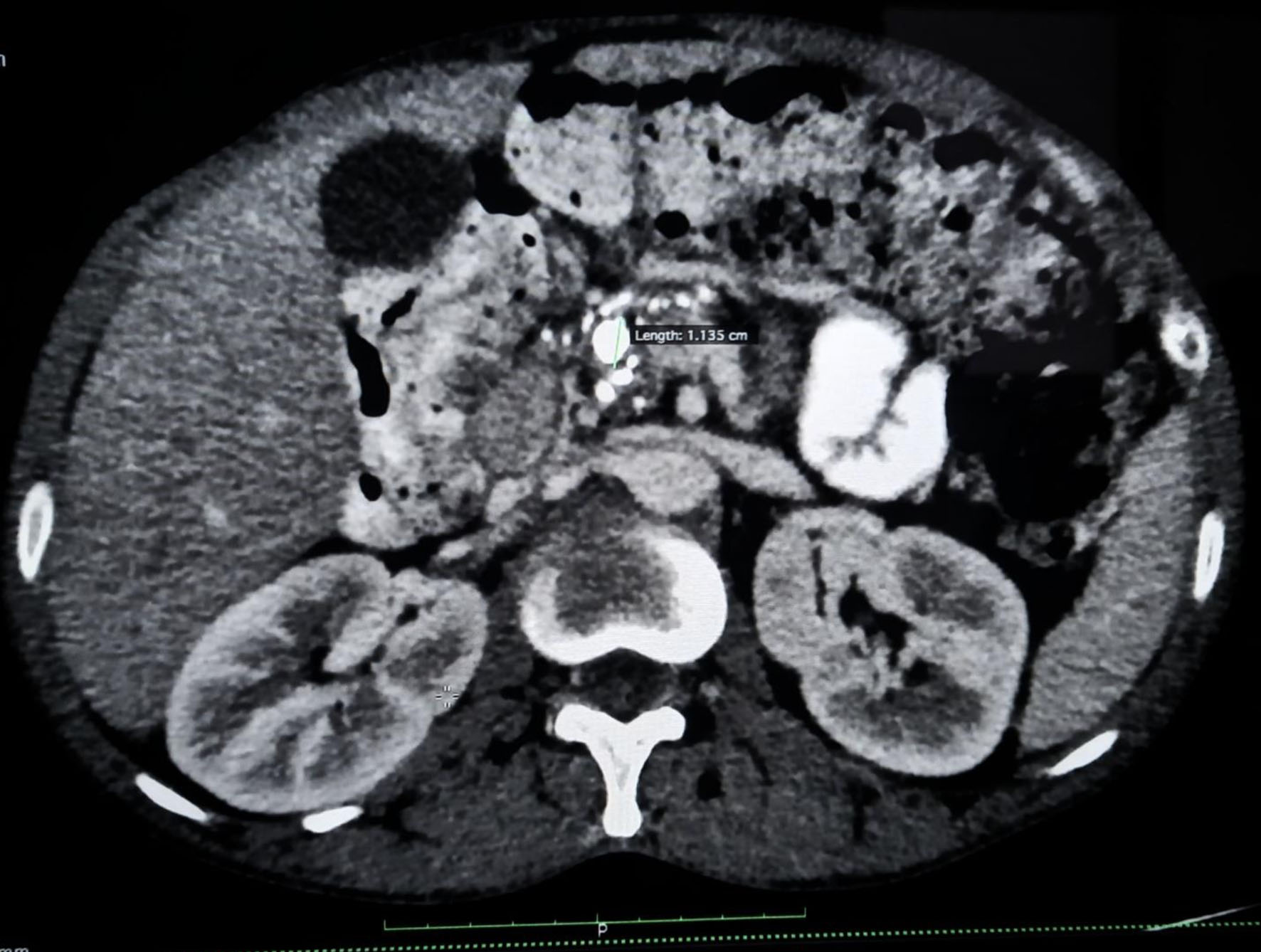 Click for large image | Figure 2. Contrast-enhanced computed tomography scan of abdomen showing chronic calcific pancreatitis with intraductal calculi. |
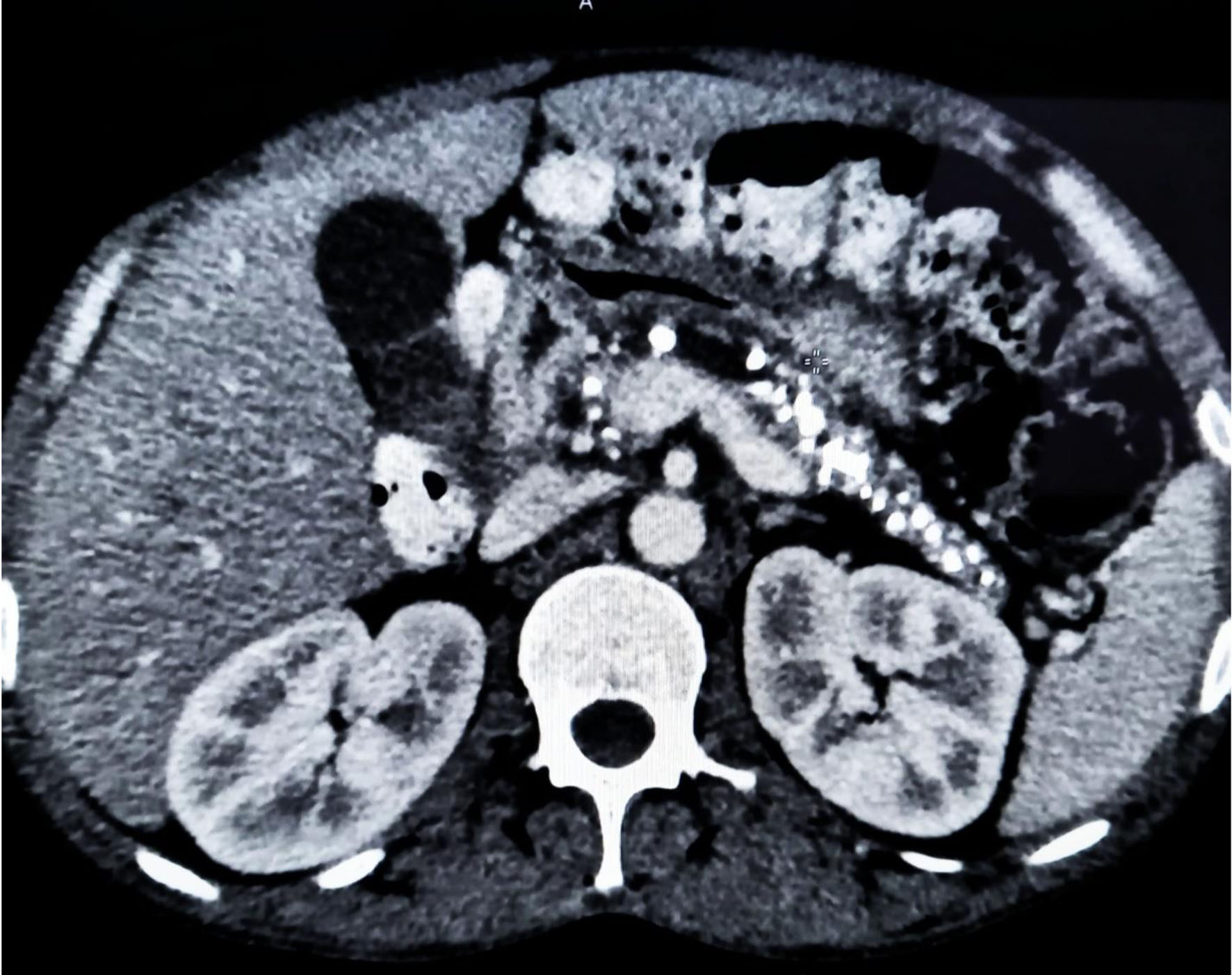 Click for large image | Figure 3. Contrast-enhanced computed tomography scan of abdomen showing multiple calcifications involving the head, body, and tail of pancreatic parenchyma. |
The patient was given intravenous fluid normal saline 1,000 mL in 2 h followed by 120 mL per hour. Insulin 12 units was started initially followed by 15 units and 20 units before breakfast and 14, 20, and 24 units before lunch, and 12, 20 and 24 units before dinner and insulin glargine (Lantus) 10 unit was started initially followed by 20 units. Also, a tablet of metformin 500 mg twice daily was given after meals. The patient was discharged with insulin regularly 20 units before breakfast, 24 units before lunch and 24 units before dinner, and 30 min before a meal, and Lantus (insulin glargine) was given 20 units subcutaneously at night. The patient was placed on a specific diet and throughout the patient’s stay in the hospital, he received instruction. The individual was advised to consult with his or her primary care provider. The patient was further advised for magnetic resonance cholangiopancreatography (MRCP).
| Discussion | ▴Top |
Globally around 10% of the population with diabetes has been diagnosed with CP, which is characterized by early-onset endocrine dysfunction of the pancreas, irreversible exocrine, and progressive fibrosis. The most prominent clinical characteristic is recurrent and severe abdominal discomfort. Diabetes develops in CP mostly as a result of pancreatic inflammation destroying islet cells. Malnutrition also impairs incretin production, leading to decreased insulin production from the surviving β cells [40].
Evaluation of the patient’s medical records, performing blood tests in order to determine fasting glucose levels and HbA1c, and analyzing the functioning of the pancreas via imaging procedures such as CT scans or endoscopic ultrasound (EUS) are all part of diagnosing secondary diabetes in the setting of CCP. Patients suffering from CP must be screened for diabetes with HbA1c. After diabetes confirmation, an autoimmune workup for T1DM must be sent to rule out the possibility of late-onset T1DM. CCP is one of the most common causes of T3cDM which is commonly underdiagnosed or misdiagnosed [41, 42]. CT and MRI are the preferred tests to detect CP at the earliest. Nutritional management of T3DM includes regular dietary assessment and monitoring to reduce hyperglycemia, prevent hypoglycemia, prevent malnutrition, and decrease the risk of diabetes-related complications [43]. The major aspects of therapeutic treatment in individuals with T3cDM include addressing exocrine pancreatic dysfunction and avoiding a shortage of fat-soluble vitamins (mostly vitamin D), as well as correcting defective fat breakdown and incretin production [6].
Once recognized, secondary diabetes in the circumstances of CCP is managed by focusing on both glycemic control and treating the root cause of pancreatic illness. A nutritious diet, frequent exercise, and weight management are all important factors in controlling blood sugar levels. To assist regulate glucose levels, medications such as insulin or oral hypoglycemic medicines may be administered.
| Conclusion | ▴Top |
Secondary diabetes management includes addressing the root of the problem or modifying drugs that might be aggravating the problem. Keeping a balanced diet, frequent exercise, and losing weight are all important in controlling blood sugar levels. If required, medications such as insulin or oral hypoglycemic medicines may be provided to assist regulate blood glucose levels.
T3cDM is a rare type of pancreatogenic diabetes resulting from pancreatic pathology and is often overlooked, underdiagnosed, or mistaken for T1DM or T2DM. So, in patients suffering from CP, it is important to consider T3cDM for planning effective long-term management. CT scans and MRIs play an important role in the diagnosis of the small subset of the population affected with T3cDM which should be considered. People with pancreatic diabetes must get continual medical treatment and surveillance. Frequent appointments with healthcare specialists, such as endocrinologists and gastroenterologists, may assist to guarantee that both the diabetes and the deeper pancreatic issue are properly managed.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient for publication of this case report including the clinical information and accompanying images.
Author Contributions
Study conception and design: Madhumati Varma. Data collection: Gurusha Bahl, Subhankar Das, Md Sadique Hussain. Analysis and interpretation of results: Dinesh K Upadhyay, Madhumati Varma, Rajveer Singh, Md Sadique Hussain. Draft manuscript preparation: Md Sadique Hussain, Gurusha Bahl. All the authors reviewed the results and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sarles H, Bernard JP. Pathogenesis of chronic pancreatitis. Canadian Journal of Gastroenterology. 1990;3(1):15-20.
- Pujahari AK. Chronic pancreatitis: a review. Indian J Surg. 2015;77(Suppl 3):1348-1358.
doi pubmed pmc - Wynne K, Devereaux B, Dornhorst A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol. 2019;34(2):346-354.
doi pubmed - Lundberg R, Beilman GJ, Dunn TB, Pruett TL, Freeman ML, Ptacek PE, Berry KL, et al. Early alterations in glycemic control and pancreatic endocrine function in nondiabetic patients with chronic pancreatitis. Pancreas. 2016;45(4):565-571.
doi pubmed pmc - Marginean CM, Popescu M, Vasile CM, Stanciu M, Popescu IA, Biciusca V, et al. Chronic calcifying pancreatitis associated with secondary diabetes mellitus and hepatosplenic abscesses in a young male patient: a case report. Gastroenterology Insights. 2022;13(3):305-312.
- Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19(42):7276-7281.
doi pubmed pmc - Johnston PC, Thompson J, McKee A, Hamill C, Wallace I. Diabetes and chronic pancreatitis: considerations in the holistic management of an often neglected disease. J Diabetes Res. 2019;2019:2487804.
doi pubmed pmc - Gudipaty L, Rickels MR. Pancreatogenic (type 3c) diabetes. Pancreapedia: The Exocrine Pancreas Knowledge Base. 2015.
- Shiratori K. Management of pancreatic diabetes secondary to chronic pancreatitis. The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery. 2018:495-502.
- Dugic A, Hagstrom H, Dahlman I, Rutkowski W, Daou D, Kulinski P, Lohr JM, et al. Post-pancreatitis diabetes mellitus is common in chronic pancreatitis and is associated with adverse outcomes. United European Gastroenterol J. 2023;11(1):79-91.
doi pubmed pmc - Sasikala M, Talukdar R, Pavan kumar P, Radhika G, Rao GV, Pradeep R, Subramanyam C, et al. beta-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012;57(7):1764-1772.
doi pubmed - Wagner-Bartak NA, Baiomy A, Habra MA, Mukhi SV, Morani AC, Korivi BR, Waguespack SG, et al. Cushing syndrome: diagnostic workup and imaging features, with clinical and pathologic correlation. AJR Am J Roentgenol. 2017;209(1):19-32.
doi pubmed - Clinton K. Cushing's syndrome. Nutritional Perspectives: Journal of the Council on Nutrition. 2017;40(4).
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567-575.
doi pubmed pmc - Culhane S, George C, Pearo B, Spoede E. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract. 2013;28(6):676-683.
doi pubmed - Coderre L, Debieche L, Plourde J, Rabasa-Lhoret R, Lesage S. The potential causes of cystic fibrosis-related diabetes. Front Endocrinol (Lausanne). 2021;12:702823.
doi pubmed pmc - Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152(4):491-499.
doi pubmed - Rogowicz-Frontczak A, Majchrzak A, Zozulinska-Ziolkiewicz D. Insulin resistance in endocrine disorders - treatment options. Endokrynol Pol. 2017;68(3):334-351.
doi pubmed - Cypress M, C-ANP CD, Spollett G. Diabetes can be associated with other endocrine diseases through a shared occur. Complete Nurse's Guide to Diabetes Care. 2009:390.
- Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab (Seoul). 2017;32(2):180-189.
doi pubmed pmc - Kwon S, Hermayer KL, Hermayer K. Glucocorticoid-induced hyperglycemia. Am J Med Sci. 2013;345(4):274-277.
doi pubmed - Fathallah N, Slim R, Larif S, Hmouda H, Ben Salem C. Drug-induced hyperglycaemia and diabetes. Drug Saf. 2015;38(12):1153-1168.
doi pubmed - Polcwiartek C, Vang T, Bruhn CH, Hashemi N, Rosenzweig M, Nielsen J. Diabetic ketoacidosis in patients exposed to antipsychotics: a systematic literature review and analysis of Danish adverse drug event reports. Psychopharmacology (Berl). 2016;233(21-22):3663-3672.
doi pubmed - Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology. 2021;2:36-50.
- Kumari R, Kaur J, Hussain S. Management of diabetes with COVID-19: a review. Int J Pharm Pharm. 2020;12:1-6.
- Maxwell DW, Jajja MR, Galindo RJ, Zhang C, Nadeem SO, Sweeney JF, Blair CM, et al. Post-pancreatectomy diabetes index: a validated score predicting diabetes development after major pancreatectomy. J Am Coll Surg. 2020;230(4):393-402.e393.
doi pubmed - Hart PA, Bradley D, Conwell DL, Dungan K, Krishna SG, Wyne K, Bellin MD, et al. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. 2021;6(8):668-675.
doi pubmed pmc - Gubergrits NB, Fomenko PG, Kolkina VY. The other way of pancreatogenic diabetes mellitus: exocrine pancreatic insufficiency upon diabetes mellitus.
- Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev. 2012;28(4):338-342.
doi pubmed - Sanyal D, Bhattacharjee K. Characteristics of children and adolescents with newly diagnosed Fibrocalculous pancreatitis diabetes (FCPD) and type 1 diabetes: A study from Eastern India. Diabetes Metab Syndr. 2022;16(7):102527.
doi pubmed - Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11(3):279-294.
doi pubmed - Kulkarni CB, Sekhar R, Moorthy S, Pullara SK, Prabhu NK, Kannan RR. Imaging of tropical chronic pancreatitis - a unique clinico-radiological entity. Journal of Gastrointestinal and Abdominal Radiology. 2020;3:144-152.
- Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)—are we neglecting an important disease? Eur J Intern Med. 2013;24(3):203-206.
doi pubmed - Jimenez-Luna C, Martin-Blazquez A, Dieguez-Castillo C, Diaz C, Martin-Ruiz JL, Genilloud O, Vicente F, et al. Novel biomarkers to distinguish between type 3c and type 2 diabetes mellitus by untargeted metabolomics. Metabolites. 2020;10(11).
doi pubmed pmc - Makuc J. Management of pancreatogenic diabetes: challenges and solutions. Diabetes Metab Syndr Obes. 2016;9:311-315.
doi pubmed pmc - Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1(3):226-237.
doi pubmed pmc - Wiwanitkit V. Diabetes type 3: A brief review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2008;2(3):223-226.
- Vonderau JS, Desai CS. Type 3c: Understanding pancreatogenic diabetes. JAAPA. 2022;35(11):20-24.
doi pubmed - Talukdar R, Sarkar P, Jakkampudi A, Sarkar S, Aslam M, Jandhyala M, Deepika G, et al. The gut microbiome in pancreatogenic diabetes differs from that of Type 1 and Type 2 diabetes. Sci Rep. 2021;11(1):10978.
doi pubmed pmc - Sasikala M, Talukdar R, Subramanyam C, Reddy DR. The enigma of type 3c diabetes in chronic pancreatitis. Pancreas Open J. 2016;1:19-21.
- Bahl G, Upadhyay DK, Varma M, Singh R, Das S, Hussain MS. Chronic calcific pancreatitis presented with secondary diabetes and diabetic ketoacidosis: a case report. Clinical Diabetology. 2023;12(3).
- Kamat R, Gupta P, Rana S. Imaging in chronic pancreatitis: State of the art review. Indian J Radiol Imaging. 2019;29(2):201-210.
doi pubmed pmc - Duggan SN, Ewald N, Kelleher L, Griffin O, Gibney J, Conlon KC. The nutritional management of type 3c (pancreatogenic) diabetes in chronic pancreatitis. Eur J Clin Nutr. 2017;71(1):3-8.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
