| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 13, Number 1, February 2023, pages 1-12
The Effect of Microgravity on Parathyroid Hormone Secretion: A Meta-Analysis
Benjamin Shepard Blue
Department of Neurology, University of California, Davis, Sacramento, CA 95816, USA
Manuscript submitted November 2, 2022, accepted December 31, 2022, published online February 25, 2023
Short title: Effect of Microgravity on PTH
doi: https://doi.org/10.14740/jem849
- Abstract
- Introduction
- Physiology in Microgravity
- Parathyroid Gland Function in Microgravity
- Methods
- Results
- Discussion
- Relationships with calcium and vitamin D
- Future Research
- References
| Abstract | ▴Top |
Background: The relationship between microgravity and parathyroid hormone (PTH), a keystone of bone mineral density, remains controversial. Bone loss is a prominent, ongoing issue in spaceflight, and PTH has been suggested as a treatment for microgravity-induced osteopenia, indicating the importance of this hormone. This systematic review and meta-analysis aimed to evaluate the association between exposure to microgravity and the production of PTH.
Methods: PubMed, Embase, Scopus, and Google Scholar were searched for studies reporting PTH levels during and after exposure to microgravity. Non-peer-reviewed articles, studies lacking control groups, and articles published earlier than 2002 were excluded. Twelve articles from 2002 to present, with a total of 145 subjects, were identified and the standardized mean differences from baseline PTH levels were combined in a random effects model. Two-way analysis of variance (ANOVA) with Tukey honestly significant difference (HSD) testing on weighted mean differences was conducted to obtain 95% confidence intervals.
Results: Compared to baseline measurements, significant changes in PTH levels are found during and after microgravity exposure. In-flight levels significantly decrease (P < 0.01), and post-flight levels show increases. Furthermore, there is evidence of an interaction between experimental condition (real or simulated microgravity) and time after removal from microgravity on PTH.
Conclusions: The findings of this systematic review and meta-analysis suggest that microgravity affects parathyroid gland function during and after spaceflight, with decreases in function in-flight and an increase at 7 days post-flight. Experimental condition also appears to play a role in the recovery timeline of PTH.
Keywords: Microgravity; Endocrine; Parathyroid; Bone metabolism
| Introduction | ▴Top |
Spaceflight grows ever ambitious. As astronauts spend longer periods in space with a future goal of a manned flight to Mars, it is critical to understand the physiological changes that occur in weightless conditions. Loss of bone density during spaceflight is a preeminent issue in aerospace medicine; upon return to the ground, astronauts are predisposed to fracture under the heavier load of Earth’s gravity and bone mineral density is recovered slowly if fully recovered at all [1, 2]. Parathyroid hormone (PTH) has gained attention in microgravity research for its role in bone density, having been used clinically to treat osteoporosis [3] and to slow bone loss in rats under simulated weightlessness [4].
Secretions of many hormones have been observed to decrease during spaceflight and in simulations of microgravity, suggesting that the human endocrine system responds to changes in ambient gravity. However, studies can be sparse and report heterogeneous results. Furthermore, several confounding variables in the spacecraft environment may alter parathyroid function separately from microgravity, such as components in the spacecraft itself. For example, iodine is no longer used as a water disinfectant in American spacecraft after observations that iodine contributed to increased thyrotropin (TSH) levels, a marker of hypothyroidism [5].
This indicates a need for a synthesis of microgravity studies to reduce the effect of confounds in single studies, emphasizing recent literature to reflect updates in gravity research. Furthermore, the effect of real versus simulated microgravity conditions on experimental results has not been thoroughly explored. This systematic review and meta-analysis gathers studies from January 1, 2002 to present concerning the effect of microgravity on PTH production to elucidate the relationship between microgravity exposure, experimental condition, and PTH levels.
| Physiology in Microgravity | ▴Top |
Decades of spaceflight research identify several physiological changes that occur in weightless conditions, many with foundations in the endocrine system. Osteopenia, primarily from load-bearing bones such as the pelvis and femur [6], is one of the most pressing and well-documented issues in spaceflight due to the potential for post-spaceflight complications, incurring similar depressed hormone profiles and high risks of bone fracture as age-related osteoporosis [7, 8]. Loss of skeletal muscle during flight compounds this risk of fracture, predisposing astronauts to unsteadiness and weakness on the ground [6].
On top of musculoskeletal changes, an assortment of other adaptations also occurs in weightless conditions. Without gravity, body fluids shift from the feet to the head, incurring reductions in blood volume. This acquired hypovolemia potentiates orthostatic hypotension when astronauts return to normal gravity [9]. Cephalad fluid shifts also increase intraocular pressure, contributing to long-term vision changes seen in astronauts on extended spaceflights [10]. Virtually every body system is affected by microgravity; for example, immune, cardiovascular, and nervous systems all show cellular changes during spaceflight, altering protein expression and the cell cycle, and incurring functional changes in cell adhesion and morphology [11, 12].
As hormones play key regulatory roles in virtually all physiological systems, it is critical to understand how glandular function changes in weightless conditions. Importantly for spacefarers, bone density is principally regulated through the hormones PTH and calcitonin [4, 8], and skeletal muscle mass is maintained in part by the thyroid hormone thyroxine [13]. Understanding how endocrine function is affected by microgravity is thus vitally important to understand, with its roles in preeminent issues such as bone and muscle loss as well as the broad scope of physiological effects seen in weightlessness. This swath of changes makes gravitational physiology research incredibly important to better understand a novel physical environment and to support the human body during long-term spaceflight.
| Parathyroid Gland Function in Microgravity | ▴Top |
PTH levels have been observed to drop during exposure to microgravity coupled with an increase above pre-flight baseline after return to Earth. This reduced hormone secretion during spaceflight is paralleled in several other endocrine organs, such as in T3, T4, and computed tomography (CT) from the thyroid [14-16]. Production of insulin and corticosteroids [8] as well as renin [14] from the pancreas, adrenal glands, and kidneys, respectively, are also all negatively impacted by exposure to microgravity.
However, multiple factors obfuscate definite conclusions about the endocrine system in weightless conditions. Complex interactions between several confounding variables in the spaceflight environment obfuscate the effect of microgravity alone, such as background radiation or psychological factors like isolation. Not only does this complicate literature analysis, but also adds technical obstacles to microgravity research by making accurate simulations more difficult to achieve. Low sample sizes and poor inter-study agreement further obfuscate clear trends in study results. These challenges perplex the formation of concrete conclusions regarding endocrine function in microgravity.
Recent discourse on thyroid function during spaceflight exemplifies these difficulties. For example, a study on American spacecraft found that iodine present in the water contributed to decreases in thyroid hormone levels [17], leading to preliminary suggestions that microgravity does not directly influence thyroid function. However, studies conducted on non-American spacecraft show a continued prevalence of decreased thyroid levels, such as on Kosmos missions [18-20], Spacelab [21-23], Euromir [24], or Mir [25] where silver was used as a water disinfectant. Furthermore, studies conducted on American spacecraft after the replacement of iodine continue to show decreased in-flight thyroid hormone levels [26-28]. Astronauts also show increased thyroid hormone levels immediately post-flight, suggesting a compensatory response after an in-flight decrease in thyroid function [24, 25, 29]. Hidden in this discourse is also the effect of technological advancement, where changes in both spaceflight research techniques and engineering subtly change the results of studies over time, especially in such a young, highly innovative field as aerospace medicine. This discussion illustrates the effect of confounding variables, high inter-study variation, and limited sample sizes in microgravity research, especially in endocrinological studies, and how these issues obfuscate clear conclusions. These challenges across all microgravity research naturally extend to PTH in microgravity, spawning ongoing knowledge gaps regarding the parathyroid gland in space. In turn, there is a need for a synthesis of modern literature to quantify the effect of weightlessness on parathyroid function.
Changes in endocrine function are paralleled by alterations to tissue and gene expression. Recent histological studies on endocrine cells cultured in microgravity show changes in gene expression, cell adhesion, and tissue morphology in a wide variety of endocrine tissues [11, 12, 26]. Again using the thyroid as an example, cellular changes to the thyroid have been suggested as a potential mechanism for microgravity-associated hypothyroidism. Both carcinoma and benign thyroid cells show increased apoptosis and decreased hormone production as well as altered protein and gene expression profiles and three-dimensional (3D) conglomeration when placed in microgravity [30-32]. In turn, there is substantial evidence to suggest that microgravity influences thyroid tissue at the cellular level. Similar histological changes have been noted in other endocrine glands such as the pancreas, including increases in vacuolation as well as islet size and number, reflecting reductions in insulin production during spaceflight [33]. However, while these structural changes to endocrine tissue have been studied fairly extensively in microgravity, little information is available regarding changes to parathyroid tissue. The parathyroid gland may undergo similar structural changes in microgravity, but further research is required to clarify the specific effect of microgravity on parathyroid tissue.
With the prevalence and strong potential for complications with microgravity-induced osteopenia, studying CT and PTH in microgravity is critical due to their roles as keystones of calcium balance. Isolated observations in the literature show a similar pattern in CT levels with exposure to microgravity, with a drop in CT levels in weightless conditions coupled with an increase after exposure. However, there are not enough data on CT levels to produce substantive results from a systematic review and quantitative analysis. Enough data exist for PTH, however, for meaningful meta-analysis. As loss of bone density continues to impact astronauts returning from space, synthesizing data on PTH levels during and after flight will elucidate the recovery timeline and establish the magnitude of change for the parathyroid gland.
| Methods | ▴Top |
Literature search
A systematic literature search was performed using Embase, Scopus, JAMA Network, and PubMed. Google Scholar was used as a secondary search tool to identify grey literature and other studies, until 19 July 2022. The bibliographies of manuscripts were also screened to obtain additional back-referenced material. Search terms used for each database are collected in Table 1.
 Click to view | Table 1. Keywords Used to Search Individual Databases Until July 19, 2022 With Records Identified From Each Source |
All inclusion and exclusion criteria were chosen a priori. To be included, articles must measure levels of PTH in either real or simulated microgravity. Articles utilizing thyroid cancer cells were excluded, as malignant thyroid cells show a different gene expression profile as well as altered aggregation and proliferative behavior in microgravity compared to nonmalignant cells [34]. Animal subject studies were excluded. Non-English articles were translated and not excluded. Articles greater than 20 years of age at the time of reading were excluded using a minimum year of publication of 2002. Studies utilizing both simulated and real microgravity conditions were included. Studies must also have been peer-reviewed. Experimental designs lacking control groups or pre-flight measurements of hormone levels were excluded. Of the initial 1,022 articles retrieved, 12 were kept and analyzed. Figure 1 depicts the selection process for these studies.
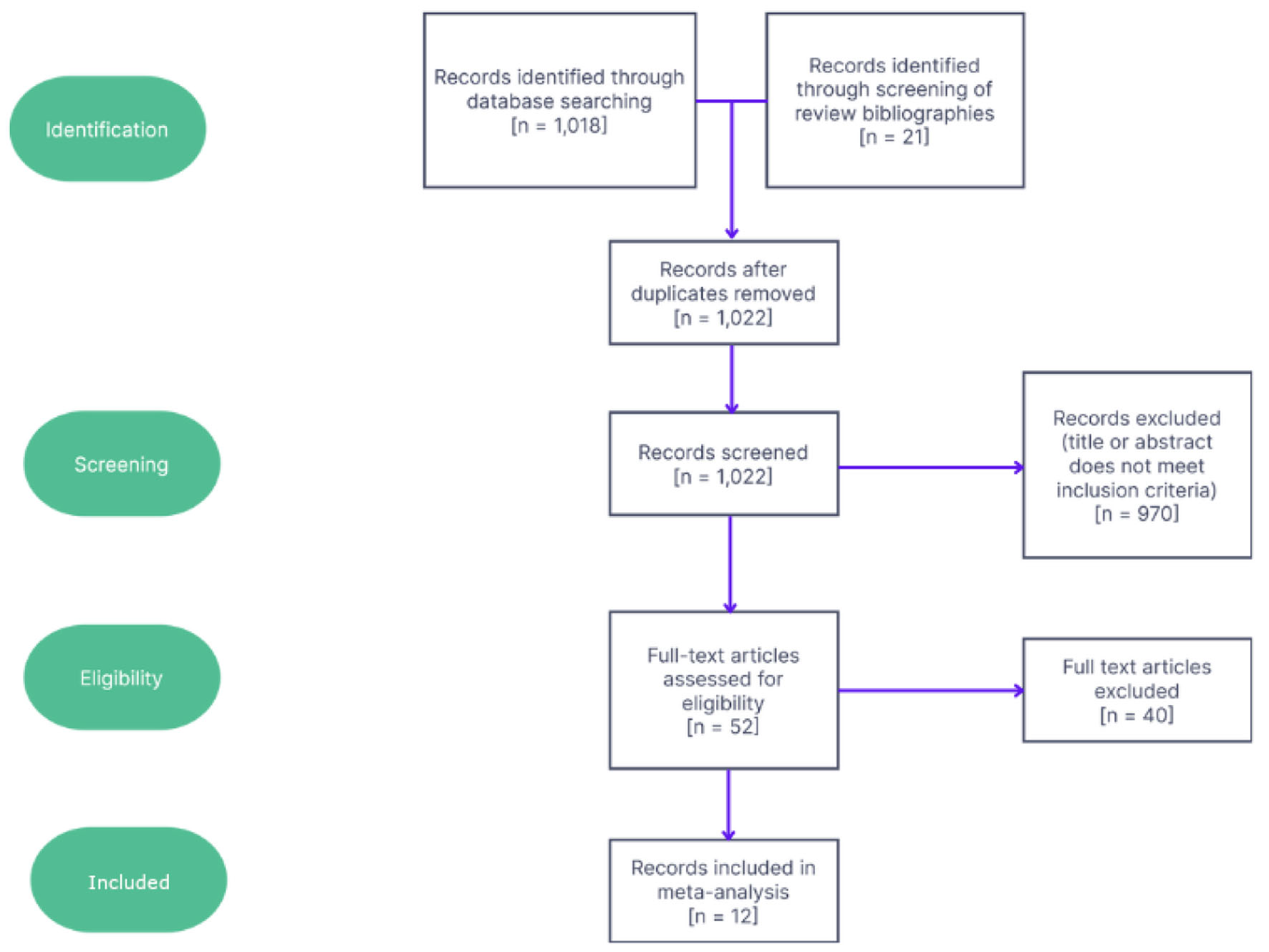 Click for large image | Figure 1. Flowchart showing screening and selection process for studies along with numbers of studies excluded at each step. |
Data extraction
The following data were extracted: author name(s), year of publication, whether the microgravity experimental conditions were real or simulated, size of treatment groups, mean or median PTH levels and variance in these measurements, mean or median calcium levels in serum and/or urine as well as variance in these measurements, mean serum vitamin D levels as both calcitriol (1,25(OH)2D) and calcifediol (25(OH)D) and variance, and timepoint of measurement: pre-, in-, or post-flight. Post-flight values were binned into categories depending on the time after removal from microgravity: < 24 h after recovery (R+0), 24 - 48 h after (R+1), between 2 and 7 days after (R+2-7), and more than 7 days after (>R+7). Data extraction from graphical data was performed using MetaLab for MATLAB R2016b [35]. In cases where both levels of intact PTH (iPTH) and mid-molecule PTH are reported, iPTH is recorded in line with more modern techniques [36]. Baseline hormone measurements were taken as either hormone levels measured before microgravity exposure or as measurements in control groups.
Table 2 [27, 28, 37-46] shows the characteristics and data extracted from the 12 studies.
 Click to view | Table 2. Data Extracted for Each of the 12 Studies That Passed the Screening Process, Including Year, Name of First Author, Sample Size (n), Microgravity Condition (Real Versus Simulated), Time Points Collected, and Databases Where the Article Is Indexed |
Analysis
Analytical methods were chosen a priori. To standardize measurements across many different units, Cohen’s d was calculated for each timepoint in an individual study, as given by the formula below [47]. Variances could reasonably be assumed to be equal between timepoints within a single study. All extracted data, along with calculated Cohen’s d for each study, are available in the Supplementary Materials 1-3 (www.jofem.org) to this article.
A weighted average of the d statistic (x) was computed using sample size to weight studies, as in the formula below. Heterogeneous variance among all studies precluded the use of inverse variance as a weighting factor.
Two-way analysis of variance (ANOVA) was then conducted to analyze the effect of experimental condition (i.e., real or simulated microgravity) and timepoint on PTH hormone levels. Tukey post hoc testing was then conducted to further characterize differences.
| Results | ▴Top |
Simple main effects analysis identified a significant difference between timepoints (P < 0.01) as well as a significant difference between experimental conditions (P < 0.01). These main effects were qualified by an interaction between experimental condition and timepoint (P < 0.05). The results are summarized in Table 3.
 Click to view | Table 3. Summary of Results From Two-Way ANOVA for a Relationship Between PTH Level and Timepoint, Experimental Condition, and the Combination of These Factors |
Post hoc testing identified significant differences from baseline in in-flight and R+7 PTH levels, as visualized in Figure 2. No significant differences from baseline were identified for R+0, R+1, and R+2-7. >R+7 shows a trend to significant increase from baseline (P < 0.1). In-flight PTH data for individual studies are depicted in Figure 3, showing the consistency of observed decreases from baseline across all studies. In both fixed and random effects models, there is also evidence of a decrease by a weighted average of one standard deviation.
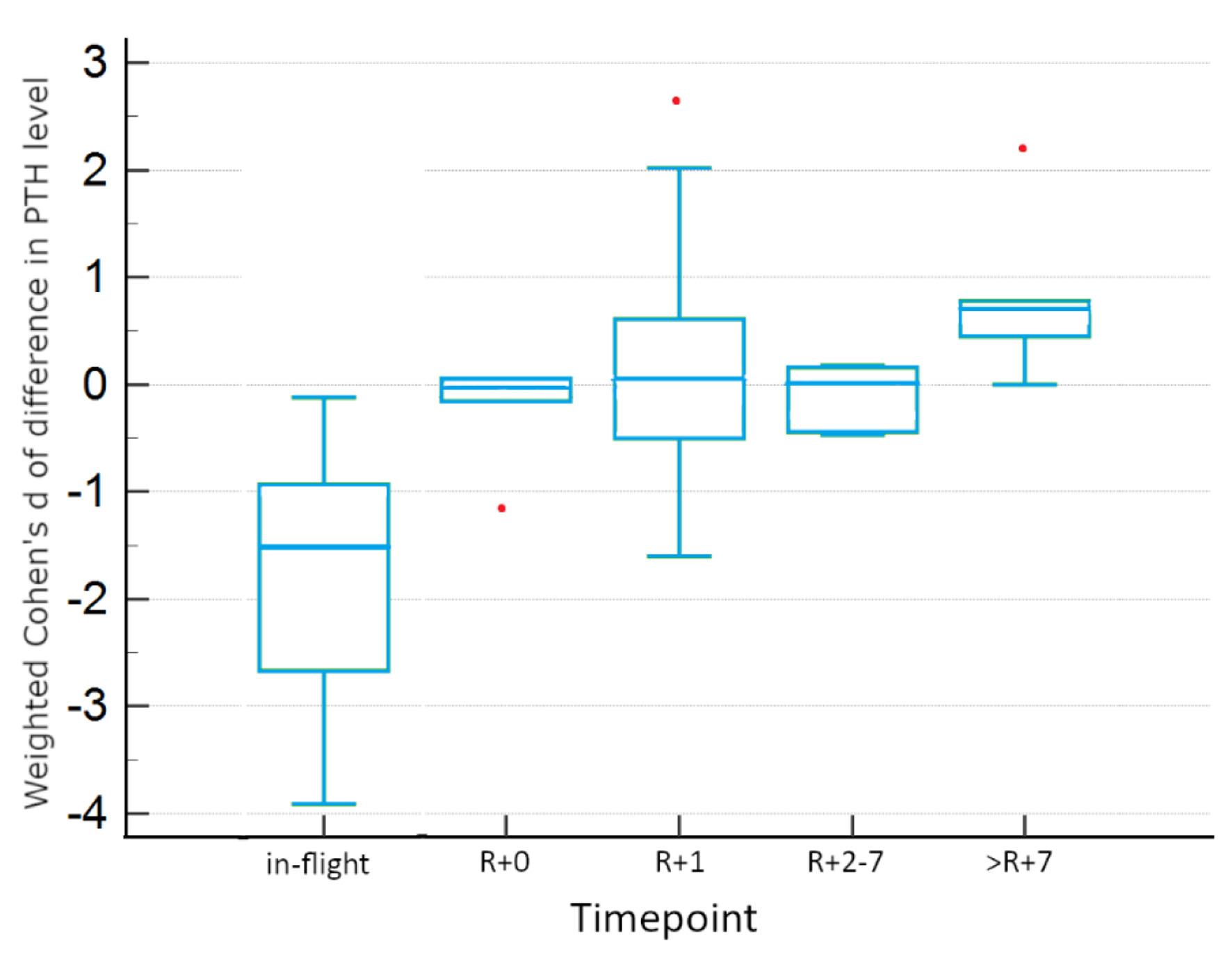 Click for large image | Figure 2. Tukey HSD plot of confidence intervals, inclusive of all studies. Red points indicate outlier data. HSD: honestly significant difference. |
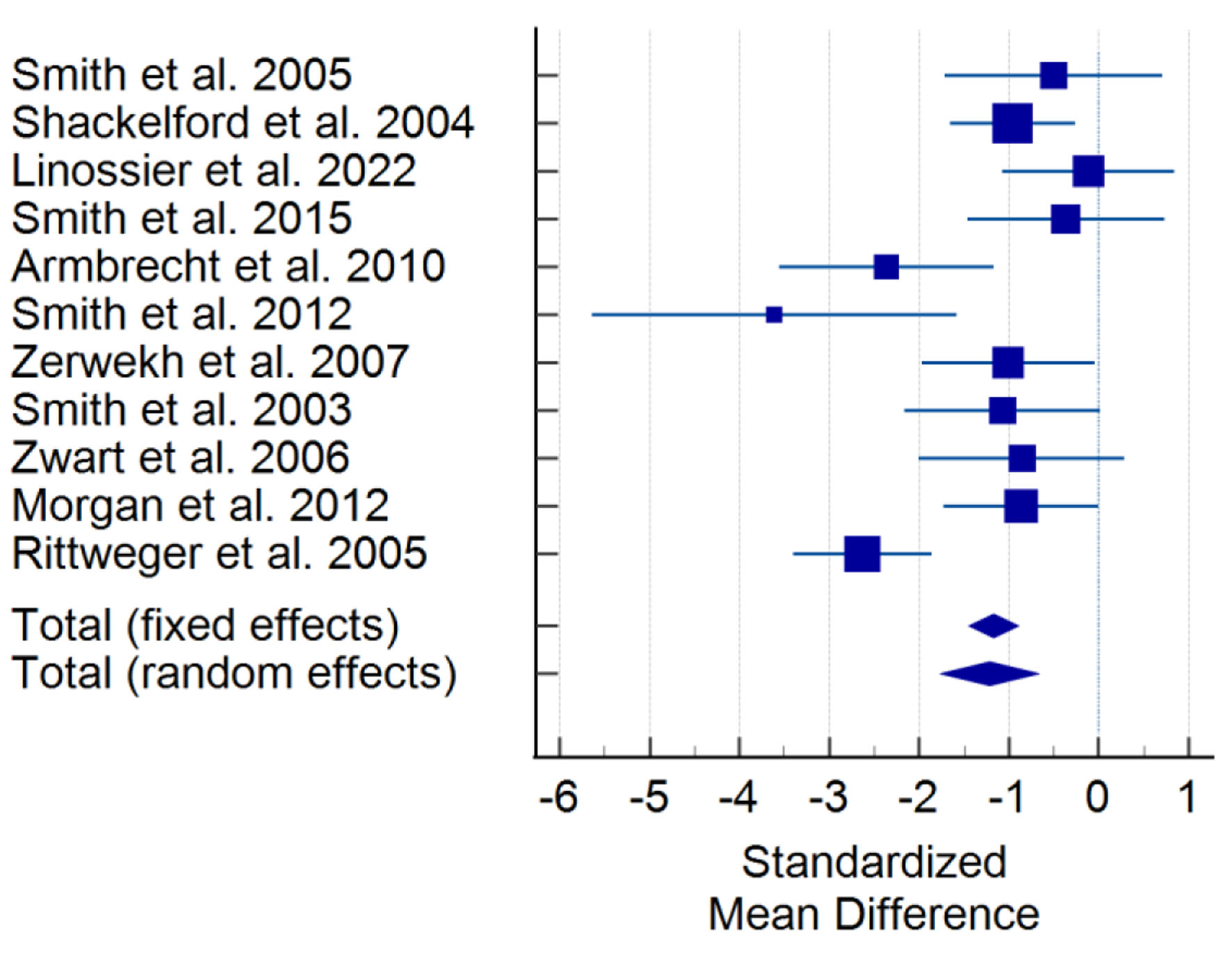 Click for large image | Figure 3. Forest plot for in-flight levels of PTH, inclusive of all studies. Squares with lines show 95% confidence intervals for a single study. Diamonds at the bottom indicate weighted average confidence interval computed from all studies. PTH: parathyroid hormone. |
There is evidence for differences between experimental conditions within timepoints, as seen in Table 4, which summarizes the results of Tukey honestly significant difference (HSD) testing for two-way ANOVA. In-flight and >R+7 measurements were not significantly different between real and simulated conditions. However, there is evidence for significant differences between experimental conditions for other post-flight timepoints. Real microgravity shows significant increases from baseline at R+0, R+1, and R+2-7, displayed in Figure 4. However, simulated microgravity shows significant decreases from baseline at these same timepoints, as visualized in Figure 5. These opposite findings may explain the apparent lack of difference at these timepoints when both conditions are incorporated.
 Click to view | Table 4. Tukey HSD Testing With 95% Confidence Intervals for Each Timepoint, Separated by Experimental Condition |
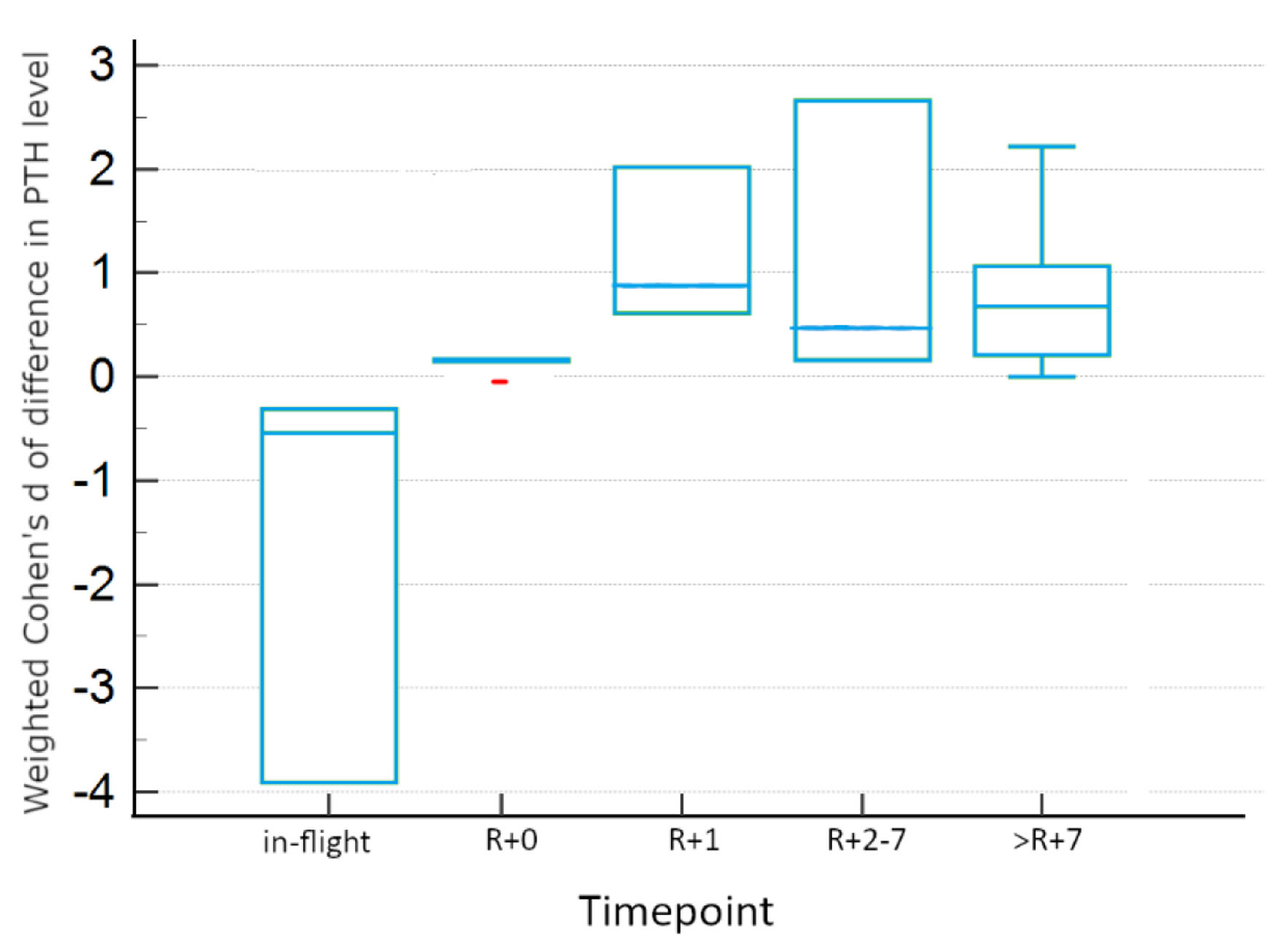 Click for large image | Figure 4. Tukey HSD plot showing 95% confidence intervals for real microgravity studies for each timepoint. Red points indicate outlier data. HSD: honestly significant difference. |
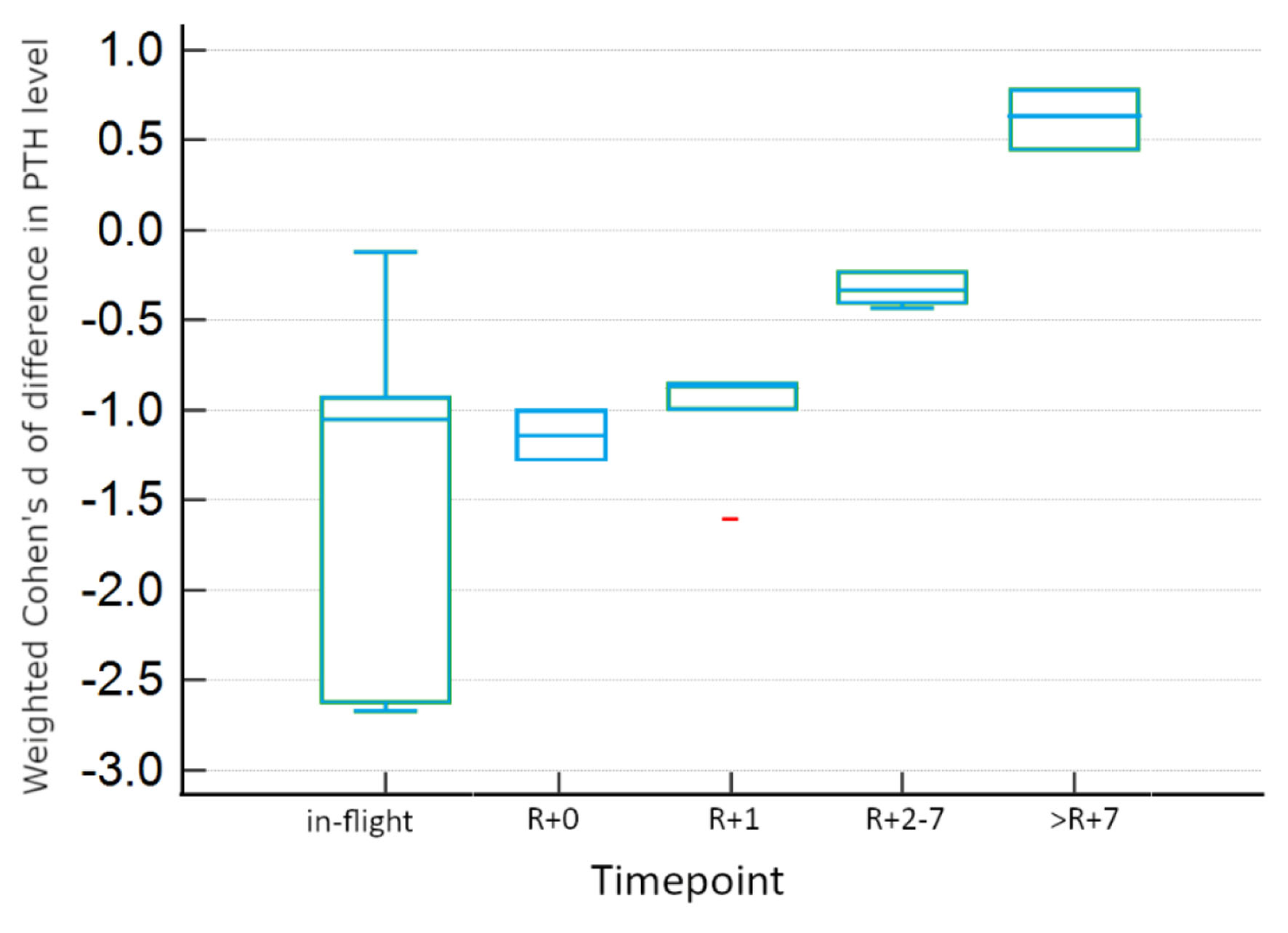 Click for large image | Figure 5. Tukey HSD plot showing 95% confidence intervals for simulated microgravity studies for each timepoint. Red points indicate outlier data. HSD: honestly significant difference. |
| Discussion | ▴Top |
Consistent throughout the data are significant increases between in-flight and post-recovery values, suggesting a compensatory rebound in PTH levels after removal from microgravity. Notably, there is a significant difference between PTH levels in-flight and within a few hours of recovery. This rebound pattern is paralleled in studies on other hormones. This provides evidence that results from in-flight and R+0 measurements should not be conflated.
All studies save for two reported a statistically significant decrease in PTH while in flight. However, the magnitude of this difference appears heterogeneous between studies, even when normalized to a statistic like Cohen’s d. This heterogeneity is characteristic of both experimental conditions. Between-study variability should be considered when attempting to pinpoint an average effect size of microgravity. Some of this may be due to the newness of gravitational physiology, where measurement methods and protocols may not be standardized.
The recovery timeline for PTH is difficult to establish in this meta-analysis, as post-flight results appear heterogeneous between studies. Furthermore, results appear to be dependent on experimental conditions: studies in real microgravity show a significant increase in hormone levels post-flight, whereas simulated studies show decreases post-flight. Continued study examining microgravity research techniques is needed to clarify the effect of different protocols on experimental results.
Overall, these findings for PTH echo what has been observed in other hormones. For example, in the thyroid, T3 and T4 [5, 33, 48] as well as CT levels [28, 49, 50] have been observed to decrease while in-flight with a post-flight rebound. These reduced hormone levels match physical changes in thyroid tissue morphology, such as increases in follicle size, cell membrane composition [51], changes in cytoskeletal elements regulating the 3D structure and cell-cell interactions [34], as well as increased expression of apoptotic proteins [31]. Thyroid carcinoma cells reduce their T3 and T4 production as they undergo greater rates of apoptosis [31, 32], and C cells that produce CT have been observed to become less active in space [52, 53] and to decrease in number while in-flight [50]. Decreased T3 and T4 levels also correlate with changes in tissue morphology. An in-depth study of parathyroid tissue is forthcoming and may follow this same pattern seen in many other endocrine tissues. Still, there is only a relatively small body of research on in-flight hormone production in human subjects, particularly in real microgravity conditions. Confounding factors also hinder the ability to draw conclusions; for example, there is evidence to suggest that the loss of C cells is at least in part due to confinement stress and not microgravity [54]. Still, the evidence that PTH production is truly reduced in microgravity is compelling. Future research will help clarify the magnitude of change in parathyroid function in microgravity, the effects on gene expression and tissue morphology, and the effect of confounding variables on observed changes in hormone secretion.
| Relationships with calcium and vitamin D | ▴Top |
Calcium
Exposure to microgravity is correlated with increased rates of bone resorption and elevated calcium excretion in urine, producing a negative calcium balance that reduces bone density over time [1]. Naturally, almost all studies included in this analysis report a significant increase in urinary calcium during flight. As calcium is a major regulator of PTH levels, it would be expected that bone demineralization during spaceflight is correlated with decreases in PTH levels [7]. Again, data from the included studies concur with this expectation, where increases in excreted calcium occur at the same time as the observed drop in PTH. A recovery timeline for urinary calcium is difficult to define due to low data availability, though many studies reporting post-flight levels appear to return to baseline by R+2-7.
As for serum calcium, data from the included studies are conflicted; a majority report no significant difference during flight while others report significant albeit modest increases, and two studies find slight decreases. A post-flight recovery timeline for serum calcium is unclear, but there is some evidence that serum calcium is depressed after spaceflight. Very limited data are available for ionized calcium, as only three of the 12 studies initially screened report any data on this factor.
However, separating real and simulated microgravity conditions suggests differences between the two regarding calcium levels. No studies conducted in real microgravity report significant changes in serum calcium, different from the near-unanimous in-flight drop in PTH seen across both types of experimental conditions. Real microgravity studies also appear to produce small magnitudes of negative change in serum calcium as opposed to the positive change that trends to significance in simulated studies. Both conditions appear to affect urinary calcium similarly, with all studies reporting a significant increase in urinary calcium and a similar magnitude of change (Table 5).
 Click to view | Table 5. Summary Data for In-Flight Serum and Urinary Calcium Levels |
Vitamin D
PTH stimulates the formation of calcitriol, the active form of vitamin D, facilitating the absorption of calcium from the intestines and increasing bone turnover to raise bone density [7]. In turn, data from five of the eight studies reporting calcitriol levels show reductions in calcitriol during flight, correlated with the in-flight drop in PTH levels. These decreases are present in both real and simulated microgravity. Reduced calcitriol prevents the effective absorption of calcium into the blood, contributing to negative calcium balance in the body and furthering bone demineralization in weightless conditions.
There is scattered evidence of a post-flight rebound in calcitriol levels, but the majority of studies report no significant difference from baseline after exposure to microgravity. As with calcium, there is a lack of data in post-flight vitamin D data to establish a clear timeline for recovery.
There is possibly an effect of experimental condition on calcitriol levels. Subjects exposed to real microgravity show no significant difference from baseline calcitriol levels during flight, reporting a far lower magnitude change in calcitriol levels than in simulation. While more data are required to clarify these distinctions, this may represent emerging evidence that real and simulated microgravity have differential effects on calcitriol levels.
Calcifediol, on the other hand, does not appear to change significantly during or after spaceflight: only one study reports a change in calcifediol levels at any timepoint. This may be due to vitamin D supplementation in both real and simulated conditions, as is common in both microgravity research subjects and astronauts outside of experiments [7]. Still, there seems to be a difference between real and simulated microgravity in calcifediol levels: real microgravity seems to decrease calcifediol, while simulated conditions appear to increase it. This conclusion is hindered by high intra-study measurements of variance and relatively small magnitudes of change, however.
The observed changes to calcitriol imply differences between real and simulated weightlessness, either as a result of experimental technique or a true physical difference between the two conditions. As calcifediol plays a major role in recovering bone mineral density, more data are needed on calcitriol in the context of decreased PTH levels to obtain a clearer picture of bone mineral density during spaceflight (Table 6).
 Click to view | Table 6. Summary Data for In-Flight Calcifediol and Calcitriol Levels |
Analysis of bias and limitations
A possible source of bias is the small sample sizes of most individual studies. Furthermore, the selection of astronauts for spaceflight introduces nonrandom sampling error in real microgravity conditions. This also hinders the generalization of these results to a broader population. Reporting issues such as unreported mean differences and measures of variance led to the exclusion of otherwise suitable studies, reducing the amount of available data. Searching and screening of articles were conducted carefully and all articles that met inclusion criteria were able to be accessed. None were unable to be located or inaccessible behind a paywall.
For in-flight changes in PTH levels, there was a trend to significance for evidence of publication bias (Egger’s test intercept = -3.4695; P = 0.07). Visual analysis of the funnel plot for all PTH studies, shown in Figure 6, reveals an asymmetrical distribution. In turn, there is potential for reporting bias in this meta-analysis.
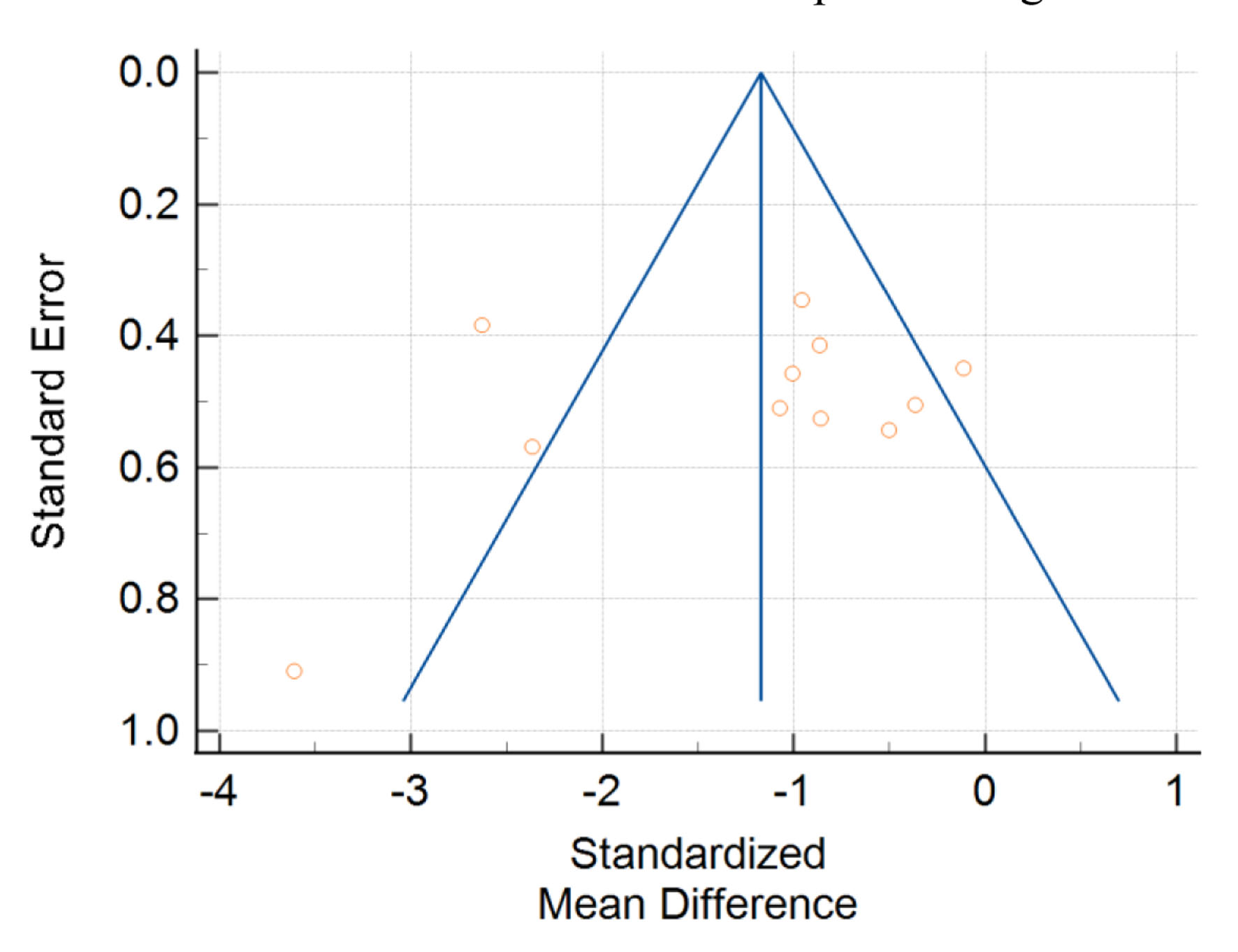 Click for large image | Figure 6. Funnel plot with standard error of studies on the y-axis plotted against standardized mean difference on the x-axis. Yellow circles represent single studies. |
Allowing the inclusion of studies in both real and simulated microgravity strengthened the power of the statistical tests but possibly introduced heterogeneity. Substudies on real and simulated conditions explore this and indicate that this could potentially bias conclusions from post-flight measurements, especially when summarizing studies from both conditions.
| Future Research | ▴Top |
A major challenge in gravitational research is isolating the effect of microgravity from a ubiquity of possible confounds. Confinement stress, loss of privacy, and disruption of social and familial ties are a few factors that contribute to physiological stress in space [41, 45]. In real microgravity, background radiation and loss of circadian cues are additional factors that may play a role in decreased endocrine function [55, 56]. Another example is common plastics: bisphenol A and phthalates, two well-known endocrine disruptors, have been shown to alter the function and tissue of the parathyroid gland and contribute to tumor formation, hinting at effects on gene expression [57]. These plastics have been known to leach and contaminate spacecraft components [58]. Also common in astronauts is a hypocaloric diet and negative energy balance [59], which may further reduce PTH levels during flight [60].
Bone loss is also subject to many confounding factors. For example, as opposed to reductions in PTH, lack of UV light on spacecraft may contribute to osteopenia [61]. Other studies find evidence that confinement stress negatively impacts calcitonin production, further reducing bone density [54]. Thus, many factors affect bone density that may exacerbate the effect of reduced PTH. With the ubiquity of potential confounds that remain unexplored, identifying and exploring these confounds is critical to isolate the effect of microgravity on parathyroid function.
The difference between real and simulated microgravity has not been thoroughly explored in endocrine literature. There is evidence that experimental condition affects the recovery timeline for PTH and may provide evidence that the experimental setting is yet another of many possible confounds affecting gravity research. Corroborating these differences appears to be the trends in vitamin D and calcium, which seem to change differently based on the experimental condition. One such example is how serum calcium appears to change less in real microgravity than in simulated microgravity, though both real and simulated conditions produce similar magnitudes of change in PTH. As the parathyroid gland is regulated by serum calcium, this may suggest that real microgravity impacts parathyroid function itself. Still, several technical obstacles such as low sample sizes and discrepancies between research techniques must be closely analyzed to better define conclusions regarding PTH and other markers of bone formation in microgravity.
This analysis provides evidence to support the hypothesis that microgravity exposure affects parathyroid gland function and raises new questions in regard to the effect of experimental condition. The implications of these issues affect not just the results of endocrine research, but all research exploring the effects of weightless conditions and should be addressed. Continuing to understand the effect of gravity on physiology is critical to preserving astronaut health; as the goals of aerospace medicine move farther into space, ensuring the safety of astronauts both during and after long-duration spaceflight is critical for manned spaceflight to continue pressing forward.
| Supplementary Material | ▴Top |
Suppl 1. PTH Data.
Suppl 2. Calcium Data.
Suppl 3. Vitamin D Data.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from an outside funding agency.
Conflict of Interest
The author declares there is no conflict of interest to report.
Author Contributions
BSB performed all research, analysis, and preparation of the manuscript.
Data Availability
The author declares that the data supporting the findings of this study are available within the article and supplementary files.
| References | ▴Top |
- Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006-1012.
doi pubmed - von Kroge S, Wolfel EM, Buravkova LB, Atiakshin DA, Markina EA, Schinke T, Rolvien T, et al. Bone loss recovery in mice following microgravity with concurrent bone-compartment-specific osteocyte characteristics. Eur Cell Mater. 2021;42:220-231.
doi pubmed - Crandall C. Parathyroid hormone for treatment of osteoporosis. Arch Intern Med. 2002;162(20):2297-2309.
doi pubmed - Moriyama I, Iwamoto J, Takeda T, Toyama Y. Comparative effects of intermittent administration of human parathyroid hormone (1-34) on cancellous and cortical bone loss in tail-suspended and sciatic neurectomized young rats. J Orthop Sci. 2002;7(3):379-385.
doi pubmed - Smith SM, Zwart SR, McMonigal KA, Huntoon CL. Thyroid status of Space Shuttle crewmembers: effects of iodine removal. Aviat Space Environ Med. 2011;82(1):49-51.
doi pubmed - Grigoriev AI, Oganov VS, Bakulin AV, Poliakov VV, Voronin LI, Morgun VV, Shnaider VS, et al. [Clinical and physiological evaluation of bone changes among astronauts after long-term space flights]. Aviakosm Ekolog Med. 1998;32(1):21-25.
- Genah S, Monici M, Morbidelli L. The Effect of Space Travel on Bone Metabolism: Considerations on Today's Major Challenges and Advances in Pharmacology. Int J Mol Sci. 2021;22(9):4585.
doi pubmed - Strollo F, Gentile S, Strollo G, Mambro A, Vernikos J. Recent progress in space physiology and aging. Front Physiol. 2018;9:1551.
doi pubmed - Buckey JC, Jr., Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, et al. Orthostatic intolerance after spaceflight. J Appl Physiol (1985). 1996;81(1):7-18.
doi pubmed - Risk of microgravity-induced visual impairment and elevated intracranial pressure (VIIP) - NASA technical reports server (NTRS). https://ntrs.nasa.gov/citations/20110012449 (Accessed Oct 23, 2022).
- Gravitational physiology of human immune cells: a review of in vivo, ex vivo and in vitro studies. Europe PMC. https://europepmc.org/article/med/11539302 (Accessed Jun 12, 2022).
- Goswami N, White O, Blaber A, Evans J, van Loon JJWA, Clement G. Human physiology adaptation to altered gravity environments. Acta Astronaut. 2021;189:216-221.
doi - Bloise FF, Cordeiro A, Ortiga-Carvalho TM. Role of thyroid hormone in skeletal muscle physiology. J Endocrinol. 2018;236(1):R57-R68.
doi pubmed - Leach CS, Johnson PC, Rambaut PC. Metabolic and endocrine studies: the second manned Skylab mission. Aviat Space Environ Med. 1976;47(4):402-410.
- Leach CS, Rambaut PC. Biochemical responses of the Skylab crewmen: An overview. 1977. Accessed Jun 17, 2022. [Online]. Available: https://ntrs.nasa.gov/citations/19770026859.
- Stein TP, Schluter MD, Moldawer LL. Endocrine relationships during human spaceflight. Am J Physiol. 1999;276(1 Pt 1):E155-162.
doi pubmed - McMonigal KA, Braverman LE, Dunn JT, Stanbury JB, Wear ML, Hamm PB, Sauer RL, et al. Thyroid function changes related to use of iodinated water in the U.S. Space Program. Aviat Space Environ Med. 2000;71(11):1120-1125.
- Loginov VI. [An immunocytochemical study of the C-cell function of the thyroid in rats exposed on the Kosmos-2044 biosatellite]. Aviakosm Ekolog Med. 1993;27(2):71-76.
- Plakhuta-Plakutina GI, Kabitskii EN, Dmitrieva NP, Amirkhanian EA. [Studies of the morphology of the thyroid gland and thyroid hormone levels in the blood of rats in experiments on "Kosmos-1667" and "Kosmos-1887"]. Kosm Biol Aviakosm Med. 1990;24(4):25-27.
- Plakhuta-Plakutina GI, Dmitrieva NP, Amirkhanian EA. [The C-cell system of the thyroid in rats following a flight on the Kosmos 1667 biosatellite]. Kosm Biol Aviakosm Med. 1988;22(2):26-32.
- Schatte C, Grindeland R, Callahan P, Funk G, Lencki W, Berry W. Animal studies on Spacelab-3. 1986. Accessed: Jun 15, 2022. [Online]. Available: https://ntrs.nasa.gov/citations/19860007419.
- Loginov VI. Histological and immunocytochemical assay of rats' thyroid after the Spacelab-1 mission. Aerosp Environ Med. 1994;28(1):21-24.
- Morey-Holton ER, Schnoes HK, DeLuca HF, Phelps ME, Klein RF, Nissenson RH, Arnaud CD. Vitamin D metabolites and bioactive parathyroid hormone levels during Spacelab 2. Aviat Space Environ Med. 1988;59(11 Pt 1):1038-1041.
- Caillot-Augusseau A, Lafage-Proust MH, Soler C, Pernod J, Dubois F, Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95). Clin Chem. 1998;44(3):578-585.
doi pubmed - Grigoriev AI, Bugrov SA, Bogomolov VV, Egorov AD, Polyakov VV, Tarasov IK, Shulzhenko EB. Main medical results of extended flights on space station Mir in 1986-1990. Acta Astronaut. 1993;29(8):581-585.
doi pubmed - Leblanc A, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, Smith SM, et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos Int. 2013;24(7):2105-2114.
doi pubmed - Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27(9):1896-1906.
doi pubmed - Smith SM, Wastney ME, O'Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, et al. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res. 2005;20(2):208-218.
doi pubmed - Lane HW, Feeback DL. Water and energy dietary requirements and endocrinology of human space flight. Nutrition. 2002;18(10):820-828.
doi pubmed - Grimm D, Bauer J, Kossmehl P, Shakibaei M, Schoberger J, Pickenhahn H, Schulze-Tanzil G, et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002;16(6):604-606.
doi pubmed - Kossmehl P, Shakibaei M, Cogoli A, Infanger M, Curcio F, Schonberger J, Eilles C, et al. Weightlessness induced apoptosis in normal thyroid cells and papillary thyroid carcinoma cells via extrinsic and intrinsic pathways. Endocrinology. 2003;144(9):4172-4179.
doi pubmed - Kossmehl P, Shakibaei M, Cogoli A, Pickenhahn H, Paul M, Grimm D. Simulated microgravity induces programmed cell death in human thyroid carcinoma cells. J Gravit Physiol. 2002;9(1):P295-296.
- Soulsby M, Johnson E, Akel N, Agarwal R, Gaddy D, Dobretsov M, Chowdhury P. Pancreatic histology and associated biocjemical changes in rats on hindlimb suspension. AIP Conference Proceedings. 2011;1326(1):93-99.
doi - Kopp S, Warnke E, Wehland M, Aleshcheva G, Magnusson NE, Hemmersbach R, Corydon TJ, et al. Mechanisms of three-dimensional growth of thyroid cells during long-term simulated microgravity. Sci Rep. 2015;5:16691.
doi pubmed - Mikolajewicz N, Komarova SV. Meta-analytic methodology for basic research: a practical guide. Front Physiol. 2019;10:203.
doi pubmed - Wood PJ. The measurement of parathyroid hormone. Ann Clin Biochem. 1992;29(Pt 1):11-21.
doi pubmed - Linossier MT, Peuriere L, Fernandez P, Normand M, Beck A, Bareille MP, Bonneau C, et al. DI-5-cuffs: bone remodelling and associated metabolism markers in humans after five days of dry immersion to simulate microgravity. Front Physiol. 2022;13:801448.
doi pubmed - Smith SM, Heer M, Shackelford LC, Sibonga JD, Spatz J, Pietrzyk RA, Hudson EK, et al. Bone metabolism and renal stone risk during International Space Station missions. Bone. 2015;81:712-720.
doi pubmed - Morgan JL, Zwart SR, Heer M, Ploutz-Snyder R, Ericson K, Smith SM. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J Appl Physiol (1985). 2012;113(10):1519-1529.
doi pubmed - Armbrecht G, Belavy DL, Gast U, Bongrazio M, Touby F, Beller G, Roth HJ, et al. Resistive vibration exercise attenuates bone and muscle atrophy in 56 days of bed rest: biochemical markers of bone metabolism. Osteoporos Int. 2010;21(4):597-607.
doi pubmed - Zerwekh JE, Odvina CV, Wuermser LA, Pak CY. Reduction of renal stone risk by potassium-magnesium citrate during 5 weeks of bed rest. J Urol. 2007;177(6):2179-2184.
doi pubmed - Zwart SR, Hargens AR, Lee SM, Macias BR, Watenpaugh DE, Tse K, Smith SM. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone. 2007;40(2):529-537.
doi pubmed - Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D. Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36(6):1019-1029.
doi pubmed - Morukov BV, Nichiporuk IA, Tret'iakov VS, Larina IM. [Biochemical markers of bone tissue metabolism in cosmonauts after a prolonged spaceflight]. Fiziol Cheloveka. 2005;31(6):73-77.
doi pubmed - Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, et al. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol (1985). 2004;97(1):119-129.
doi pubmed - Smith SM, Davis-Street JE, Fesperman JV, Calkins DS, Bawa M, Macias BR, Meyer RS, et al. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: a bed rest study with identical twins. J Bone Miner Res. 2003;18(12):2223-2230.
doi pubmed - Diener MJ. Cohen's d. The Corsini Encyclopedia of Psychology. 2010:1.
doi pubmed - Afonin BV, Sedova EA. Digestive system functioning during simulation of microravity effects on humans by means of immersion. Hum Physiol. 2012;38(7):776-780.
doi - Arnaud SB, Navidi M, Deftos L, Thierry-Palmer M, Dotsenko R, Bigbee A, Grindeland RE. The calcium endocrine system of adolescent rhesus monkeys and controls before and after spaceflight. Am J Physiol Endocrinol Metab. 2002;282(3):E514-521.
doi pubmed - Albi E, Curcio F, Spelat R, Lazzarini A, Lazzarini R, Cataldi S, Loreti E, et al. Loss of parafollicular cells during gravitational changes (microgravity, hypergravity) and the secret effect of pleiotrophin. PLoS One. 2012;7(12):e48518.
doi pubmed - Albi E, Curcio F, Spelat R, Lazzarini A, Lazzarini R, Loreti E, Ferri I, et al. Observing the mouse thyroid sphingomyelin under space conditions: a case study from the MDS mission in comparison with hypergravity conditions. Astrobiology. 2012;12(11):1035-1041.
doi pubmed - Oss-Ronen L, Redden RA, Lelkes PI. Enhanced induction of definitive endoderm differentiation of mouse embryonic stem cells in simulated microgravity. Stem Cells Dev. 2020;29(19):1275-1284.
doi pubmed - Perrella G, Meli A, Curcio F, Ambesi-Impiombato F. TSH stimulation of differentiated thyroid cultured cells FRTL5 in simulated microgravity (clinostat). Astrobiology. 2011;11(1):57-64.
doi pubmed - Albi E, Ambesi-Impiombato FS, Lazzarini A, Lazzarini R, Floridi A, Cataldi S, Loreti E, et al. Reinterpretation of mouse thyroid changes under space conditions: the contribution of confinement to damage. Astrobiology. 2014;14(7):563-567.
doi pubmed - White RJ, Averner M. Humans in space. Nature. 2001;409(6823):1115-1118.
doi pubmed - Baldini E, D'Armiento M, Sorrenti S, Del Sordo M, Mocini R, Morrone S, Gnessi L, et al. Effects of ultraviolet radiation on FRTL-5 cell growth and thyroid-specific gene expression. Astrobiology. 2013;13(6):536-542.
doi pubmed - Guarnotta V, Amodei R, Frasca F, Aversa A, Giordano C. Impact of chemical endocrine disruptors and hormone modulators on the endocrine system. Int J Mol Sci. 2022;23(10):5710.
doi pubmed - An etiological study of phthalate self-contamination of spacecraft and contamination from their earthly environs - NASA Technical Reports Server (NTRS). https://ntrs.nasa.gov/citations/19720020495 (Accessed Aug 7, 2022).
- Stein TP, Leskiw MJ, Schluter MD, Hoyt RW, Lane HW, Gretebeck RE, LeBlanc AD. Energy expenditure and balance during spaceflight on the space shuttle. Am J Physiol. 1999;276(6 Pt 2):R1739-1748.
doi pubmed - Heer M. Nutritional interventions related to bone turnover in European space missions and simulation models. Nutrition. 2002;18(10):853-856.
doi pubmed - Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25(4):585-591.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
