| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 12, Number 6, December 2022, pages 168-177
Combination of a Glucagon-Like Peptide 1 Analog and a Sodium-Glucose Cotransporter 2 Inhibitor Improves Lipid Metabolism Compared to the Monotherapies in Experimental Metabolic Syndrome
Isaias dos Santos Silvaa , Luciano Pinto Souzaa
, Priscila Gomes Pereirab
, Jorge Jose de Carvalhob
, Adalgiza Mafra Morenoa
, Hugo C. Castro-Faria-Netoc
, Rodrigo de Azeredo Siqueiraa
, Joana da Costa d’Avilaa, c
, Aluana Santana Carlosa, d
aLaboratory of Pre-clinical Research, Iguacu University, Nova Iguacu, RJ, Brazil
bLaboratory of Ultrastructure and Tissue Biology, State University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil
cLaboratory of Immunopharmacology, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, RJ, Brazil
dCorresponding Author: Aluana Santana Carlos, Faculty of Biological Sciences and Health, Iguacu University (UNIG), Nova Iguacu, RJ 26260-045, Brazil
Manuscript submitted October 10, 2022, accepted October 19, 2022, published online December 1, 2022
Short title: Liraglutide and Canagliflozin Improve Lipid Metabolism
doi: https://doi.org/10.14740/jem843
| Abstract | ▴Top |
Background: Obesity is a risk factor for insulin resistance, dyslipidemia, fatty liver disease, and all disorders associated with metabolic syndrome. Here we evaluated the association of the glucagon-like peptide 1 (GLP-1) analog, liraglutide, and the sodium-glucose cotransporter-2 (SGLT-2) inhibitor, canagliflozin, on the improvement of metabolic syndrome symptoms in a high-fat diet (HFD)-induced obesity rat model.
Methods: Male Wistar rats received either a control diet or HFD ad libitum for 5 months. After 4 months of diet, HFD rats were randomly divided into four experimental groups (HFD, HFD + liraglutide, HFD + canagliflozin, and HFD + liraglutide + canagliflozin). Treatment groups received liraglutide (100 µg/kg) and/or canagliflozin (10 mg/kg) once daily for one month. Body mass and food intake were monitored throughout the experiment. An oral glucose tolerance test, biochemical parameters, epididymal and liver fat, and adipocyte morphology were assessed after the treatment period.
Results: Rats on the HFD developed obesity, glucose intolerance, dyslipidemia, and fatty liver. Liraglutide reduced food intake and body weight, normalized the lipid profile, and reduced abdominal and liver fat. Canagliflozin slightly reduced body mass and improved glucose tolerance and dyslipidemia. The combination therapy was more effective than the monotherapies in normalizing the lipid profile.
Conclusions: The combination of liraglutide and canagliflozin was more effective than the monotherapies in improving dyslipidemia and liver fat. These results indicate that the combination of GLP-1 receptor agonists and SGLT-2 inhibitors is a promising therapeutic strategy to treat dyslipidemia, and possibly prevent fatty liver disease in metabolic syndrome and obese patients.
Keywords: Obesity; Dyslipidemia; Fatty liver; Liraglutide; Canagliflozin
| Introduction | ▴Top |
The prevalence of obesity and metabolic syndrome is increasing worldwide [1]. Excessive accumulation of adipose tissue is a risk factor for metabolic syndrome, cardiovascular diseases, and type 2 diabetes [2]. Metabolic syndrome encompasses various features (hyperglycemia, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, hypertension, central obesity, and fatty liver) that together increase the risk of cardiovascular disease and diabetes mellitus [2, 3]. Intra-abdominal adiposity has a well-known association with an increased risk of insulin resistance and fatty liver disease [3, 4]. Furthermore, high-fat diets (HFDs) are consistently associated with metabolic alterations, central obesity, and negative changes in lipid profile and glucose metabolism, contributing to insulin resistance and obesity [4, 5].
Canagliflozin is a competitive and reversible inhibitor of renal sodium-glucose cotransporter-2 (SGLT-2). SGLT-2 inhibitors, as a class effect, reduce plasma glucose concentration by reducing glucose reabsorption to 40-50%, enhancing glycosuria and improving blood glucose control in type 2 diabetes patients [6]. In addition, SGLT-2 inhibitors present a low risk of hypoglycemia because their mechanism is independent of insulin [7]. However, the effects of SGLT-2 inhibitors on body mass, food intake, and dyslipidemia are modest [8].
Liraglutide is a glucagon-like peptide 1 (GLP-1) receptor agonist and a potent insulin secretagogue used to treat type 2 diabetes and obesity [9]. Liraglutide improves glycemic control and body weight combined with cardioprotection and is considered a safe drug to treat diabetes, obesity, and related cardiometabolic disorders [10].
We hypothesized that combining drugs with distinct pharmacodynamics, a GLP-1 receptor agonist and an SGLT-2 inhibitor, would be more effective than the monotherapies of these drugs in improving features of metabolic syndrome, including glucose metabolism, body weight, liver adiposity, and lipid metabolism. For this study, an animal model of metabolic syndrome was generated by feeding Wistar rats with a HFD, which were then used to test the combination of liraglutide and canagliflozin compared with the monotherapies.
| Materials and Methods | ▴Top |
Animals
A total of 50 male Wistar rats weighing 200 - 210 g, bred and maintained in the animal facility of Iguacu University, were used in this study. All the animals received food and water ad libitum under a 12 h light-dark cycle (lights on from 7:00 am to 7:00 pm) and controlled temperature (25 ± 1 °C). This study was conducted in compliance with all the applicable institutional ethical guidelines for the care, welfare and use of animals. All the protocols used in this study were approved by the Ethics Committee on Animal Use of Iguacu University (PEBIO/UNIG No. 010/2017), which based their analysis on the principles adopted and promulgated by Brazilian law [11].
Experimental groups
Animals were fed with commercial food (Purina®, Sao Paulo, Brazil) and filtered water ad libitum until 21 days of age and then were separated into two experimental groups: control rats fed with the regular commercial diet and rats fed with a HFD (Table 1), which was prepared according to the suggestion of the American Institute of Nutrition (AIN93-M), for 5 months.
 Click to view | Table 1. Composition of the Experimental High-Fat Diet |
Drug treatments
After 4 months of HFD, the HFD rats were separated into four experimental groups (n = 10 per group): HFD, HFD treated with liraglutide (HFD-L), HFD treated with canagliflozin (HFD-C), and HFD treated with liraglutide plus canagliflozin (HFD-LC). Liraglutide at 100 µg/kg/day was administered subcutaneously (Victoza®; Novo Nordisk, Denmark), and canagliflozin at 10 mg/kg/day was administered orally (Invokana®; Janssen-Cilag Farmaceutica Ltda, Brazil). The HFD rats, and the control group on the regular diet, received a 0.9% saline solution (vehicle) subcutaneously and by gavage. Drugs were administered at the beginning of the circadian cycle’s light phase daily for 1 month. Average food intake (g) was measured every 4 days, and body weight (g) was verified every week.
Glucose tolerance test
The oral glucose tolerance test (OGTT) was performed following 12 h of fasting to determine insulin sensitivity changes. Blood samples were collected from the tail vein before and 30, 60, 90, and 120 min after oral glucose administration (2 g/kg of a 50% glucose solution). The OGTT was performed before drug administration on the last day of drug treatment. Blood glucose concentrations were measured with an Accu-Chek Glucose Testing device (Roche, Switzerland). The incremental area under the glucose tolerance curve was calculated as the integrated area under the curve (AUC) above the basal value (time 0) over the 120 min sampling period.
Euthanasia and sample collection
After 1 month of drug treatment, all the animals were euthanized (between 8:00 am and 2:00 pm) by thiopental overdose. The urine was aspirated directly from the bladder, and glucose concentration was measured with the Accu-Chek Glucose Testing device (Roche, Switzerland). Blood samples were collected from the heart and centrifuged at 1,500 × g for 20 min at 4 °C to obtain serum, which was kept at -20 °C until the assay. The following organs were collected: intra-abdominal epididymal adipose tissue and liver. Tissues were weighed and liver fragments of approximately 1 cm3 were immediately frozen in liquid nitrogen then stored at -70 °C until analysis.
Biochemical measurements
The lipid profile (total cholesterol, triglycerides, HDL cholesterol, and low-density lipoprotein (LDL) cholesterol), and levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in the serum samples (in duplicate) in a Vitros® 250 Chemistry Analyzer equipment (Ortho Clinical Diagnostics, Raritan, NJ, USA) at the Clinical Analysis of Laboratory Animals subunit of the Oswaldo Cruz Foundation. Triglycerides and total cholesterol measurements were replicated using a colorimetric assay kit (Bioclin-Quibasa, Belo Horizonte, MG, Brazil) and evaluated in a spectrophotometer (UV-1600; Pro-analise, Porto Alegre RS, Brazil).
Oil red O (ORO) staining
Frozen sections of 10-µm thickness from the liver fragments were obtained in a Leica cryostat (model CM1850; Wetzlar, Germany), air-dried at room temperature, fixed with 4% paraformaldehyde for 10 min, and then washed with phosphate-buffered saline (PBS). The sections were incubated in 100% propylene glycol for 3 min, stained with a pre-heated 0.5% ORO solution in propylene glycol for 10 min at 60 °C, then differentiated in 85% propylene glycol for 3 min, washed in distilled water twice, counterstained with hematoxylin and mounted with glycerin. The intensity of hepatic steatosis was studied in the histological slices as described. For each section, 10 nonconsecutive random digital images were obtained per animal in an Olympus optical microscope (model BX51, 100 × plan-achromatic objective, DP71 camera; Olympus Corporation, Tokyo, Japan) in TIFF format, 36-bit, and 1,280 × 1,024 pixels resolution. For each slide, 10 images were acquired, and a blinded experimenter performed all the quantitative analyses using the Image J/NIH software.
Morphometry of adipose tissue
Samples of adipose tissue were fixed in buffered formaldehyde. After 24 h of fixation, samples were dehydrated with increasing concentration of ethanol (1 × 80%, 1 × 90%, 2 × 100%), incubated with xylol (3 ×) and liquid paraffin (3 ×), and embedded into paraffin. Sections of 5 µm were obtained using a microTec microtome (model CUT 4050; microTec Laborgerate, Walldorf, Germany), placed onto slides, and stained with hematoxylin/eosin. Ten images were acquired per animal with an Olympus light microscope (model BX40; Olympus Corporation, Tokyo, Japan) coupled to a digital camera (Olympus DP71). A blinded experimenter performed all morphometric analyses, and for each image, randomly selected all the visible adipocytes in each field. At least five fields of each slide were analyzed per animal, and the area was measured using the Image J/NIH software.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism version 9.1.0 (San Diego, CA, USA). Food intake, body mass, and OGTT were analyzed by repeated measures two-way analysis of variance (ANOVA). To evaluate the differences between the experimental groups and the efficacy of the monotherapies compared to the combination therapy with liraglutide and canagliflozin, we used one-way ANOVA and Tukey’s multiple comparisons test for parametric analysis. A P value lower than 0.05 was considered significant.
| Results | ▴Top |
Effects of liraglutide and canagliflozin on the body mass gain and food intake of HFD rats
Body mass and food intake of Wistar rats were monitored over the 5 months of experiment (Fig. 1). After 4 months of HFD, rats on the HFD presented a 16% increment in body mass compared to control rats on the regular commercial diet (P < 0.05). The HFD rats treated with liraglutide showed a significant decrease in mass gain (HFD-L, -16.6% and HFD-LC, -17.8%, compared to the other groups, P < 0.001) but the association of liraglutide and canagliflozin did not have an additional effect on body weight (Fig. 1b). Accordingly, the food intake evaluated in the same period was significantly decreased in the liraglutide-treated groups only (Fig. 1c).
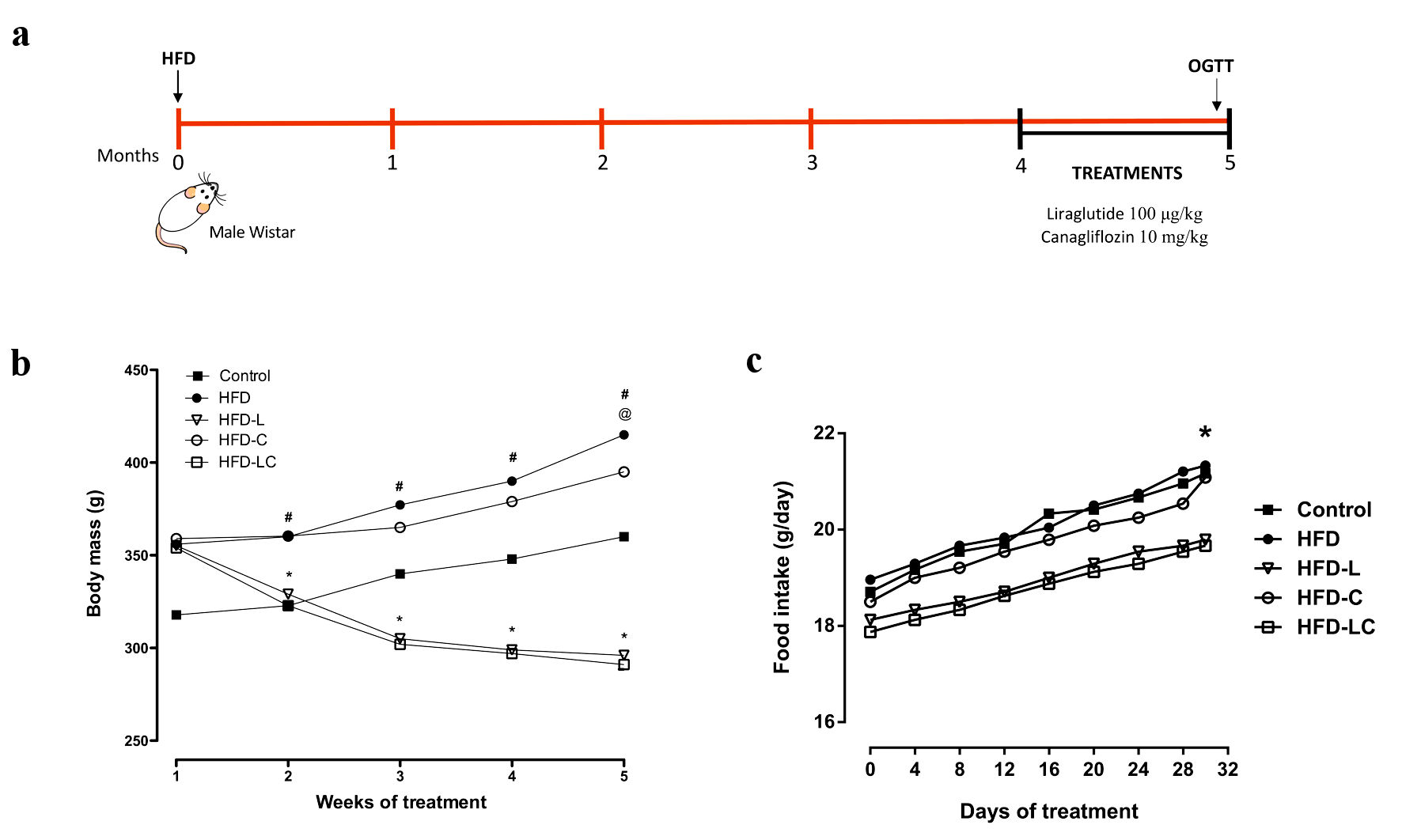 Click for large image | Figure 1. Effects of liraglutide and canagliflozin on body mass and food intake of high-fat diet-fed rats. (a) Experimental design. (b) Body mass evolution, #P < 0.001 HFD vs. control, HFD-L, and HFD-LC. *P < 0.001 HFD-L vs. HFD-C. @P < 0.01 HFD-C vs. HFD, HFD-L, and HFD-LC. (c) Food intake evolution, expressed as the amount consumed per rat per day. *P < 0.05 HFD vs. HFD-LC. Values are means and SEM (n = 10 per experimental group). Control: rats on a regular diet; HFD: high-fat diet; HFD-L: HFD treated with liraglutide; HFD-C: HFD treated with canagliflozin; HFD-LC: HFD treated with liraglutide plus canagliflozin; SEM: standard error of the mean. |
Effects of liraglutide and canagliflozin on the glucose metabolism of HFD rats
Non-treated HFD rats developed severe hyperglycemia during the glucose tolerance test (HFD peak of 490 mg/dL, P < 0.001) (Fig. 2a). The HFD rats exhibited glucose intolerance, with blood glucose levels at 38.6% higher than control rats on the regular diet (P < 0.001), as evidenced by a larger AUC of blood glucose compared to control rats (Fig. 2b). The mean values of fasting blood glucose during the OGTT were 87 mg/dL in control rats, 159 mg/dL in HFD rats, 158 mg/dL in the HFD-L group, 106 mg/dL in the HFD-C group, and 88 mg/dL in the HFD-LC group (Fig. 2c).
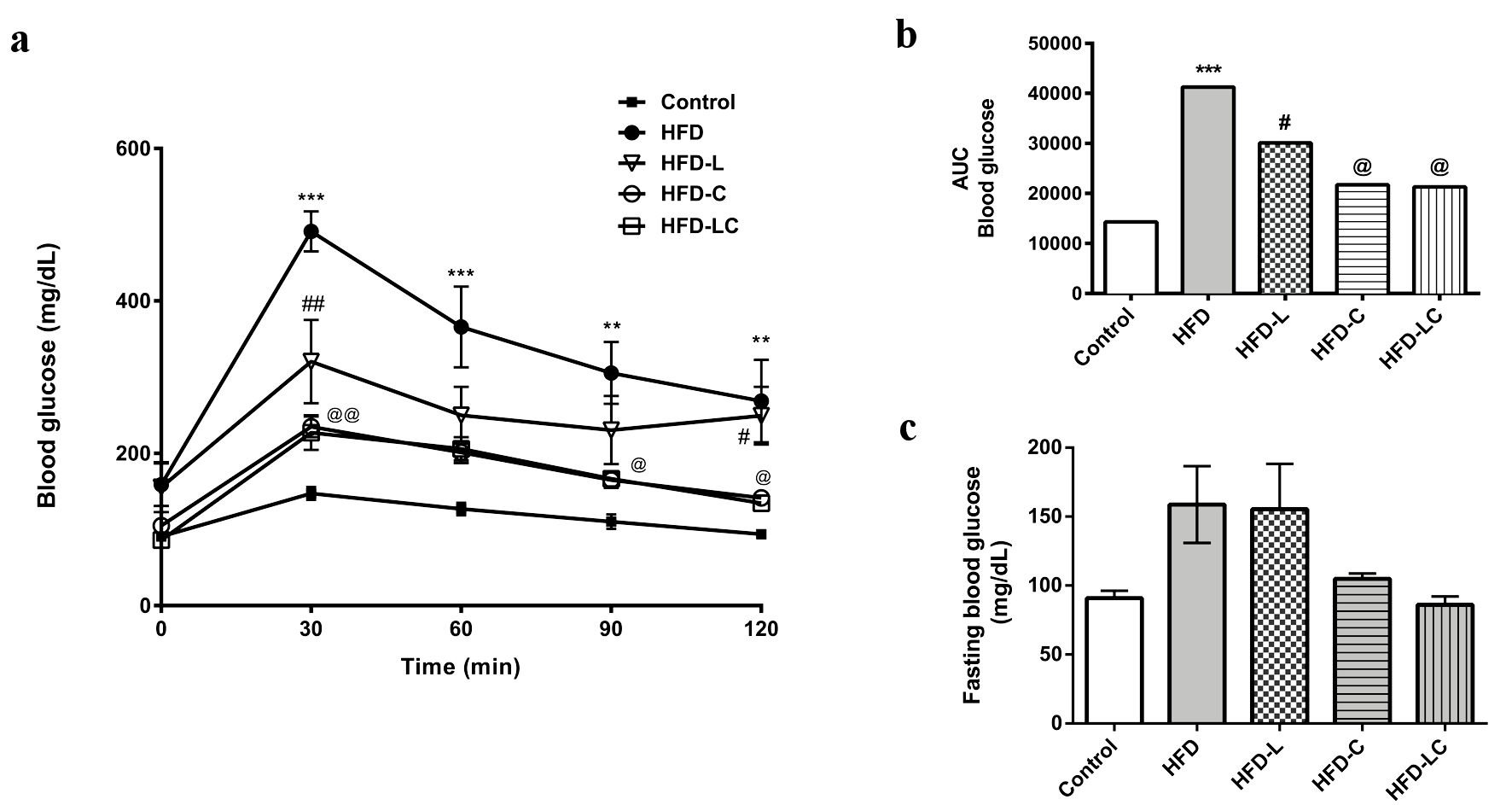 Click for large image | Figure 2. Effects of liraglutide and canagliflozin on the glucose metabolism of high-fat diet-fed rats. (a) Oral glucose tolerance test (OGTT) performed after the treatment period. (b) Area under the curve (AUC) of the OGTT graph. ***P < 0.001 HFD vs. control, HFD-C, and HFD-LC. **P < 0.01 HFD vs. HFD-C and HFD-LC. ##P < 0.001 HFD-L vs. HFD. @@P < 0.001 HFD-C and HFD-LC vs. HFD. @P < 0.01 HFD-C and HFD-LC vs. HFD. #P < 0.05 HFD-L vs. HFD-C and HFD-LC. (c) Fasting blood glucose before OGTT. Values are means and SEM (n = 10 per experimental group). Control: rats on a regular diet; HFD: high-fat diet; HFD-L: HFD treated with liraglutide; HFD-C: HFD treated with canagliflozin; HFD-LC: HFD treated with liraglutide plus canagliflozin; SEM: standard error of the mean. |
All drug treatments significantly reduced blood glucose compared to the untreated HFD rats (HFD-L, -40.9%; HFD-C, -49.2%; HFD-LC, -51%; P < 0.05) in the OGTT. Canagliflozin was more effective than liraglutide in normalizing glycemia; however, the association of these drugs did not have any additive effects on glycemia in our experimental model. As expected, HFD rats treated with canagliflozin, either as monotherapy or combined with liraglutide, had increased glycosuria (control 65.7 ± 3.3 mg/dL vs. HFD-C 185.3 ± 2.8 and HFD-LC 184.7 ± 4.1; P < 0.001) (Table 2).
 Click to view | Table 2. Effects of Liraglutide and Canagliflozin in Metabolic Parameters of HFD Rats |
Effects of liraglutide and canagliflozin on the lipid metabolism of HFD rats
In addition to glucose intolerance, HFD rats also developed dyslipidemia (Table 2), presenting higher levels of blood total cholesterol (HFD 110.6 ± 14.5 vs. control 60.1 ± 4.8, P < 0.001), triglycerides (HFD 215.9 ± 16.1 vs. control 82.9 ± 5.9, P < 0.001), and LDL cholesterol (HFD 92.5 ± 3.4 vs. control 44.5 ± 8.0, P < 0.01), and lower levels of HDL cholesterol (HFD 38.7 ± 5.2 vs. control 93.5 ± 7.9, P < 0.001). Liraglutide treatment decreased total cholesterol (HFD 110.6 ± 14.5 vs. HFD-L 79.5 ± 4.3, non-significant (ns)), triglycerides (HFD 215.9 ± 16.1 vs. HFD-L 94.4 ± 9.3, P < 0.05), and LDL cholesterol (HFD 92.5 ± 3.4 vs. HFD-L 47.0 ± 11.0, P < 0.05), and increased HDL cholesterol (HFD 38.7 ± 5.2 vs. HFD-L 72.3 ± 8.6, P < 0.05) in comparison to the untreated HFD rats. Canagliflozin improved the lipid profile of HFD rats in a less extent, significantly increasing HDL cholesterol (HFD 38.7 ± 5.2 vs. HFD-L 64.1 ± 5.1, P < 0.05). Interestingly, the combination of liraglutide and canagliflozin normalized all parameters of the lipid profile to levels comparable to control rats that had been on the regular diet: total cholesterol (control 60.1 ± 4.8 vs. HFD-LC 63.3 ± 3.4), triglycerides (control 82.9 ± 5.9 vs. HFD-LC 70.3 ± 10.3), LDL cholesterol (control 44.5 ± 8.0 vs. HFD-LC 33.3 ± 5.5) and HDL cholesterol (control 93.5 ± 7.9 vs. HFD-LC 91.5 ± 8.9). Comparing the three treatment regimens, we observed that the combination therapy significantly reduced blood triglycerides compared to canagliflozin (HFD-LC 70.3 ± 10.3 vs. HFD-C 138.9 ± 11.5, P < 0.001) and to liraglutide monotherapy (HFD-LC 70.3 ± 10.3 vs. HFD-L 94.4 ± 9.3, ns). The combination therapy was more effective to increase HDL cholesterol levels than canagliflozin (HFD-LC 91.5 ± 8.9 vs. HFD-C 64.1 ± 5.1, P < 0.05) and liraglutide (HFD-LC 91.5 ± 8.9 vs. HFD-L 72.3 ± 8.6, ns).
Effects of liraglutide and canagliflozin on liver fat of HFD rats
HFD rats presented hepatic steatosis, as evidenced by the numerous ORO-stained lipid drops in the liver tissue (Fig. 3). However, it was not enough to produce tissue injury and steatohepatitis since the blood levels of ALT and AST enzymes were normal in all groups of rats (Table 2). Quantitative analysis revealed a four-fold increase in liver ORO staining of untreated HFD rats compared to control rats (Fig. 3b). All drug treatments reduced liver ORO staining of the HFD rats, especially the liraglutide-treated rats (HFD 18.5 ± 0.8 vs. HFD-L 9.2 ± 0.4, P < 0.0001), while the canagliflozin treatment was found to be less effective when quantified (HFD 18.5 ± 0.8 vs. HFD-C 16.8 ± 0.4, ns). Nonetheless, the combination of liraglutide and canagliflozin significantly reduced liver fat compared to the canagliflozin monotherapy (HFD-LC 7.2 ± 0.1 vs. HFD-C 16.8 ± 0.4, P < 0.0001). The reduction in liver fat produced by the combination therapy was also evidenced by a significant decrease in liver weights of HFD-LC rats compared to untreated HFD rats (Fig. 3c).
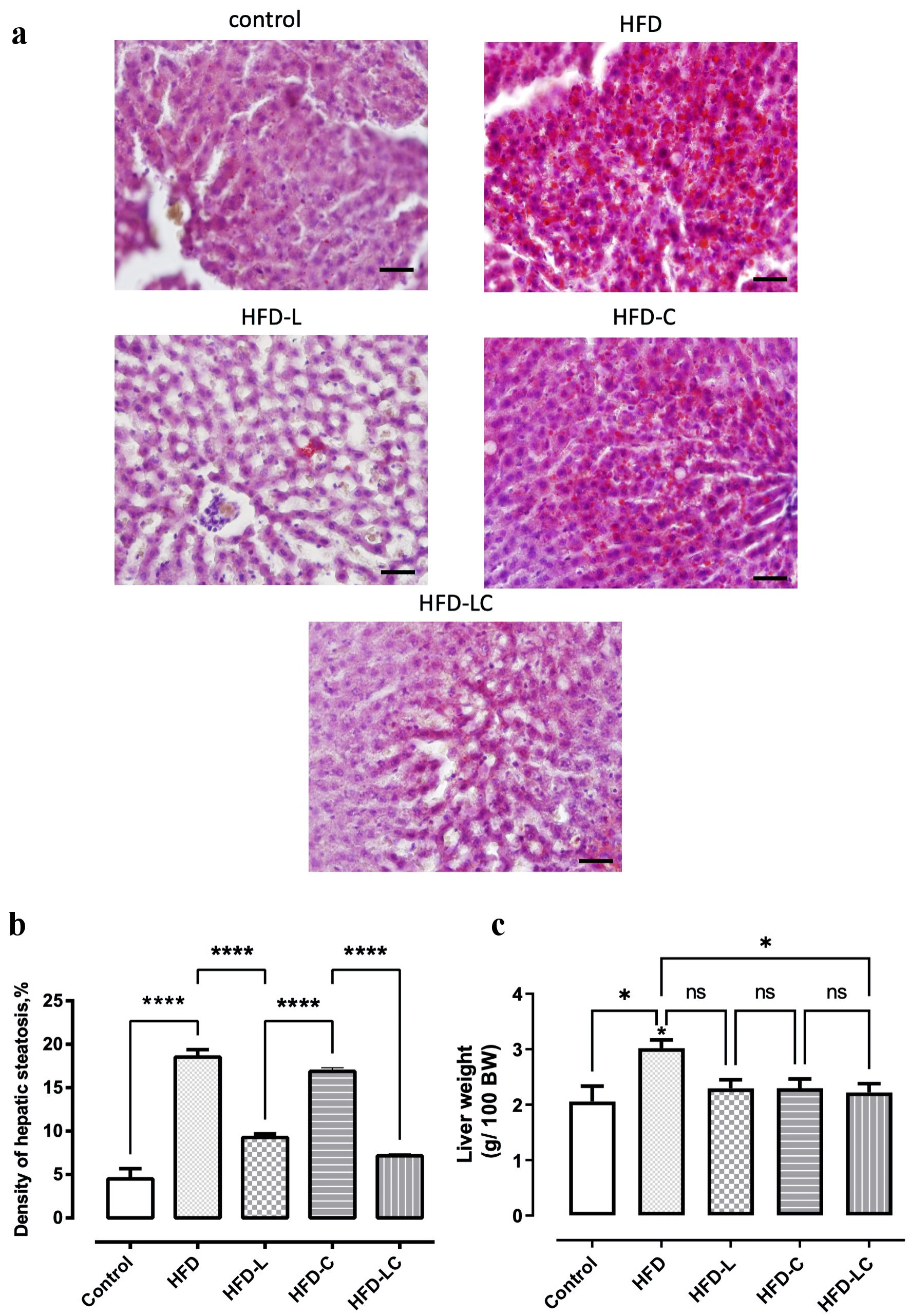 Click for large image | Figure 3. Treatments with liraglutide and canagliflozin reduced liver fat of high-fat diet-fed rats. Liver slices were stained with oil red O to enable observation of hepatic steatosis (red). (a) Rats on a regular diet (control). (b) High-fat diet (HFD). (c) HFD + liraglutide (HFD-L). (d) HFD + canagliflozin (HFD-C). (e) HFD + canagliflozin + liraglutide (HFD-LC). (f) Morphometric analysis of liver steatosis after treatment period. (g) Liver weights after treatment period. Values are means and SEM. *P < 0.05, ****P < 0.0001. Scale bar = 50 µm. SEM: standard error of the mean; ns: non-significant. |
Effects of liraglutide and canagliflozin on the adipose tissue of HFD rats
HFD rats presented increased adipocyte size (HFD +98%, P < 0.0001) compared to the control rats fed with the regular diet (Fig. 4). Liraglutide treatments significantly decreased adipocyte area relative to the untreated HFD group (HFD-L -31%, P < 0.05 and HFD-LC -41%, P < 0.01), while canagliflozin treatment did not have a significant effect on reducing adipocyte size (HFD-C -15%, ns) (Fig. 4b). The association of liraglutide and canagliflozin was slightly more effective than liraglutide monotherapy in reducing adipocyte size (HFD-LC -13% relative to HFD-L, ns). HFD rats presented a 54% increase in epididymal fat accumulation compared to the control rats on the regular diet (Fig. 4c). All treatments had some effect in decreasing epididymal fat, but only treatments involving liraglutide showed statistically significant reductions of epididymal fat related to the untreated HFD rats (HFD-L -40%, P < 0.05; and HFD-LC -44%, P < 0.01).
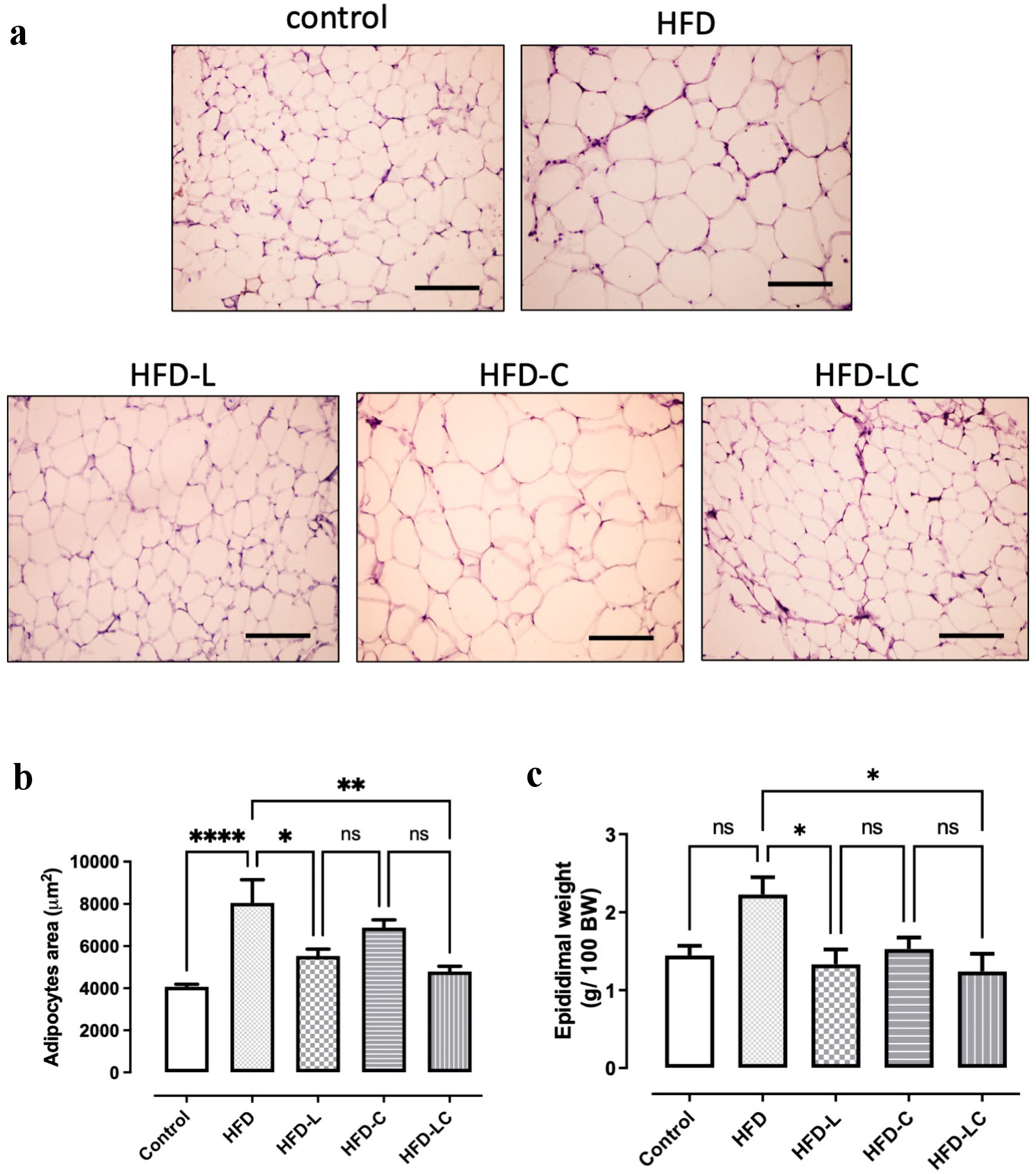 Click for large image | Figure 4. Effects of liraglutide and canagliflozin in the epididymal adipose tissue of high-fat diet-fed rats. Histology of the epididymal adipose tissue of: (a) rats on a regular diet (control), (b) high-fat diet (HFD), (c) HFD + liraglutide (HFD-L), (d) HFD + canagliflozin (HFD-C), (e) HFD + canagliflozin + liraglutide HFD-LC. (f) Morphometric analysis of adipocyte area after treatment period. (g) Epididymal weight after treatment period. ****P < 0.0001, **P < 0.001, *P < 0.05. Scale bar = 100 µm. |
| Discussion | ▴Top |
Here we evaluated the combination of the GLP-1 receptor agonist liraglutide and the SGLT-2 inhibitor canagliflozin on improving features of metabolic syndrome in HFD-induced obese rats. Overall, the results indicate that the combination of liraglutide and canagliflozin was more effective than the monotherapies in improving dyslipidemia and fatty liver in our experimental model of metabolic syndrome (Table 3).
 Click to view | Table 3. Summary of Effects on Metabolic Syndrome Parameters |
Our experimental model reproduced most features of metabolic syndrome, such as body mass gain, glucose intolerance, increased abdominal and liver fat, and dyslipidemia. The HFD-induced obesity in rodents produced symptoms comparable to other models that use a high carbohydrate diet, genetic or drug-induced obesity, and very similar to human metabolic syndrome [12].
Liraglutide was more effective than canagliflozin in decreasing body weight, food intake (Fig. 1), hepatic steatosis (Fig. 3), adipocyte size, epidydimal fat (Fig. 4), and lipid profile (Table 2). On the other hand, canagliflozin was more effective than liraglutide in normalizing blood glucose (Fig. 2). The combination therapy and liraglutide monotherapy showed significant advantages in normalizing lipid profiles compared to canagliflozin alone. The reduction of adipocyte size and liver fat was more expressive in the rats treated with the combination therapy.
Liraglutide is currently approved to treat obesity [13]. Its primary action is to increase satiety [14], consistent with our findings of reduced food intake in the liraglutide-treated groups (Fig. 1). In the present study, liraglutide improved lipid metabolism and reduced intra-abdominal and liver fat of obese rats (Table 2, Figs. 3, 4). These results are consistent with clinical findings in humans treated with liraglutide, which have demonstrated body weight reduction associated with a reduction in visceral fat [15], as well as an improvement in lipid profile with high doses of liraglutide [16]. However, since we used a relatively low dose of liraglutide in this study (100 µg/kg/day), the effect of the treatment on body mass reduction did not reflect a strong beneficial effect on glycemia of HFD rats. Other experimental studies have used higher doses (from 200 µg/kg/day up to 1,200 µg/kg/day), which were shown to significantly reduce visceral fat and insulin resistance [17-20].
Liraglutide controls insulin release by directly binding to GLP-1 receptors in β-cells and neural pathways [21]. Therefore, a GLP-1 receptor agonist is expected to enhance β-cell glucose sensitivity [9]. A recent clinical study with type 2 diabetes patients compared the effects of 16 weeks of treatment with canagliflozin and liraglutide and their combination on β-cell function. They found that liraglutide and canagliflozin significantly lowered glycated hemoglobin (HbA1c) with no significant additive effect of the combination therapy in these patients [22]. Furthermore, the decrease in HbA1c was reported to be strongly and inversely correlated with the increase in β-cell glucose sensitivity [22].
Canagliflozin is the first-in-class SGLT-2 receptor blocker approved for type 2 diabetes by increasing urinary glucose excretion without inducing hypoglycemia, thereby promoting body weight reduction due to the loss of about 300 kcal per day [7]. In a recent review, SGLT-2 inhibitors appeared to reduce the risk of cardiovascular events and mortality, suggesting that the benefits of these drugs seen in people with diabetes may apply to a broader population [23]. One significant advantage of SGLT-2 inhibitors is that they eliminate excess glucose through urine instead of stimulating tissue glucose utilization by tissues, avoiding glucose toxicity. High glucose levels in the blood and within cells have a toxic effect on mitochondria and other organelles, generating oxidative stress and damage to cells and tissues, known as glucotoxicity [24]. Chronic hyperglycemia and glucotoxicity can be especially damaging to particular cell types more vulnerable to elevated plasma glucose levels, such as pancreatic β-cells, neuronal cells, and vascular endothelial cells, which equilibrate their intracellular glucose level to that of their extracellular environment [25].
Intra-abdominal fat accumulation is an essential predictor of metabolic syndrome, even in young adult subjects [26]. Our results show that canagliflozin had a small but significant effect on reducing body weight and improving lipid metabolism, and a substantial effect on normalizing glucose in obese rats. Together, these findings reinforce the beneficial effect of canagliflozin on improving body weight, glucose, and lipid metabolism observed in the clinical treatment of type 2 diabetic patients [27].
A limitation of our study was that we did not evaluate the blood pressure and insulin of the rats. However, it is well-established that rats fed a HFD develop hypertension and increase blood insulin levels [28]. Epidemiological studies have shown that obesity predicts the future development of hypertension and insulin resistance [29]. According to the National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III), metabolic syndrome is characterized by the presence of at least three of the following symptoms: increased waist circumference (> 102 cm in men and > 88 cm in women), elevated blood pressure (130/85 mm Hg or higher), elevated fasting blood glucose (> 100 mg/dL), elevated blood triglycerides (> 150 mg/dL), and decreased blood HDL cholesterol (< 40 mg/dL in men and < 50 mg/dL in women). Therefore, blood insulin concentration is not necessary to diagnose metabolic syndrome.
Conclusions
The present study showed that combining the beneficial effects of canagliflozin on glucose metabolism with a low dose of liraglutide was advantageous to improve lipid metabolism and visceral adiposity in our experimental model of metabolic syndrome (Table 3). Treating dyslipidemia and reducing visceral fat in parallel to glycemic control is an important measure to avoid metabolic syndrome complications, preventing diabetes, fatty liver, and cardiovascular diseases. Therefore, the data presented here support the advantages of the association of low doses of GLP-1 agonists with SGLT-2 inhibitors in the prevention of metabolic complications of obesity and encourage further investigations to test the clinical benefit of this therapy.
Acknowledgments
We want to thank the biologist Marli Amaro da Silva for helping take care of the animals and our laboratory.
Financial Disclosure
This work was supported by Fundacao de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
ISS, LPS, PGP performed experiments and collected data. JJC, AMM, HCCFN critically reviewed the manuscript for important intellectual content and provided resources. RAS performed analysis and interpreted data. JCD performed data analysis and wrote the paper. ASC conceived and designed the analysis, performed data analysis.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- de Siqueira Valadares LT, de Souza LSB, Salgado Junior VA, de Freitas Bonomo L, de Macedo LR, Silva M. Prevalence of metabolic syndrome in Brazilian adults in the last 10 years: a systematic review and meta-analysis. BMC Public Health. 2022;22(1):327.
doi pubmed - Denisenko YK, Kytikova OY, Novgorodtseva TP, Antonyuk MV, Gvozdenko TA, Kantur TA. Lipid-induced mechanisms of metabolic syndrome. J Obes. 2020;2020:5762395.
doi pubmed - Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. 2017;960:1-17.
doi pubmed - Meneghel P, Pinto E, Russo FP. Physiopathology of nonalcoholic fatty liver disease: from diet to nutrigenomics. Curr Opin Clin Nutr Metab Care. 2022;25(5):329-333.
doi pubmed - Cariou B. The metabolic triad of non-alcoholic fatty liver disease, visceral adiposity and type 2 diabetes: Implications for treatment. Diabetes Obes Metab. 2022;24(Suppl 2):15-27.
doi pubmed - Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13(7):669-672.
doi pubmed - Haas B, Eckstein N, Pfeifer V, Mayer P, Hass MD. Efficacy, safety and regulatory status of SGLT2 inhibitors: focus on canagliflozin. Nutr Diabetes. 2014;4:e143.
doi pubmed - Filippas-Ntekouan S, Tsimihodimos V, Filippatos T, Dimitriou T, Elisaf M. SGLT-2 inhibitors: pharmacokinetics characteristics and effects on lipids. Expert Opin Drug Metab Toxicol. 2018;14(11):1113-1121.
doi pubmed - Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab. 2021;46:101090.
doi pubmed - Konwar M, Bose D, Jaiswal SK, Maurya MK, Ravi R. Efficacy and Safety of Liraglutide 3.0 mg in Patients with Overweight and Obese with or without Diabetes: A Systematic Review and Meta-Analysis. Int J Clin Pract. 2022;2022:1201977.
doi pubmed - Marques RG, Morales MM, Petroianu A. Brazilian law for scientific use of animals. Acta Cir Bras. 2009;24(1):69-74.
doi pubmed - Wong SK, Chin KY, Suhaimi FH, Fairus A, Ima-Nirwana S. Animal models of metabolic syndrome: a review. Nutr Metab (Lond). 2016;13:65.
doi pubmed - Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606-1616.
doi - Narayanaswami V, Dwoskin LP. Obesity: Current and potential pharmacotherapeutics and targets. Pharmacol Ther. 2017;170:116-147.
doi pubmed - Ishii S, Nagai Y, Sada Y, Fukuda H, Nakamura Y, Matsuba R, Nakagawa T, et al. Liraglutide reduces visceral and intrahepatic fat without significant loss of muscle mass in obese patients with type 2 diabetes: a prospective case series. J Clin Med Res. 2019;11(3):219-224.
doi pubmed - Peradze N, Farr OM, Perakakis N, Lazaro I, Sala-Vila A, Mantzoros CS. Short-term treatment with high dose liraglutide improves lipid and lipoprotein profile and changes hormonal mediators of lipid metabolism in obese patients with no overt type 2 diabetes mellitus: a randomized, placebo-controlled, cross-over, double-blind clinical trial. Cardiovasc Diabetol. 2019;18(1):141.
doi pubmed - Kaji N, Takagi Y, Matsuda S, Takahashi A, Fujio S, Asai F. Effects of liraglutide on metabolic syndrome in WBN/Kob diabetic fatty rats supplemented with a high-fat diet. Animal Model Exp Med. 2020;3(1):62-68.
doi pubmed - Liberini CG, Lhamo R, Ghidewon M, Ling T, Juntereal N, Chen J, Cao A, et al. Liraglutide pharmacotherapy reduces body weight and improves glycaemic control in juvenile obese/hyperglycaemic male and female rats. Diabetes Obes Metab. 2019;21(4):866-875.
doi pubmed - Chai W, Fu Z, Aylor KW, Barrett EJ, Liu Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2016;311(3):E640-648.
doi pubmed - Zhao L, Zhu C, Lu M, Chen C, Nie X, Abudukerimu B, Zhang K, et al. The key role of a glucagon-like peptide-1 receptor agonist in body fat redistribution. J Endocrinol. 2019;240(2):271-286.
doi pubmed - Muller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72-130.
doi pubmed - Ali AM, Mari A, Martinez R, Al-Jobori H, Adams J, Triplitt C, DeFronzo R, et al. Improved beta cell glucose sensitivity plays predominant role in the decrease in HbA1c with Cana and Lira in T2DM. J Clin Endocrinol Metab. 2020;105(10):dgaa494.
doi pubmed - Aftab S, Vetrivel Suresh R, Sherali N, Daniyal M, Tsouklidis N. Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors: Benefits in Diabetics With Cardiovascular Disease. Cureus. 2020;12(10):e10783.
doi - Giaccari A, Sorice G, Muscogiuri G. Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutr Metab Cardiovasc Dis. 2009;19(5):365-377.
doi pubmed - Campos C. Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med. 2012;124(6):90-97.
doi pubmed - Kobayashi M, Ogawa S, Tayama J, Sagara I, Takeoka A, Bernick P, Kawano T, et al. Intra-abdominal fat accumulation is an important predictor of metabolic syndrome in young adults. Medicine (Baltimore). 2020;99(37):e22202.
doi pubmed - Chen MB, Wang H, Cui WY, Xu HL, Zheng QH. Effect of SGLT inhibitors on weight and lipid metabolism at 24 weeks of treatment in patients with diabetes mellitus: A systematic review and network meta-analysis. Medicine (Baltimore). 2021;100(6):e24593.
doi pubmed - Obadia N, Lessa MA, Daliry A, Silvares RR, Gomes F, Tibirica E, Estato V. Cerebral microvascular dysfunction in metabolic syndrome is exacerbated by ischemia-reperfusion injury. BMC Neurosci. 2017;18(1):67.
doi pubmed - Vaneckova I, Maletinska L, Behuliak M, Nagelova V, Zicha J, Kunes J. Obesity-related hypertension: possible pathophysiological mechanisms. J Endocrinol. 2014;223(3):R63-78.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
