| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 12, Number 2, April 2022, pages 53-58
Influence of Age on the Effects of Sodium-Glucose Cotransporter 2 Inhibitors in Japanese Patients With Type 2 Diabetes Mellitus
Masataka Kusunokia, f, Fumiya Hisanob, Shinichi Matsudac, Naomi Wakazonoa, Kazuhiko Tsutsumid, Tetsuro Miyatae
aDepartment of Diabetes, Motor Function and Metabolism, Research Center of Health, Physical Fitness and Sports, Nagoya University, Furou-cho, Chigusa-ku, Nagoya City, Aichi 464-0814, Japan
bDepartment of Integrated Health Sciences, Graduate School of Medicine, Nagoya University, Higashi-ku, Nagoya City, Aichi 461-8673, Japan
cDepartment of Data Science, Faculty of Science and Technology, Nanzan University, Showa-ku, Nagoya City, Aichi 466-8673, Japan
dOkinaka Memorial Institute for Medical Research, Minato-ku, Tokyo 105-8470, Japan
eOffice of Medical Education, School of Medicine, International University of Health and Welfare, Narita City, Chiba 286-8686, Japan
fCorresponding Author: Masataka Kusunoki, Department of Diabetes, Motor Function and Metabolism, Research Center of Health, Physical Fitness and Sports, Nagoya University, Furou-cho, Chigusa-ku, Nagoya City, Aichi 464-0814, Japan
Manuscript submitted March 7, 2022, accepted March 23, 2022, published online April 23, 2022
Short title: SGLT2 Inhibitor and Aging
doi: https://doi.org/10.14740/jem799
| Abstract | ▴Top |
Background: Selective inhibitors of sodium-glucose cotransporter 2 (SGLT2) inhibitors have been reported to improve glucose metabolism, lipid metabolism and liver dysfunction in patients with type 2 diabetes mellitus. However, few studies have investigated if the effects of this class of drugs vary according to the age of the patients. This study was conducted to determine the influence of age on the effects of SGLT2 inhibitors in Japanese patients with diabetes mellitus.
Methods: SGLT2 inhibitors were administered for 6 months to 199 patients with type 2 diabetes mellitus. The body weight, body mass index (BMI), visceral fat area, serum glucose, hemoglobin A1c (HbA1c), blood lipid levels, serum uric acid, liver function parameters, and bone mineral density were measured before and after 6 months of treatment, and regression analysis was performed to examine the relationship between age and the effects of SGLT2 inhibitors.
Results: The results of the regression analysis showed that the effects of SGLT2 inhibitors on the serum levels of high-density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GTP) were influenced by the patient age. The effect of SGLT2 inhibitors in increasing the serum HDL-C was more pronounced in older patients. There was an interaction between age and the baseline levels of the serum AST and γ-GTP; reductions of the serum AST and γ-GTP levels by SGPLT2 inhibitors were more pronounced in younger patients with higher baseline levels of these parameters, but not pronounced in aged patients.
Conclusions: The effects of SGLT2 inhibitor treatment on the serum HDL-C, AST and γ-GTP levels were affected by the patient age: their beneficial effect on the serum HDL-C increased with the patient age and their effects in reducing the serum AST and γ-GTP decreased with the patient age. The effects of SGLT2 inhibitor treatment on the blood glucose and serum HbA1c were not affected by the patient age, suggesting that SGLT2 inhibitors can be used as antidiabetic drugs in patients over a wide age range, from the young to the elderly.
Keywords: SGLT2 inhibitor; Aging; Glycemic control; Lipid metabolism; Hepatic function
| Introduction | ▴Top |
Sodium-glucose cotransporter 2 (SGLT2) is a transporter that is expressed in the proximal tubules of the kidney and is responsible for approximately 90% of the reabsorption of the glucose filtered by the glomeruli [1, 2]. In patients with type 2 diabetes mellitus, SGLT2 inhibitors are thought to selectively inhibit SGLT2 to promote urinary excretion of excess blood glucose, and thereby exert a hypoglycemic effect [3]. In addition to lowering the blood glucose, SGLT2 inhibitors have also been reported to reduce the body weight, lower the blood pressure, and improve lipid metabolism, uric acid metabolism, and liver dysfunction [4, 5]. Because of these pleiotropic effects, SGLT2 inhibitors are known to significantly suppress the cardiovascular risk, as compared with placebo, in patients with high cardiovascular risk [6]. However, few studies have investigated the differences in the effects of SGLT2 inhibitors according to the patient age. Therefore, the present study was conducted to investigate the influence of age on the effects of SGLT2 inhibitors in Japanese patients with type 2 diabetes mellitus.
| Materials and Methods | ▴Top |
Subjects
This study was conducted in compliance with the ethical standards of the responsible institution for studies in human subjects as well as with the Declaration of Helsinki. The protocol of this study was reviewed and approved by the Institutional Review Board (IRB) of the Medical Corporation Odakai Ethics Committee (IRB approval number 2020-01). Patients who participated in the study were provided an explanation about the purpose of the study by the physicians in charge and gave informed consent. This prospective clinical study is officially registered as an open-label study (ID: UMIN000021584). A total of 199 Japanese patients with type 2 diabetes mellitus (142 males and 57 females), all of whom were attending the outpatient clinic, were enrolled in the study.
Study design
The patients enrolled in this study received one of the following five SGLT2 inhibitors: luseogliflozin (Taisho Pharmaceuticals Co., Ltd, Tokyo, Japan), dapagliflozin (Astra Zeneca, Osaka, Japan), tofogliflozin (Kowa Pharmaceutical Co., Ltd, Tokyo, Japan), empagliflozin (Boehringer Ingelheim Japan, Inc., Tokyo, Japan), and canagliflozin (Mitsubishi Tanabe Pharm Corporation, Osaka, Japan). The above drugs were given at the following doses: luseogliflozin, 2.5 mg/day; dapagliflozin, 5 mg/day; tofogliflozin, 20 mg/day; empagliflozin, 10 mg/day; canagliflozin, 100 mg/day. The SGLT2 inhibitors were administered once daily before or after breakfast for 6 months. Each patient received only one of the SGLT2 inhibitors.
Serum hemoglobin A1c (HbA1c), glucose, insulin, lipids, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), uric acid, visceral fat area, and bone strength measurement
Blood was collected before and after 6 months of treatment with the SGLT2 inhibitors to measure various serum parameters. The measurement of serum levels of HbA1c, glucose, insulin, AST, ALT, γ-GTP, uric acid and blood lipid levels was entrusted to Handan Medical Association Health Center (Aichi, Japan). Insulin was measured with an enzyme-linked immunosorbent assay (ELISA), glucose, AST, ALT, γ-GTP, blood lipids and uric acid were measured using an auto-analyzer (JCA-BM8000 series, JAOL, Tokyo, Japan), and HbA1c was measured with automated high-performance liquid chromatography (HPLC) (HLC-723GX, Tosoh Corporation, Tokyo, Japan).
Visceral fat area was measured using DUALSCAN HDS-2000 (Omron Healthcare Co., Ltd, Tokyo, Japan). Bone strength was measured in the calcaneus by ultrasonography; Achilles Express A-1000 (GE Healthcare UK Ltd, England) was used as an ultrasonic generator. Bone strength measurements were expressed as percentages of the values in young individuals (100%) [7].
Statistical analysis
Data are shown as means ± standard deviation (SD). A paired t-test was used to statistically compare the parameters measured before and after SGLT2 treatment; and the level of significance was set at P < 0.05. Regression analysis was performed with the results after 6 months of treatment as the dependent variables, and the baseline values, age, and their interaction as independent variables; the optimal model was obtained by selecting the independent variables using backward elimination based on the Akaike information criterion (AIC) [8]. In addition, the values of parameters that were not normally distributed were also logarithmically transformed for the analyses, to determine whether different conclusions would be drawn from the untransformed and logarithmically transformed data.
| Results | ▴Top |
Patient characteristics
Table 1 shows the patient characteristics. The concomitantly administered antidiabetic drugs, lipid-lowering drugs, and antihypertensive drugs in the patients are shown in Table 1.
 Click to view | Table 1. Patient Characteristics at Baseline |
Effects of SGLT2 inhibitor treatment on the body weight, BMI, visceral fat area, blood glucose, serum HbA1c, plasma insulin, blood lipid, and serum uric acid levels, and liver function parameters
Table 2 shows the values of the parameters before and after 6 months of treatment with an SGLT2 inhibitor.
 Click to view | Table 2. Effects of SGLT2 Inhibitors on Various Parameters (N = 199) |
Body weight, BMI, and visceral fat area
The subjects showed significant decreases, as compared with the baseline values, of the body weight, BMI, and visceral fat area after 6 months of SGLT2 inhibitor treatment.
Glucose, HbA1c and insulin
The subjects showed significant decreases of the blood glucose, serum HbA1c and plasma insulin, as compared with the baseline values, after 6 months of SGLT2 inhibitor treatment.
Blood lipid levels
SGLT2 inhibitor treatment significantly decreased the serum triglyceride levels, as compared with the baseline values, but had no effect on the serum total cholesterol or low-density lipoprotein cholesterol (LDL-C) levels. On the other hand, SGLT2 inhibitor treatment significantly increased the serum high-density lipoprotein cholesterol (HDL-C) levels; in addition, the ratio of the LDL-C/HDL-C (atherosclerosis index) also decreased significantly as compared with baseline.
Serum uric acid
SGLT2 inhibitor treatment significantly decreased the serum uric acid levels as compared with the baseline values.
Liver function parameters
SGLT2 inhibitor treatment was associated with significant decreases of the serum AST, ALT and γ-GTP levels as compared with the baseline values.
Bone mineral density
SGLT2 inhibitor treatment significantly increased the calcaneal bone mineral density as compared with the baseline value.
Influence of patient age on the effects of SGLT2 inhibitors
Regression analysis between age and parameters at the baseline and after 6 months of SGLT2 inhibitor treatment in the patients revealed that the effect of SGLT2 inhibitor treatment on the serum HDL-C was positively correlated with the age, i.e., it increased with age. There was an interaction between age and the baseline levels of AST and γ-GTP; when the baseline levels were low, no age differences in the effects of SGLT2 inhibitor treatment were found, while when the baseline levels were high, the effects of the drugs in reducing the levels of these parameters were more pronounced in younger patients but decreased with advancing age. Serum AST and γ-GTP measurements were also analyzed after logarithmic transformation of the values from the perspective of normality, and similar results were obtained for both AST and γ-GTP.
Scatter plots of HDL-C, AST and γ-GTP before and after 6 months of treatment, and the regression line for representative age, are shown in Figures 1, 2, and 3, respectively. No significant influence of age was observed on the effects of SGLT2 inhibitor treatment on the body weight, BMI, visceral fat area, blood glucose, serum HbA1c, plasma insulin, serum triglycerides, serum LDL-C/HDL-C ratio (atherosclerosis index), serum ALT, serum uric acid or bone mineral density.
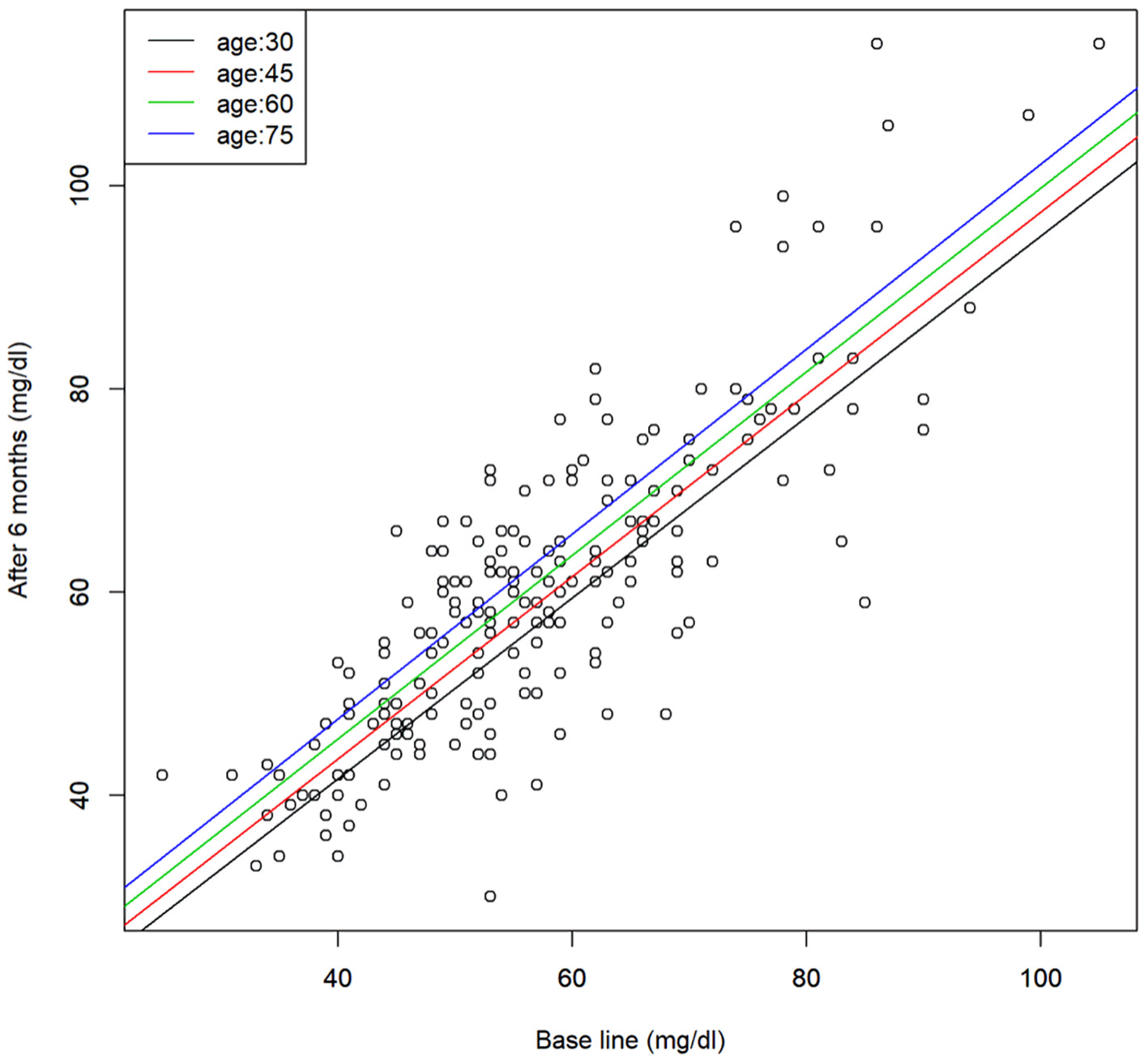 Click for large image | Figure 1. Scatter plot of the serum HDL-C levels before and after 6 months of SGLT2 inhibitor treatment and the regression line for representative age. Regression model, y = 1.26 + 0.9x + 0.13z (x: base line, y: after 6 months, and z: age). HDL-C: high-density lipoprotein cholesterol; SGLT2: sodium-glucose cotransporter 2. |
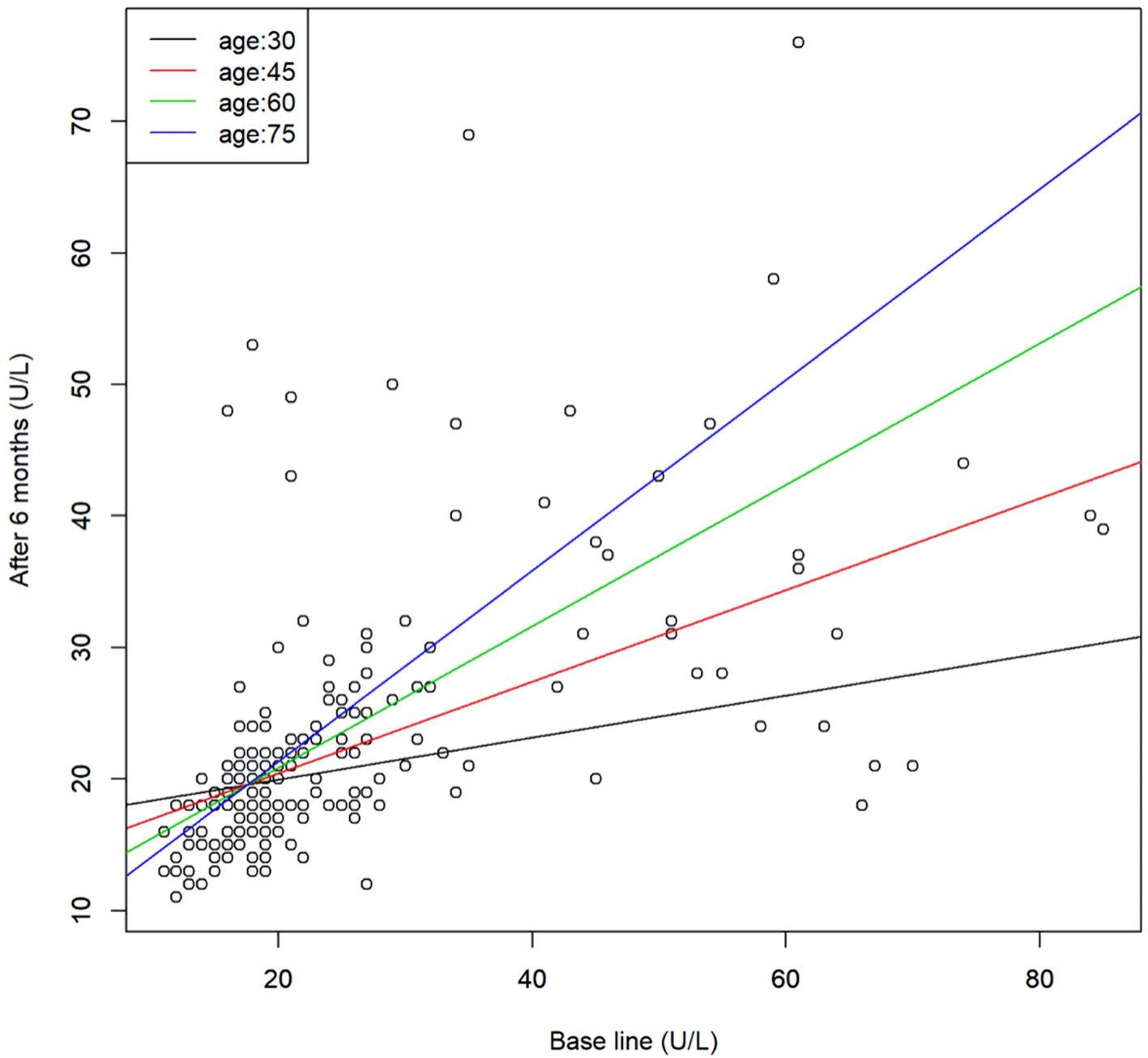 Click for large image | Figure 2. Scatter plot of the serum AST levels before and after 6 months of SGLT2 inhibitor treatment and the regression line for representative age. Regression model, y = 23.4 - 0.218x - 0.22z + 0.013xz (x: base line, y: after 6 months, and z: age). AST: aspartate aminotransferase; SGLT2: sodium-glucose cotransporter 2. |
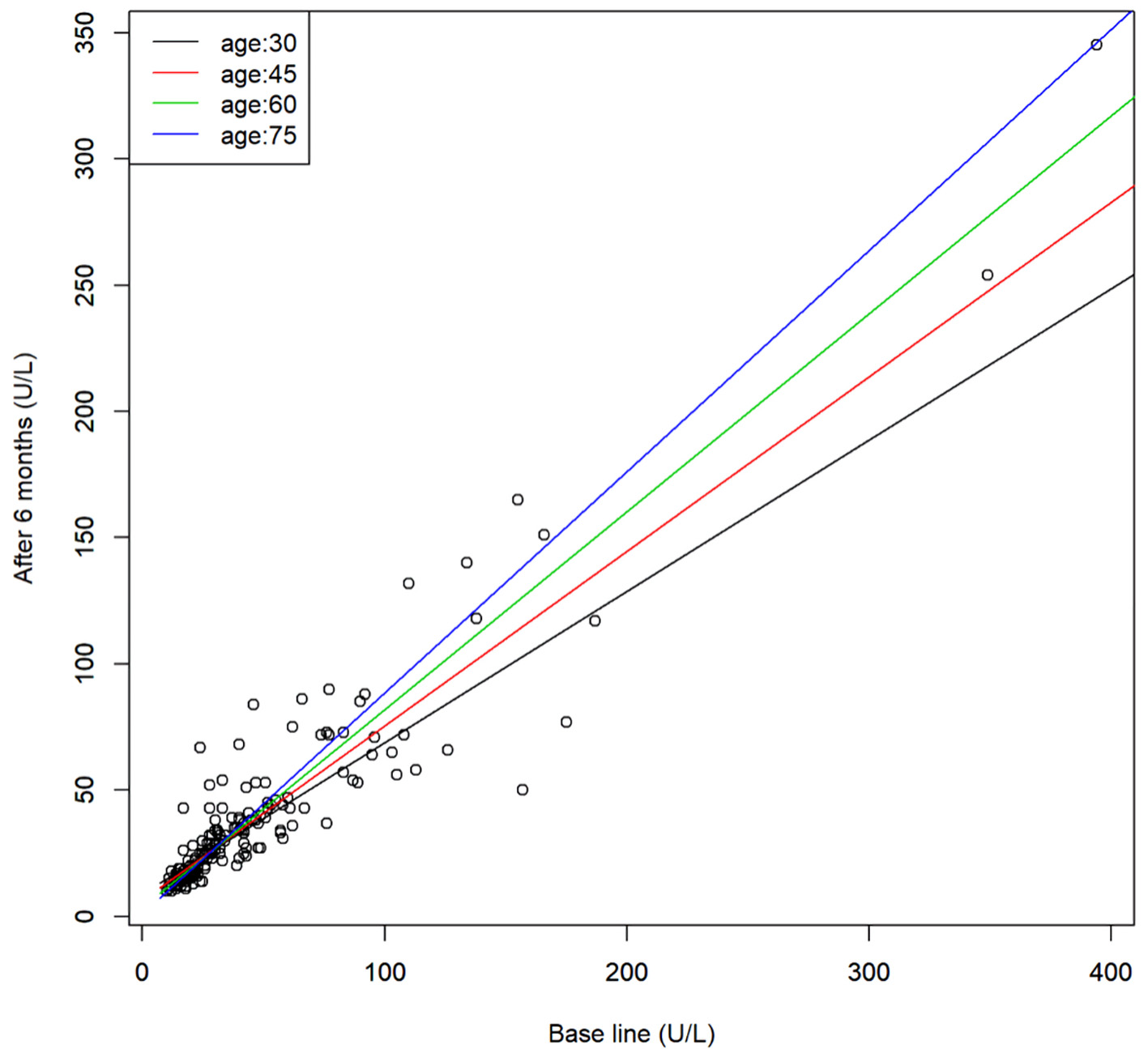 Click for large image | Figure 3. Scatter plot of the serum γ-GTP levels before and after 6 months of SGLT2 inhibitor treatment and the regression line for representative age. Regression model, y = 14.3 + 0.41x - 0.17z + 0.006xz (x: base line, y: after 6 months, and z: age). γ-GTP: γ-glutamyl transpeptidase; SGLT2: sodium-glucose cotransporter 2. |
| Discussion | ▴Top |
The purpose of this study was to clarify how the effects of SGLT2 inhibitors are influenced by the patient age in Japanese patients with type 2 diabetes mellitus.
The results of the study showed that treatment with SGLT2 inhibitors decreased the body weight, BMI, visceral fat area, blood glucose, serum HbA1c, plasma insulin, serum triglycerides, serum LDL-C/HDL-C ratio (atherosclerosis index), liver function parameters (serum ALT, AST, and γ-GTP), and serum uric acid. On the other hand, the treatment was associated with increase of the serum HDL-C. In addition, the bone mineral density also increased. These results are consistent with the results of previously reported clinical studies [4, 5].
Regression analysis of the relationship between age and the changes in the values of the parameters after SGLT2 inhibitor treatment revealed that the effects of the drugs on three parameters, namely, serum HDL-C, AST, and γ-GTP, varied with the patient age.
The effect of SGLT2 inhibitor treatment in increasing the serum HDL-C was positively correlated with the patient age, suggesting that the effect of SGLT2 inhibitors of increasing the serum HDL-C is more pronounced in older patients. HDL-C, which has an antiatherogenic effect, tends to decrease with age, and SGLT2 inhibitor treatment is considered as being useful in the elderly.
The effects of SGLT2 inhibitor treatment in reducing the liver dysfunction parameters AST and γ-GTP were more pronounced in younger patients and decreased with age. This phenomenon was not observed for the effects on the liver function parameter, namely, the serum ALT.
Patients with diabetes mellitus often have decreased liver function and are reported to have a high incidence of hepatic steatosis [9]. Progression of hepatic steatosis has been reported to increase the risk of non-alcoholic steatohepatitis (NASH) [10, 11]. Clinical studies [4, 5] and an animal study [12] have reported that administration of SGLT2 inhibitors improves the liver function, and in this respect, SGLT2 inhibitors are superior to other antidiabetic drugs. However, the results of the present study suggest that the effects of SGLT2 inhibitors in improving the serum AST and γ-GTP may decrease with age; therefore, when SGLT2 inhibitors are administered to elderly diabetic patients with liver dysfunction, it is necessary to monitor the serum AST and γ-GTP levels.
The results of this study showed that SGLT2 inhibitor treatment is associated with significant decrease of the main targets of treatment, namely, the blood glucose, serum HbA1c and plasma insulin, with no influence of the patient age on these effects. SGLT2 inhibitor treatment also decreased the values of variables that affect the blood glucose levels, i.e., the body weight, BMI, and visceral fat, with no effect of the patient age on these effects. The results suggest that SGLT2 inhibitors can be used to treat diabetes mellitus in patients over a wide age range, from the young to the elderly.
In conclusion, our study revealed that the patient age influenced the effects of SGLT2 inhibitor treatment on some factors, but not others, in Japanese patients with type 2 diabetes mellitus. The effects of SGLT2 inhibitor treatment on the serum HDL-C, AST and γ-GTP levels, in particular, were affected by the patient age: their beneficial effect on the serum HDL-C (increase of the level) increased with the patient age and their effects in reducing the serum AST and γ-GTP decreased with the patient age. The effects of the SGLT2 inhibitors on the body weight, BMI, visceral fat area, blood glucose, serum HbA1c, plasma insulin, serum total cholesterol, serum LDL-C, serum triglycerides, serum LDL-C/HDL-C ratio, serum uric acid, and bone mineral density were not affected by the patient age.
The effects of SGLT2 inhibitor treatment on the blood glucose and serum HbA1c were not affected by the patient age, suggesting that SGLT2 inhibitors can be used as antidiabetic drugs in patients over a wide age range, from the young to the elderly.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents were obtained from the patients.
Author Contributions
Mistake Kusunoki: principal investigator, study planning. Fumiya Hisano: data management. Shinichi Matsuda: data analysis. Naomi Wakazono: data management. Kazuhiko Tsutsumi: data management and data analysis. Tetsuro Miyata: co-investigator, data management and data analysis.
Data Availability
Any inquiries regarding the availability of the supporting data from this study should be directed to the corresponding author.
| References | ▴Top |
- Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med. 2013;34(2-3):183-196.
doi pubmed - Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733-794.
doi pubmed - DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14(1):5-14.
doi pubmed - Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30(7):1245-1255.
doi pubmed - Kusunoki M, Natsume Y, Sato D, Tsutsui H, Miyata T, Tsutsumi K, Suga T, et al. Luseogliflozin, a sodium glucose co-transporter 2 inhibitor, alleviates hepatic impairment in Japanese patients with type 2 diabetes. Drug Res (Stuttg). 2016;66(11):603-606.
doi pubmed - Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117-2128.
doi pubmed - Kusunoki M, Sato D, Miyata T, Tsutsumi K, Oshida Y. SGLT2 inhibitors increased serum levels of total P1NP and bone strength in Japanese patients with type 2 diabetes. Journal of Endocrinology and Metabolism. 2020;10(3-4):89-93.
doi - Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716-723.
doi - Bhatt HB, Smith RJ. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr. 2015;4(2):101-108.
- Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age? World J Gastroenterol. 2014;20(27):9072-9089.
- Le TA, Loomba R. Management of Non-alcoholic Fatty Liver Disease and Steatohepatitis. J Clin Exp Hepatol. 2012;2(2):156-173.
doi - Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715(1-3):246-255.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
