| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 13, Number 3, August 2023, pages 121-125
Metastatic Papillary Thyroid Carcinoma Gone Wild
Khulood Talpura, d, Ambreen Soomrob, Afrah Talpurc
aDepartment of Internal Medicine, Liaquat University of Medical and Health Sciences, Jamshoro, Pakistan
bChandka Medical College, Larkana, Pakistan
cJefferson Abington Hospital, Abington, PA, USA
dCorresponding Author: Khulood Talpur, Department of Internal Medicine, Liaquat University of Medical and Health Sciences, Jamshoro, Pakistan
Manuscript submitted August 3, 2021, accepted July 21, 2023, published online August 25, 2023
Short title: Metastatic Papillary Thyroid Carcinoma
doi: https://doi.org/10.14740/jem764
| Abstract | ▴Top |
Thyroid cancers account for only 1.5% of all cancers in adults and 3% of all cancers in children. In females, however, thyroid cancers are the fifth most common cancer, comprising 4% of all cases. Of all thyroid cancers, 74-80% of cases are papillary cancer. The prevalence of thyroid cancer is rising worldwide. Although thyroid cancer has a favorable prognosis, up to 20% of patients experienced recurrent disease during the follow-up period. Extra-thyroidal extension occurs in 8% to 32% of cases. Local or regional recurrences occur in 5-15% of patients with papillary thyroid carcinoma (PTC). Distant metastasis occurs in only 1% to 25% mostly in the lungs and bones. Our patient is a 68-year-old woman with a past medical history of hypertension and endometriosis. She has metastatic PTC with recurrent metastasis. She had a subtotal thyroidectomy in 2004; radioactive iodine ablation of residual cancer with two recurrent metastases at the right lateral supraclavicular mass and skull base; underwent multiple neck surgeries and resection of skull base lesion in 2014; and local neck resection of right lateral supraclavicular neck mass in 2017. She presented this time with a 6-month history of right submaxillary and right infra-auricular growth. The patient underwent an ultrasound-guided biopsy of tonsillar and submaxillary masses with pathology consistent with PTC. In 2020, the patient was found to have parotid gland involvement. Biopsy of the parotid gland confirmed PTC cells. Recently in 2021 whole-body positron emission tomography/computed tomography (PET/CT) showed early bone metastasis. This case report exemplifies a highly aggressive PTC with recurrent metastases to sites that have rarely been reported in the past. It also draws our attention to the unknown prognosis of such aggressive metastatic PTC. The presence of metastatic lesions at uncommon sites such as the tonsils, vocal cord, parotid gland, oropharynx and bone metastasis should direct our focus toward a need for further research on metastatic PTC to establish improved management strategies.
Keywords: Papillary thyroid cancer; Recurrent metastatic cancer; Vocal cord; Bone metastasis
| Introduction | ▴Top |
Thyroid cancers account for only 1.5% of all cancers in adults and 3% of all cancers in children. In females, however, thyroid cancers are the fifth most common cancer, comprising 4% of all cases. Of all thyroid cancers, 74-80% of cases are papillary cancer [1]. The prevalence of thyroid cancer is rising worldwide. Although thyroid cancer has a favorable prognosis, up to 20% of patients experienced recurrent disease during the follow-up period [2]. Extra-thyroidal extension occurs in 8% to 32% of cases. Local or regional recurrences occur in 5% to 15% of patients with papillary thyroid carcinoma (PTC) [3].
Distant metastasis occurs in only 1% to 25% mostly in the lungs and bones. Other less common sites are the brain, liver, and skin [4]. The 5-year relative survival rates by stage of diagnosis are as follows: all stages 98%, local disease > 99%, regional metastasis 98% and distant metastasis 55% [1]. Both recurrences and death from PTC can occur more than 30 years after being treated, thus lifelong follow-up of patients with PTC is necessary [5].
A study by Seejore et al (2019) [6] suggested that patients with delayed risk stratification (DRS) low-risk thyroid cancer could be safely discharged after 5 years of follow-up; however, the ideal duration of follow-up of individuals categorized as having low-risk thyroid carcinoma, who account for most differentiated thyroid cancer (DTC) patients, is as yet not entirely clear. The 2014 British Thyroid Association (BTA) guidelines advise lifelong follow-up [7].
| Case Report | ▴Top |
Investigations
Our patient is a 68-year-old female with past medical history of hypertension, endometriosis, metastatic PTC with recurrent metastasis, status post subtotal thyroidectomy in 2004 to avoid the risk of hypoparathyroidism, status post radioactive iodine (iodine-131) (RAI) dose of 70 millicuries ablation of residual cancer for with two recurrent metastasis of PTC at the right lateral supraclavicular mass and skull base status post modified right radical neck dissection with salvage of submandibular gland with superior mediastinal dissection and resection of skull base lesion 2014 status post local neck resection of right lateral supraclavicular neck mass in 2017 (Fig. 1). She presented in 2019 with a 6-month history of right submaxillary and right infra-auricular growth. Both masses were painful, gradually increased in size, and were associated with difficulty swallowing. She denied fever, discharge, recent trauma, other masses, weight changes, or appetite changes. She was hemodynamically stable, and on examination, the neck masses were soft, tender, 2 × 2 cm in size, and cold to touch. Rigid laryngoscopy showed right vocal cord paralysis and right tonsillar enlargement (Fig. 2). Labs were unremarkable.
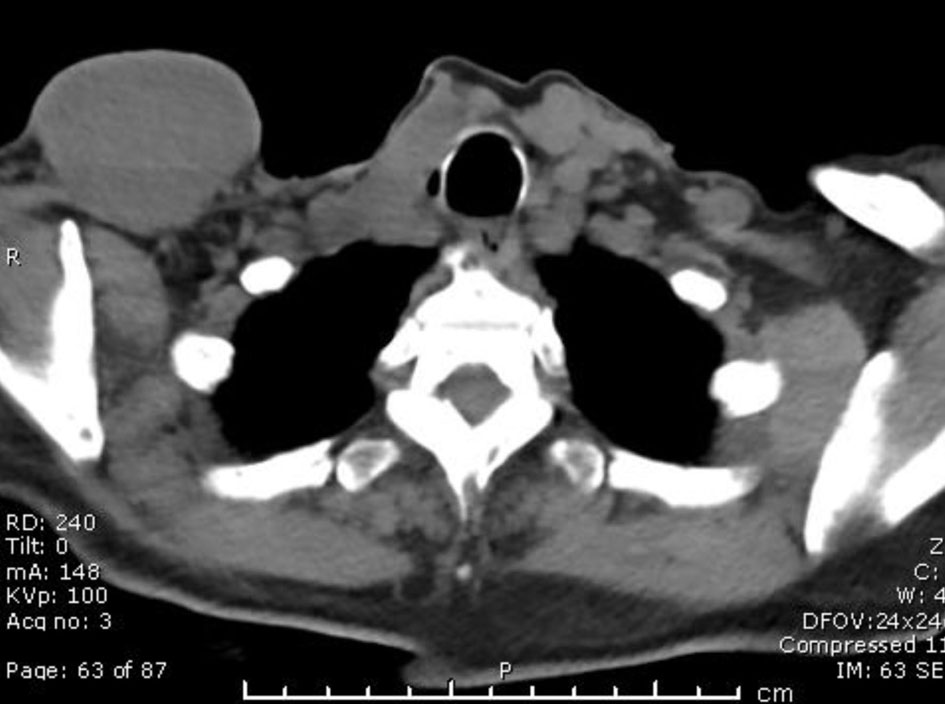 Click for large image | Figure 1. Computed tomography scan w/o contrast of neck and soft tissue in 2017. |
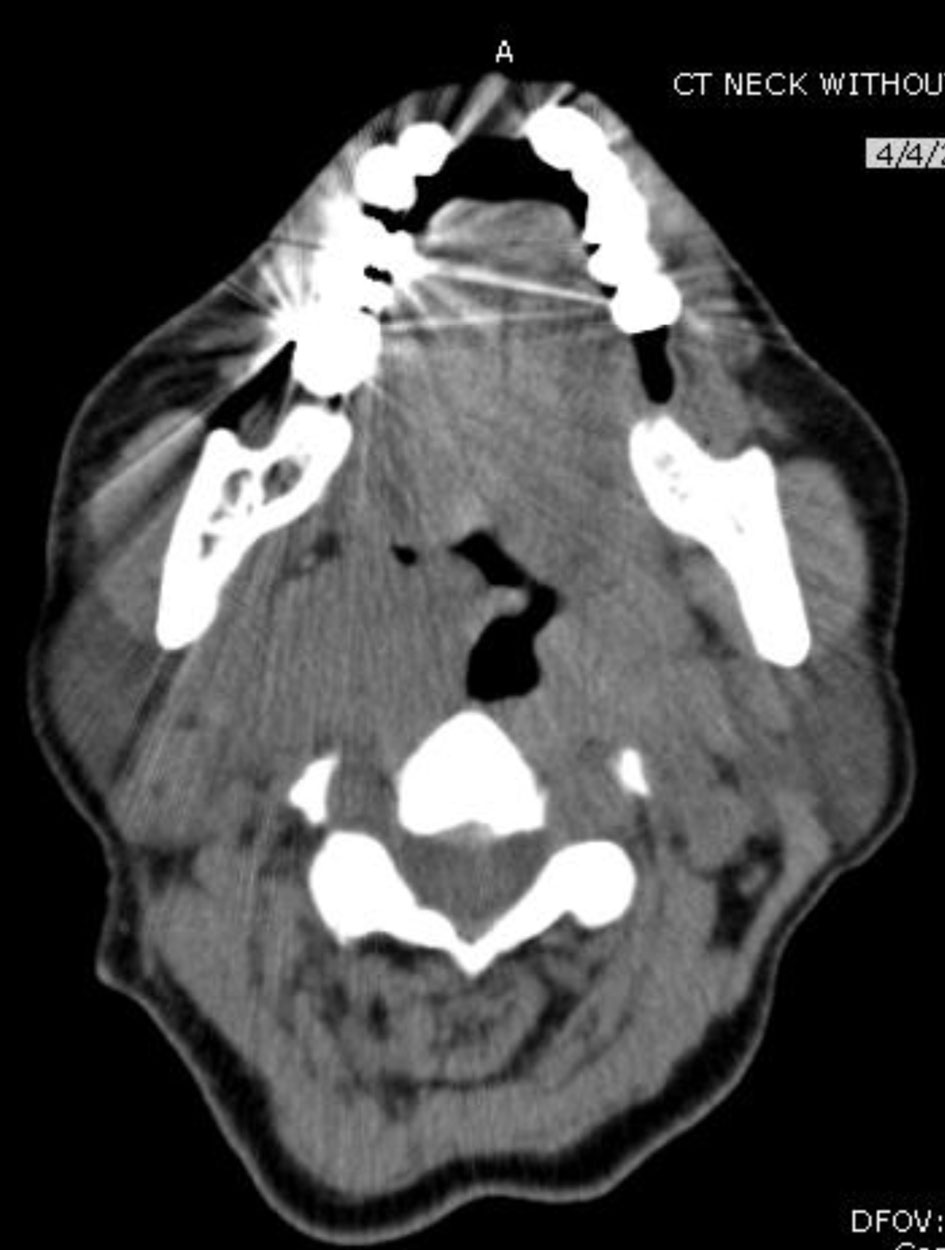 Click for large image | Figure 2. Oropharyngeal mass on computed tomography scan in 2019. |
Diagnosis
The patient underwent an ultrasound-guided biopsy of tonsillar and submaxillary mass. The hematoxylin and eosin (H&E) stain showed classical microscopic features of PTC which included papillae with fibrovascular cores, psammoma bodies and Orphan Annie nuclei consistent with PTC (Figs. 3, 4). The H&E stain of the parotid gland tissue done in 2020 showed classical microscopic features of PTC which included papillae with fibrovascular cores, psammoma bodies, Orphan Annie nuclei, nuclear groove formation and overlapping of nuclei which are shown in Figures 5 and 6.
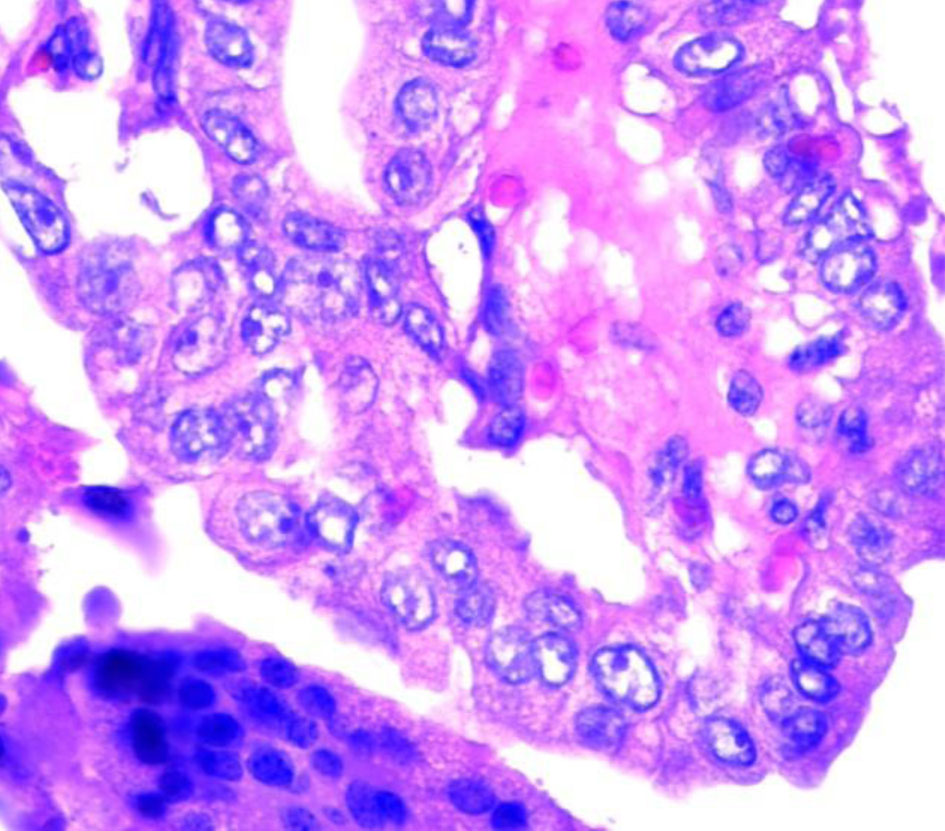 Click for large image | Figure 3. Orphan Annie nuclei. |
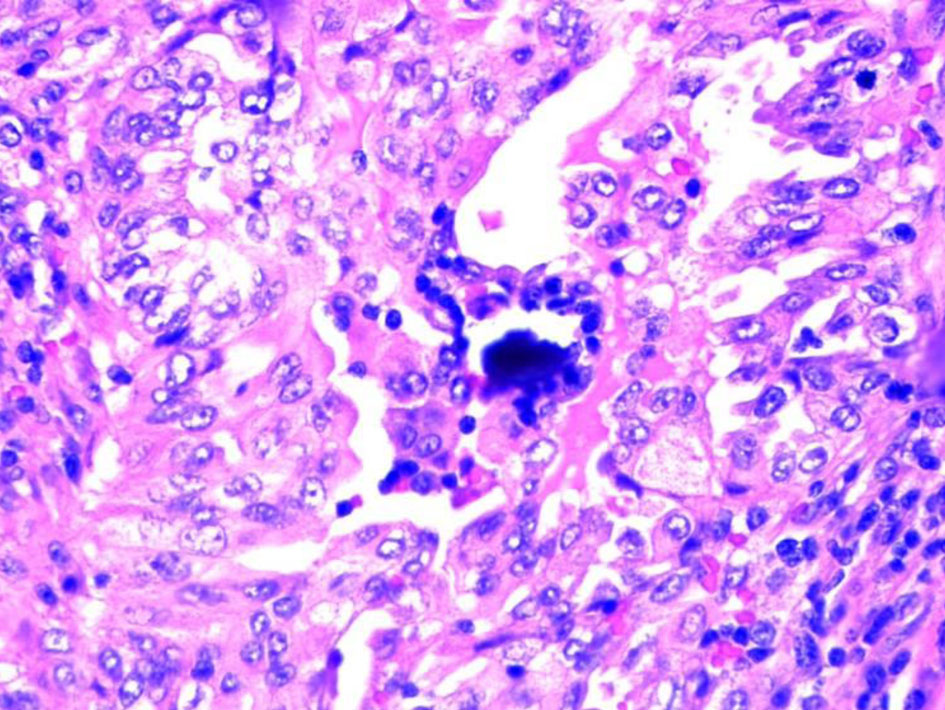 Click for large image | Figure 4. Psammoma body. |
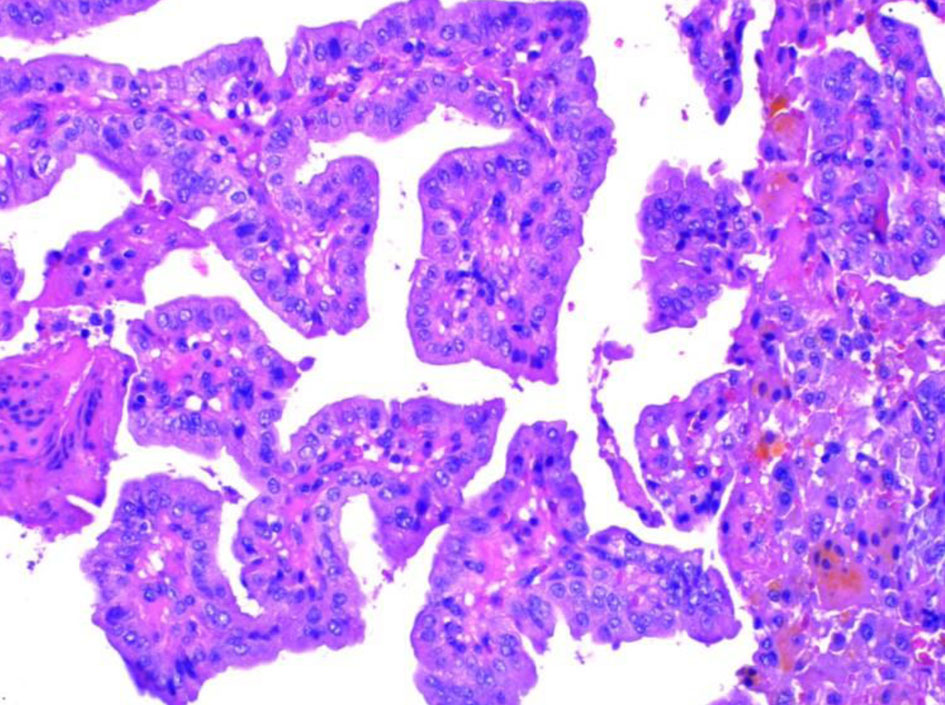 Click for large image | Figure 5. Papillae with fibrovascular cores. |
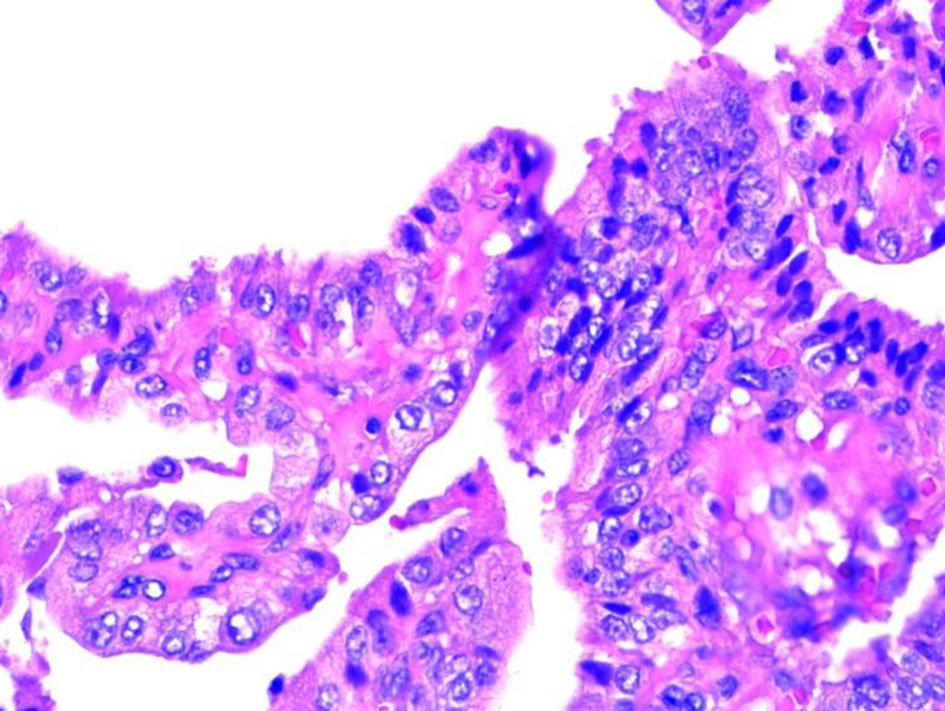 Click for large image | Figure 6. Nuclear grove. |
Treatment
To treat PTC in this patient, subtotal thyroidectomy was done to avoid the risk of hypoparathyroidism. RAI ablation of residual cancer was done in six different sessions; however, after that, the PTC became iodine refractory. She also underwent modified right radical neck dissection with salvage of submandibular gland with superior mediastinal dissection and resection of skull base lesion in 2014 and local neck resection of right lateral supraclavicular neck mass in 2017. Patient was started with anti-cancerous medication 3 months after the last surgery and is currently on lenvatinib. First, all surgeries were done then lenvatinib was started. She follows up with Hematology/Oncology. A gene survey of the patient was also advised.
Follow-up and outcomes
During her follow-up, a whole-body skull-thigh positron emission tomography/computed tomography (PET/CT) scan was done in 2021, which showed a new hypermetabolic lesion in the region of clivus suggesting early osseous metastatic disease. Patient continued to have progressive metastatic disease. Anti-thyroglobulin antibodies investigation was done after surgery in 2004 which was 400 IU/mL but after 2018 they were undetectable; however, she followed up with anti-thyroglobulin antibodies and a CT scan to see any recurrence.
| Discussion | ▴Top |
PTC accounts for 80% of thyroid malignancies and tends to metastasize to the regional lymph nodes [8]. Many patients who are less than 45 years of age that have thyroid cancer with only regional lymph node involvement usually have an excellent prognosis. The lung is the most reported distant secondary metastasis location occurring at a frequency of only around 3% to 15% [9]. PTC tends to metastasize through the lymphatic system and hematogenous spread is rare [10, 11]. We believe this to be one of the rare presentations of metastatic PTC with spread to the tonsils, parotid gland and oropharynx. The newest PET scan shows detailed clear picture of the entire body, which is helpful in diagnosis of metastatic disease.
Most commonly, PTC manifests as an asymptomatic thyroid nodule or mass. Occasionally, it can also present as dyspnea, dysphagia or persistent cough. Rarely, symptoms such as new onset hoarseness, vocal cord paralysis, rapid enlargement or cervical lymphadenopathy point to local metastasis. In around 50% of cases, diagnosis of PTC is a result of a thyroid nodule identified on physical examination or an incidental finding detected on an imaging study. The rest of cases are a result of patients identifying an asymptomatic nodule by themselves [12]. Age less than 15 or more than 45, male gender, previous head or neck radiation and a family history of thyroid cancer in a first-degree relative are some of the risk factors associated with increased risk of malignancy. A history of radiation exposure is a very common finding associated with PTC. Thyroid nodules may develop within 5 years of radiation exposure and reach a peak within 30 years, but incidence remains high even after 40 years of exposure [13, 14].
However, there is the emergence of novel treatments for thyroid carcinoma, including tyrosine kinase inhibitors (TKIs) such as lenvatinib and sorafenib, pretreatment with mitogen-activated protein kinase inhibitors such as trametinib and selumetinib, FDA-approved TKIs vandetanib and cabozantinib to treat medullary thyroid cancer (MTC). Other cutting-edge treatments for MTC include peptide receptor radionuclide therapy and the carcinoembryonic antigen (CEA) vaccine. In clinical trials, selective rearranged-during-transfection (RET) protooncogene inhibitors, such as LOXO-292 and BLU-667, demonstrated promising efficacy in the treatment of metastatic MTC resistant to nonselective TKIs. The FDA-approved combination of BRAF/MEK inhibitors dabrafenib and trametinib has revolutionized the treatment of BRAFV600E-positive anaplastic thyroid carcinoma [3].
In terms of staging, the good prognostic factors include young age (< 45 years), control locally, micronodular pulmonary metastasis and disease that is sensitive to RAI [2, 9]. If a 45-year-old patient has metastatic thyroid cancers confined to the lungs only then their classification is stage II with a 5-year disease-specific survival (DSS) of 100%. If it was the same scenario with a patient greater than 45 years of age, their classification becomes stage IV with a 5-year DSS that drops by half at 51%. The prognostic factors that are consistent with a poor prognosis include age > 70 years, distant metastasis beyond the lungs, follicular histology, poor differentiation and lymph node metastasis > 3 cm [9].
Diagnostic measures for any patient presenting with a thyroid nodule include a complete history and physical exam and a routine neck ultrasound examination to help differentiate the nodule and assess the thyroid gland itself. The American Thyroid Association (ATA), the American Association of Clinical Endocrinologists and the National Comprehensive Cancer Network (NCCN) all recommend an ultrasound as a first step to determine the location, characteristics, and extent of the thyroid nodule. The sonographic features of a malignant thyroid nodule include hypoechogenicity, incomplete peripheral halo, irregular margins, microcalcifications, invasion of surrounding tissue, and intramodular hypervascularity. Ultrasound can also detect additional sites of local or metastatic lesions. Based on ultrasound findings and clinical symptoms, a fine-needle aspiration (FNA) biopsy may be warranted. It is the most accurate and valuable procedure in assessing a thyroid nodule. The sensitivity of FNA depends on the sample collection technique and the experience of the cytopathologist [15]. The results of the biopsy are divided into the following categories: malignant, suspicious, indeterminate, benign, and nondiagnostic. Accuracy of FNA can be as high as 90-95%. Immunostains such as cytokeratin-19, HMBE-1 and galectin-3 can prove to be useful in differentiating benign and malignant lesions. BRAF mutations could also play an important role in indeterminate cytology. Forty to fifty percent of PTCs have reported BRAF point mutations [16]. A screening serum thyroid-stimulating hormone (TSH) level is also measured to determine the functionality of the thyroid gland. Thyroid scintigraphy can be useful in cases of low serum TSH levels which would point to a hyperfunctioning nodule that is rarely malignant.
Management of PTC entails total or near-total thyroidectomy in patients with a nodule sized 1 cm or greater. For patients with PTC of less than 1 cm, total thyroidectomy or near-total thyroidectomy is only recommended if they have contralateral thyroid disease, regional or distant metastases, personal history of radiation therapy to the head and neck region or a family history of PTC in a first degree relative or else a lobectomy or isthmus ectomy is suitable. While the ATA guideline of 2015 states that lobectomy may also be sufficient as initial therapy [7, 17]. In patients with macroscopic nodal disease, a lymphadenectomy could reduce the risk of recurrence and mortality. There is also a possible role of post-operative radioiodine administration which in theory destroys the normal thyroid tissue or microscopic tumor lesions elsewhere. Nevertheless, the usefulness of this procedure routinely is controversial or yet to be determined as some studies have shown empiric benefits of reducing the rate of recurrence while others have shown no added benefit in terms of recurrence or mortality. However, there are adequate data that demonstrate the value of radioiodine therapy in reducing recurrence and improving mortality in patients with incomplete resections, extra-thyroidal tumor spread, nodal or distant metastasis or aggressive histology. External beam radiation is indicated when a complete resection is not possible, and the PTC does not concentrate radioiodine [15].
There is an important role in post-operative thyroid hormone replacement therapy as it inhibits TSH-dependent growth of residual tumor cells and understandably treats post-thyroidectomy hypothyroidism. The ATA recommends serum TSH levels to be suppressed below 0.1 mU/L for patients with persistent or residual disease but for patients with low risk of PTC, the levels should be maintained within the normal range [15], post-operative surveillance is recommended based on the recurrence risk levels of patients. Physical examination, serum TSH, basal thyroglobulin and anti-thyroglobulin antibody measurements are recommended every 6 months. At the least, patients should have a TSH-stimulated thyroglobulin level and a neck ultrasound performed a year after total thyroidectomy and remnant ablation [13].
The National Cancer database shows that the 10-year survival rate for patients of PTC was 93% [10]. Despite high success rates of surgery with or without RAI and suppressive doses of thyroid hormone, some cases are unresponsive to treatment with continued progression of disease leading to metastasis like our patient. Several molecular markers and therapies are being investigated for treatment of PTCs. Inhibition of tumor growth by inhibiting cell signaling and angiogenesis and induction of redifferentiation of thyroid tumor tissue are the points of research. These therapies are currently in clinical trials [13]. Overall, this case illustrates a rare presentation of metastatic PTC.
Learning point
PTC can be an aggressive cancer with multiple recurrences. This unique case reminds us that although our patient had surgeries and was in remission, this cancer can come back anytime during the next several years. Patients need to be counseled about need for follow-up regularly. Survival rates are high, but patients’ quality of life is affected significantly due to multiple follow-up scans, surgeries and clinic visits. Our case report points out the need for further research for better treatment options to specifically reduce recurrence.
Conclusion
This case report exemplifies a highly aggressive PTC with recurrent metastasis to sites that have rarely been reported in the past. It also draws our attention to the unknown prognosis of such aggressive metastatic PTC. The presence of metastatic lesions at uncommon and unreported sites such as the tonsils and oropharynx should direct our focus towards a need for further research on metastatic PTC to establish improved management strategies.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was taken from the patient.
Author Contributions
Case presentation: Khulood Talpur. Research and data gathering: Khulood Talpur, Afrah Talpur, Ambreen Soomro. Review of article and images of histology findings: Khulood Talpur, Ambreen Soomro, Afrah Talpur.
Data Availability
We declare that data supporting the finding of this study are available in this article and it can be requested from the corresponding author.
| References | ▴Top |
- Viale PH. The American Cancer Society's Facts & Figures: 2020 Edition. J Adv Pract Oncol. 2020;11(2):135-136.
doi pubmed pmc - Chatchomchuan W, Thewjitcharoen Y, Karndumri K, Porramatikul S, Krittiyawong S, Wanothayaroj E, Vongterapak S, et al. Recurrence factors and characteristic trends of papillary thyroid cancer over three decades. Int J Endocrinol. 2021;2021:9989757.
doi pubmed pmc - Xue S, Wang P, Liu J, Chen G. Total thyroidectomy may be more reasonable as initial surgery in unilateral multifocal papillary thyroid microcarcinoma: a single-center experience. World J Surg Oncol. 2017;15(1):62.
doi pubmed pmc - Sebastian SO, Gonzalez JM, Paricio PP, Perez JS, Flores DP, Madrona AP, Romero PR, et al. Papillary thyroid carcinoma: prognostic index for survival including the histological variety. Arch Surg. 2000;135(3):272-277.
doi pubmed - Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154(6):1436-1446; discussion 1446-1437.
doi pubmed - Seejore K, Gerrard GE, Gill VM, Murray RD. Can we discharge dynamically risk-stratified low-risk (Excellent response to treatment) thyroid cancer patients after 5 years of follow-up? Clin Oncol (R Coll Radiol). 2019;31(4):219-224.
doi pubmed - Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, Gilbert J, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81(Suppl 1):1-122.
doi pubmed - Alameh A, Shubham A, Elizabeth V, Ahmad J, Jason L, Hussein H. A rare case of papillary thyroid carcinoma with pleural metastasis. Chest Annual Meeting. 2020;158:1289-1290.
doi - Sacks W. Management of aggressive metastatic papillary thyroid cancer involves multiple treatment methods. Clinic Thyroidol. 2013;25:20-23.
- Ozdemir M, Lee YS, Makay O, Duenas JP, Yazici B, Akgun A, Icoz G, et al. Clinical behavior and outcome of papillary T1 thyroid cancers: South Korea vs. Turkey vs. Colombia in a cohort study analyzing oncological outcomes. Asian J Surg. 2020;43(8):795-798.
doi pubmed - Varghese BT, Mathews A, Pandey M, Pradeep V. Unusual metastasis of papillary thyroid carcinoma to larynx and hypopharynx a case report. World J Surg Oncol. 2003;1(1):7.
doi pubmed pmc - So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg. 2018;50:94-103.
doi pubmed - Rosenbaum MA, McHenry CR. Contemporary management of papillary carcinoma of the thyroid gland. Expert Rev Anticancer Ther. 2009;9(3):317-329.
doi pubmed - Zhu J, Zheng J, Li L, Huang R, Ren H, Wang D, Dai Z, et al. Application of machine learning algorithms to predict central lymph node metastasis in T1-T2, non-invasive, and clinically node negative papillary thyroid carcinoma. Front Med (Lausanne). 2021;8:635771.
doi pubmed pmc - Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, European Thyroid Cancer T. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787-803.
doi pubmed - Alexander EK. Approach to the patient with a cytologically indeterminate thyroid nodule. J Clin Endocrinol Metab. 2008;93(11):4175-4182.
doi pubmed - Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109-142.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
