| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Review
Volume 10, Number 3-4, August 2020, pages 57-59
The Points of Action of Drugs for Treating COVID-19
Hidekatsu Yanai
Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, 1-7-1 Kohnodai, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted June 16, 2020, accepted June 22, 2020, published online August 26, 2020
Short title: Points of Action of Drugs for Treating COVID-19
doi: https://doi.org/10.14740/jem659
- Abstract
- Introduction
- SSCV May Contribute to the Development of Severe COVID-19
- The Points of Action of Drugs for Treating COVID-19
- Conclusions
- References
| Abstract | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has reached a pandemic level. Very recently, I reported a significantly higher prevalence of diabetes and hypertension in severe COVID-19 as compared with non-severe COVID-19 by the meta-analysis. Considering that both diabetes and hypertension are risk factors for atherosclerosis, I further studied the prevalence of cardiovascular disease (CVD) in COVID-19 and found a significantly higher prevalence of CVD in severe patients than in non-severe patients. I speculate that the pre-existing vascular damage is associated with severity of COVID-19. A recent study showed that obese patients with COVID-19, despite their younger age, required more frequently assisted ventilation and access to intensive care units than normal weight patients. I thought that if the reason that COVID-19 is likely to become severe in obese people could be elucidated, the mechanism for aggravation of COVID-19 would be understood. As a result of considering a model of aggravation in obese people, I came up with the notion that pre-existing risk factors in obese people such as their vascular high-affinity for SARS-CoV-2, pro-inflammatory and pro-coagulant state and endothelial dysfunction may be likely to induce the development of “systemic severe coagulopathic vasculitis (SSCV)” in obese people. I believe that SSCV may largely contribute to the development of severe COVID-19. Here, I will describe the points of action of drugs for treating COVID-19 by using the SSCV model.
Keywords: SARS-CoV-2; COVID-19; Points of action; Drugs
| Introduction | ▴Top |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has reached a pandemic level. Very recently, I reported a significantly higher prevalence of diabetes (odds ratio (OR) 3.52, 95% confidence interval (CI) 2.65 - 4.67) and hypertension (OR 2.69, 95% CI 2.16 - 3.34) in severe COVID-19 as compared with non-severe COVID-19 [1]. Considering that both diabetes and hypertension are risk factors for atherosclerosis, I further studied the prevalence of cardiovascular disease (CVD) in COVID-19, and found the prevalence of CVD in severe patients was remarkably higher than that in non-severe patients (OR 5.37, 95% CI 3.73 - 7.74) [1].
I speculate that the pre-existing vascular damage is associated with severity of COVID-19. Aging is one of important risk factors for vascular damage such as arteriosclerosis. Evidence has emerged to show that elderly people may be at higher risk of developing severe health outcomes from COVID-19 [2], supporting a significance of pre-existing vascular damage in severe COVID-19. I believe that “systemic severe coagulopathic vasculitis (SSCV)” which I named may contribute to the development of severe COVID-19. Here, I will introduce the concept of SSCV and describe the points of action of drugs for treating COVID-19 by using this SSCV model.
| SSCV May Contribute to the Development of Severe COVID-19 | ▴Top |
Both diabetes and hypertension are closely related to insulin resistance commonly observed in obese people. A recent study showed that obese patients with COVID-19, despite their younger age, required more frequently assisted ventilation and access to intensive care units (ICUs) than normal weight patients [3], suggesting that obesity/insulin resistance can be the risk for severe COVID-19 even in young people. I think that elucidating the mechanism by which COVID-19 is aggravated in obese individuals may lead to elucidation of the mechanism for the aggravation of COVID-19.
Angiotensin-converting enzyme 2 (ACE2) is the cellular entry receptor of SARS-CoV-2 [4]. Increased ACE2 expression in bronchial epithelial cells in chronic obstructive pulmonary disease (COPD) patients who are overweight compared to those who are not overweight was observed [5], indicating that SARS-CoV-2 is more likely to enter into the human body in obese people as compared with non-obese people. ACE2 is also present in vascular endothelial cells, and its expression is increased due to obesity [6]. Such high-affinity of SARS-CoV-2 for cardiovascular system may explain frequent cardiovascular involvement in severe COVID-19 [7]. Obesity/insulin resistance is likely to induce vascular damage such as endothelial dysfunction and procoagulant state due to increased secretion of inflammatory cytokines (interleukin-6 (IL-6), etc) and plasminogen activator inhibitor-1 (PAI-1) from adipose tissues [8]. In severe COVID-19 patients, elevation of von Willebrand factor (VWF, the marker for endothelial injury) and D-dimer (the marker for thrombosis) was observed, indicating the existence of hypercoagulability together with severe endothelial injury in severe COVID-19 [9], which I named as SSCV [8]. Pre-existing risk factors in obese people such as their vascular high-affinity for SARS-CoV-2 via ACE2, pro-inflammatory and pro-coagulant state and endothelial dysfunction may be likely to induce the development of SSCV in obese people. I believe that SSCV may largely contribute to the development of severe COVID-19. The SARS-CoV-2 epidemic was reported to be associated with high incidence of a severe form of Kawasaki disease known as systemic vasculitis [10], supporting a significant association of SARS-CoV-2 infection with systemic severe vasculitis.
| The Points of Action of Drugs for Treating COVID-19 | ▴Top |
I will think of the points of action of drugs for treating COVID-19 by using the SSCV model (Fig. 1).
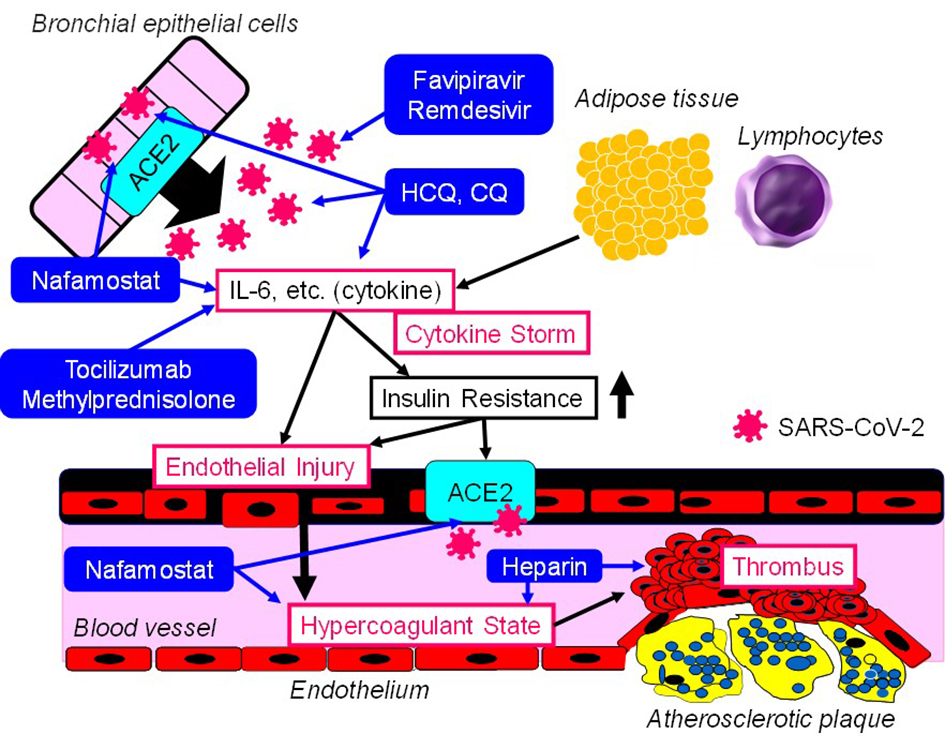 Click for large image | Figure 1. The points of action of drugs for treating COVID-19 in the SSCV model. SSCV: systemic severe coagulopathic vasculitis; ACE2: angiotensin-converting enzyme 2; IL-6: interleukin-6; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; HCQ: hydroxychloroquine; CQ: chloroquine. |
Drugs which can directly target the virus replication
Favipiravir plays as an inhibitor of the RNA-dependent RNA polymerase by structurally resembling the endogenous guanine [11]. Through competitive inhibition, the efficacy of viral replication can be largely reduced. Favipiravir may be a relatively safe and effective drug for COVID-19 at present [12]. Remdesivir is an adenosine analogue, which incorporates into nascent viral RNA chains and results in pre-mature termination [13]. Remdesivir was highly effective in the control of SARS-CoV-2 infection in vitro [14]. Favipiravir and remdesivir can be classified into pharmaceutical agents that can directly target the virus replication.
Drugs based on immunotherapy which improve damage induced by inflammatory responses
Hydroxychloroquine (HCQ) is often used in chronic inflammatory diseases, including systemic lupus erythematosus and rheumatoid arthritis. Several potential mechanisms of action of HCQ against SARS-CoV-2 include inhibition of viral attachment to the host cells, inhibition of viral release into the intracellular space by disruption of lysosome-endosome fusion, and inhibition of the release of pro-inflammatory cytokines [15]. Chloroquine (CQ) was highly effective in the control of SARS-CoV-2 infection in vitro [14]. Tocilizumab is a humanized monoclonal antibody which selectively targets the IL-6 receptor. Recently, tocilizumab has become one of the therapeutic options for the management of cytokine release syndrome [16], indicating that tocilizumab may improve cytokine storm in COVID-19. Among COVID-19 patients with acute respiratory distress syndrome (ARDS), the treatment with methylprednisolone decreased the risk of death (hazard ratio, 0.38; 95% CI 0.20 - 0.72) [17]. CQ, HCQ, tocilizumab and methylprednisolone can be classified into pharmaceutical agents based on immunotherapy which improve damage induced by inflammatory responses.
Anticoagulant
Nafamostat (serine protease inhibitor), known as anticoagulant, has potential anti-inflammatory and anti-viral activities against COVID-19 [18]. Nafamostat can prevent the fusion of the envelope of the virus with host cell surface membranes [19, 20]. In the study which investigated the association between the treatment with heparin known as anticoagulant and mortality in 2,075 patients with COVID-19, heparin was associated with lower mortality when the model was adjusted for age and gender (OR 0.55, 95% CI 0.37 - 0.82; P = 0.003) [21]. This association remained significant when saturation of oxygen < 90% and temperature > 37 °C were added to the model (OR 0.54, 95% CI 0.36 - 0.82; P = 0.003) [21].
| Conclusions | ▴Top |
Successful suppression of viral replication by favipiravir or remdesivir can prevent the development of cytokine storm and SSCV. If cytokines and markers for inflammation continue to increase, the additional use of CQ and HCQ may be considered. If cytokine storm or ARDS develops, the additional use of tocilizumab or methylprednisolone may be supportive. It may be better to consider the additional use of nafamostat or heparin when the development of SSCV accompanied by elevation of VWF and D-dimer is observed.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
HY collected literatures and wrote and approved the final paper.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Yanai H. A significance of high prevalence of diabetes and hypertension in severe COVID-19 patients. J Clin Med Res. 2020;12(6):389-392.
doi - Matsushita K, Ding N, Kou M, Hu X, Chen M, Gao Y, et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: A systematic review and meta-analysis. medRxiv. 2020:20054155.
doi - Busetto L, Bettini S, Fabris R, Serra R, Dal Pra C, Maffei P, Rossato M, et al. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring). 2020.
doi pubmed - Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382(17):1653-1659.
doi pubmed - Higham A, Singh D. Increased ACE2 Expression in the bronchial epithelium of COPD patients who are overweight. obesity (silver spring). 2020.
doi pubmed - Engin AB, Engin ED, Engin A. Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking. Environ Toxicol Pharmacol. 2020;78:103411.
doi pubmed - Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. COVID-19 and the Cerebro-Cardiovascular Systems: What do we Know so Far? J Am Heart Assoc. 2020:e016793.
doi pubmed - Yanai H. Adiposity is the crucial enhancer of COVID-19. Cardiol Res. 2020 (in press).
- Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, et al. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020.
doi pubmed - Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778.
doi - Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446-454.
doi pubmed - Yanai H. Favipiravir: a possible pharmaceutical treatment for COVID-19. J Endocrinol Metab. 2020;10(2):33-34.
doi - Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381-385.
doi pubmed - Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271.
doi pubmed - Das S, Bhowmick S, Tiwari S, Sen S. An updated systematic review of the therapeutic role of hydroxychloroquine in coronavirus disease-19 (COVID-19). Clin Drug Investig. 2020.
doi pubmed - Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943-947.
doi pubmed - Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020.
doi - Jang S, Rhee JY. Three cases of treatment with Nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int J Infect Dis. 2020.
doi pubmed - Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue JI, Matsuda Z. Identification of Nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60(11):6532-6539.
doi pubmed - Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280 e278.
doi pubmed - Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. 2020;31;1-4.
doi
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
