| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 9, Number 4, August 2019, pages 108-112
Food Neophobia in Patients With Phenylketonuria
Tassia Tonona, b, f, Chenia Martineza, b, Soraia Polonia, b, Tatiele Nalinc, Anita MacDonaldd, Ida Vanessa D. Schwartza, b, e
aGraduate Program in Medicine: Medical Sciences, Universidade Federal do Rio Grande do Sul, Brazil
bDepartment of Medical Genetics, Hospital de Clinicas de Porto Alegre, Brazil
cUltragenyx Brazil Pharmaceutical Ltda, Sao Paulo, Brazil
dDietetic Department, Birmingham Children’s Hospital, Birmingham, UK
eDepartment of Genetics, Universidade Federal do Rio Grande do Sul, Brazil
fCorresponding Author: Tassia Tonon, Graduate Program in Medicine: Medical Sciences, Universidade Federal do Rio Grande do Sul, Brazil
Manuscript submitted May 17, 2019, accepted May 28, 2019
Short title: Food Neophobia in Patients With PKU
doi: https://doi.org/10.14740/jem581
| Abstract | ▴Top |
Background: In phenylketonuria (PKU), only a limited range of natural foods, such as fruits, vegetables, oils and sugars, can be consumed without restrictions. The present study aimed to study if food neophobia (i.e. an unwillingness to eat novel food) occurs in patients with PKU and to compare in non-PKU healthy controls and to identify any associated factors.
Methods: This cross-sectional case-control study used a convenience sampling strategy to recruit patients diagnosed with PKU and healthy controls matched by gender and age. Patients and controls were invited to complete the food neophobia scale (FNS) questionnaire, and clinical and treatment data were collected through a review of medical records.
Results: Twenty-five patients (mean age 19.3 ± 4.7 years, 13 women) with PKU and 25 controls (mean age 19.9 ± 4.9 years; P = 0.676, 13 women) were evaluated. The mean age of treatment onset in patients with PKU was 52.8 ± 29.7 days. The mean phenylalanine (Phe) level in the 12-month preceding neophobia study was 710.5 ± 346 µmol/L. The mean FNS score of patients with PKU was significantly higher (47.2 ± 9.7) than controls (29.4 ± 12.5, P < 0.001). Food neophobia was associated more with male gender (P = 0.039).
Conclusions: Food neophobia is frequent in patients with PKU. It does not appear to be associated with female gender, with Phe levels and not just in children.
Keywords: Phenylketonuria; Food neophobia; Inborn errors of metabolism
| Introduction | ▴Top |
Phenylketonuria (PKU) is an inborn error of metabolism with an autosomal recessive inheritance pattern caused by the presence of mutations in the gene that codes for phenylalanine hydroxylase (PAH, EC 1.14.16.1). PAH catalyzes the conversion of phenylalanine (Phe) to tyrosine by means of a reaction dependent on tetrahydrobiopterin, iron and molecular oxygen [1]. In untreated patients, serum Phe concentrations are raised to neurotoxic levels, resulting in progressive, irreversible neurological impairment [2, 3]. The worldwide prevalence of PKU is estimated at 1 per 10,000 live births [4]. In Brazil, the most recent survey found a prevalence of 1 per 24,780 newborns [5].
The treatment consists of a Phe-restricted diet and suplementation with a Phe-free metabolic formula to ensure adequate intake of other amino acids and micronutrients. If a strict diet is started early (ideally before 10 days of life) and maintained carefully, normal development is expected [2]. Although nutritional management is effective, only a very limited range of natural foods can be offered without any restrictions; these include fruits, vegetables, fats and sugars [6]. In Brazil, medical foods with low Phe content are not provided by the public health system. Although they can be bought independently, their out-of-pocket cost is high and availability is limited, contributing to a lack of variety in the diet of PKU patients.
According to MacDonald and colleagues (1994), it is important to support the development of normal eating behavior in patients with PKU, within the limits of dietary management, so that all their nutritional needs are met [6]. Food neophobia, defined as an unwillingness to eat novel foods [7], may further limit the variety of foods and, consequently, nutrients ingested by patients with PKU [6]. In recent years, neophobic eating behavior in the general population has been investigated since it can affect dietary preferences, food quality and variety, thus increasing the risk of developing several chronic diseases [8].
However, to date, little is known about the relationship between the presence of food neophobia in patients with PKU and clinical and treatment-related variables, such as age, blood Phe control, anthropometry, lifestyle measures, breastfeeding in infancy and age of solid introduction of foods. Within this context, the present study aimed to compare the presence of food neophobia in patients with PKU and in non-PKU controls and to elucidate the factors possibly associated with this condition.
| Materials and Methods | ▴Top |
This was a cross-sectional, controlled study. A convenience sampling strategy was used to enroll patients with PKU followed up at the Outpatient Metabolic Disorders Clinic of the Medical Genetics Service at Hospital de Clinicas of Porto Alegre (HCPA), Brazil. The study protocol was approved by the local Research Ethics Committee. Seventy-five patients with PKU are followed by the HCPA clinic. The inclusion criteria were age ≥ 9 years (deemed as the minimum for understanding the applied questionnaire), and all patients on continuous dietary treatment with a low phenylalanine diet with Phe-free metabolic formula and absence of any intellectual impairment. Thirty patients met the inclusion criteria; of these, 25 agreed to participate in the study. The sample also included 25 healthy controls, matched by age and gender. Enrollment took place from 2016 to 2018.
Clinical and demographic information, such as date of birth, gender, mean age at diagnosis, mean age at introduction of solid foods and mean Phe levels at diagnosis and during the year prior to study inclusion were obtained by a chart review. PKU subtype was classified as mild or classical as described by Nalin and colleagues (2010) [9]. Good metabolic control was defined according to the European PKU guidelines [2]; for patients < 13 years of age, a mean Phe level < 360 µmol/L on at least three measurements obtained in the 12 months prior to study enrollment was considered satisfactory. For patients over 13 years of age, mean Phe levels < 600 µmol/L were required. Data on breastfeeding (in infancy) and smoking were collected through a specific questionnaire. No patients were on sapropterin or PEG-PAL.
Weight and height were used to calculate the body mass index (BMI), which was expressed as the z-score for age and sex; patients were then classified as underweight, normal weight, overweight, or obese, according to the World Health Organization criteria (2007) [10].
Food neophobia was evaluated through the food neophobia scale (FNS), a questionnaire first developed in English by Pliner and Hobden (1992) [7] and later translated into Portuguese and validated for use in Brazil by Previato (2015) [8]. The FNS consists of a 10-item self-report questionnaire. A high average score (obtained by adding the individual scores of each item on a Likert-type scale) indicates less willingness to try new foods (neophobia), while a low average score indicates greater willingness to try new foods (neophilia). Individuals with scores ≤ 16.4 were classified as neophilic, those with scores between 16.5 and 38.5 were considered neutral and those with scores > 38.6 were classified as neophobic [8].
Additionally, patients and controls were asked about their self-perception about the rejection of new foods through a specific question: “Do you reject new foods?”
Statistical analyses were carried out in PASW Statistics, version 18.0 (SPSS Inc., Chicago, IL). Descriptive analysis consisted of absolute frequencies. Continuous variables were expressed as mean and standard deviation. Student’s t-test and the Chi-square test for paired or independent samples were used. For the univariate analyses presented in the chart, Fisher’s exact test was used. The level of significance was set at 5%.
| Results | ▴Top |
Fifty participants (25 patients and 25 controls) were included in this study (Table 1). In the patient group, the mean Phe levels were 1360.3 ± 671.3 µmol/L at diagnosis and 710.5 ± 346.4 µmol/L in the 12 months prior to the study (range, 215.1 - 1,408.9). The mean age at treatment onset was 52.8 ± 29.7 days.
 Click to view | Table 1. Demographic and Clinical Characteristics of Patients With Phenylketonuria and Controls |
No patient was exclusively breastfed due to treatment, while 21 controls had exclusive breastfeeding until 6 months of age. No patients and controls report being smokers.
According to the FNS, 20 patients and seven controls were classified as neophobic (P = 0.001); five patients and 15 controls as neutral (P = 0.001); and three controls and no patients as neophilic (P < 0.001). The mean FNS score was 47.2 ± 9.7 for patients with PKU and 29.4 ± 12.5 for controls (P < 0.001).
Figure 1 shows the possible factors associated with food neophobia in patients with PKU. Female gender was the only factor significantly (P = 0.039) associated with neophobia (in this case, as a protective factor against the occurrence of food neophobia).
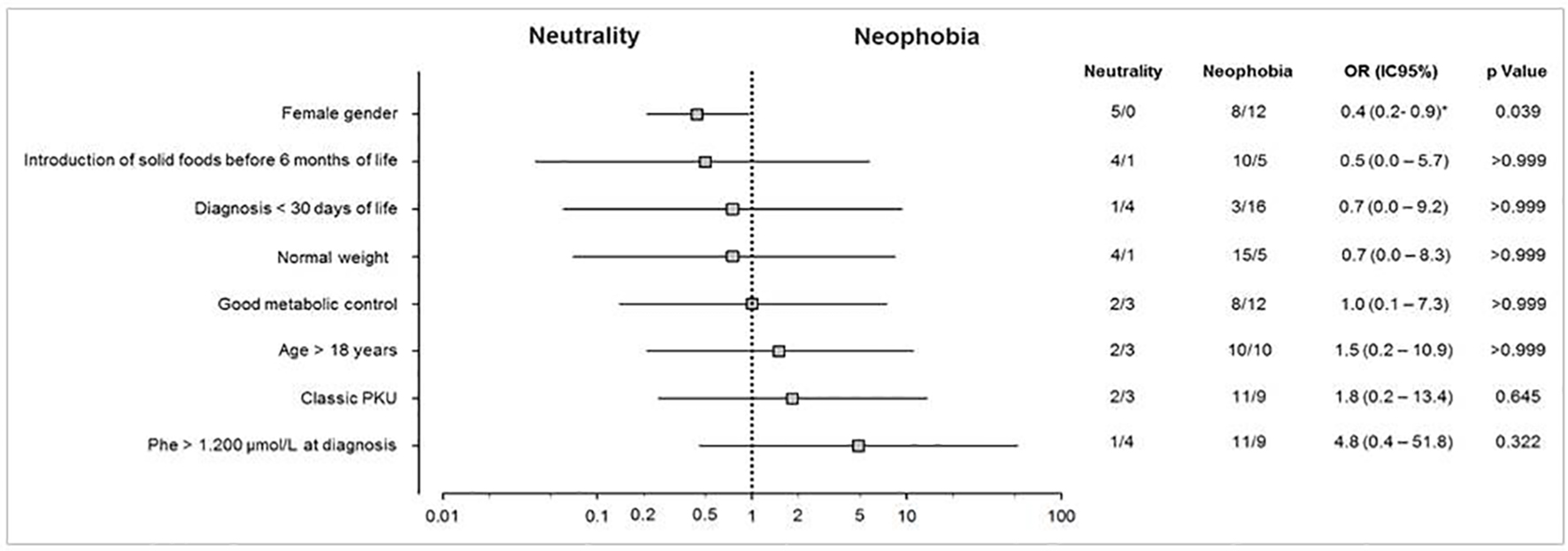 Click for large image | Figure 1. Potential factors associated with food neophobia in patients with PKU. Univariate analysis representing the association between patients with PKU and the factors involved with food neophobia. Odds ratios are represented by a log scale with their respective confidence intervals (95%) and statistical significance (P) obtained by Fisher’s exact test. *Values estimated by the likelihood ratio with Agresti correction. PKU: phenylketonuria. |
| Discussion | ▴Top |
In this study, a high prevalence of food neophobia was found among this older group of patients with PKU. Food neophobia does not appear to be associated with female gender, and is not associated with Phe metabolic control. According to Knaapila and colleagues (2007) [11], from an evolutionary point of view, food neophobia is a protective phenomenon, designed to prevent humans from eating foods that are potentially harmful to health. Thus, it is unsurprising that, in our sample, PKU patients were classified as neophobic, corroborating the findings of Evans and colleagues (2016) [12], and showing that not only children with PKU have food neophobia, but that this condition remains even in adult life.
In the past, these restrictions were recommended to PKU patients only until early adulthood, which allowed a greater variety in their choice of foods after the treatment period. Over the years, however, guidelines have been updated, and currently recommend “diet for life” [2]. In the present study, food neophobia was not only a characteristic of children, as described by Evans and colleagues (2016) [12]; it persisted even into adulthood.
Theoretically, because it allows intake of fruits and most vegetables (which do not affect metabolic control), the PKU diet is considered a healthy one. However, it is among the most restrictive of all known nutritional therapies. Given the central role of food in the life of a developing child, extremely strict dietary therapies can adversely affect the eating behaviors and nutritional status of these patients in long term [13].
Few studies have addressed this issue, but patients with PKU are known to be apprehensive when trying new foods, limiting themselves to the intake of few foods that are within their dietary restrictions. The study by Evans and colleagues (2016) [12] evaluated 35 children with PKU (age 4 - 13 years) through a parental-report questionnaire about dietary habits. Our study is novel in so far as patients were evaluated directly (they completed their own assessments), and was the first, to our knowledge, to investigate the potential association between presence of food neophobia in patients with PKU and metabolic control, as well as other variables that can influence food choices in this population. In our study, diagnosis was late in the majority of patients (mean age of treatment onset was 52.8 ± 29.7 days), unlike in the population assessed by Evans and colleagues (2016) [12], which was diagnosed by neonatal screening.
Some determinants of food neophobia
Gender and age
Hursti and Sjooden (1997) [14] reported higher rates of food neophobia in healthy boys when compared to girls (n = 722, age 7 - 17 years). Greater food neophobia was also observed in the children’s fathers as opposed to their mothers. In a recent German study which evaluated adolescents, food phobia levels did not differ between boys and girls [15], but the authors noted that food phobia levels between the sexes were age-dependent. The literature shows that, in childhood and adulthood, men appear to be more neophobic, whereas Cooke and Wardle (2005) [16], who evaluated 1,291 children aged 4 - 16 years, believe that in adolescence body weight and physical appearance are more relevant topics for girls, making them more neophobic than boys. The present study is consistent with the literature, evincing a higher level of food neophobia in boys than in girls.
Phe levels
This was the first study to associate Phe levels with food neophobia in patients with PKU. Good metabolic control, that is having Phe levels within reference range, was not a determinant of greater rejection of novel foods. Regarding the Phe level at diagnosis, we were unable to verify any significant influence of Phe > 1,200 µmol/L on later food neophobia; however, the sample size may have limited the power of this analysis.
Breastfeeding and diet
Another potential determinant of food neophobia is the duration of the breastfeeding period. Babies who are breastfed for longer discover new flavors as early in their first days of life, as flavors from the mother’s diet are conveyed to them via the breast milk [17]. Children who were breastfed for at least 6 months demonstrated lower levels of food neophobia compared to children with shorter breastfeeding times [18]. In addition, exclusive breastfeeding may be associated with reduced food neophobia, as early introduction of food may lead to increased gastrointestinal discomfort and food allergies [18].
The World Health Organization and the Brazilian Ministry of Health recommend exclusive breastfeeding for 6 months, with complementary feeding for 2 years or longer [19, 20]. However, in patients with PKU, the Phe-free metabolic formula should ideally be introduced as early as the first month of life, substantially reducing their breast milk intake after diagnosis and thereby modifying their taste perceptions into childhood. The Phe-restricted diet for PKU patients is rife with obstacles, such as limited food variety, the unpalatability of the metabolic formula (which tastes strong and bitter) and the rigid dietary management routine. MacDonald and colleagues (1997) [13] associated the food neophobia found in children with PKU to factors such as an innate fear of eating foods they consider “unsafe”, limited food choices, palatability issues and a lack of appetite due to use of the metabolic formula.
We believe that use of the metabolic formula, the significant, early reduction of breast milk intake and the limited choice of foods are all factors that trigger food neophobia in patients with PKU. In Brazil, these issues are compounded by the fact that medical foods with low Phe content are not provided by the public health system. Although they can be bought independently, their out-of-pocket cost is high and availability is limited, contributing to a lack of variety in the diet of PKU patients. Furthermore, problems related to the metabolic formula are frequent, such as shortages of government supply and the inability to choose more palatable formulations, making treatment even more difficult.
Food neophobia may be an issue when new treatments will be available to Brazilian patients with PKU, such as PEG-PAL and sapropterin, designed to improve protein intake, so should be addressed as early as possible in life.
Conclusions
Long-term good metabolic control, that is, having Phe levels within target range, without impairing dietary behavior is a challenge for patients with PKU, their families and healthcare providers. We believe that the introduction of adjuvant therapies could contribute to better management of Phe levels in patients, allowing greater exposure to novel foods. The importance of developing normal eating behavior within the constraints of a Phe-restricted diet, encouraging intake of a wide range of flavors to maximize healthy food choices and to promote better social interaction, cannot be underestimated. Education strategies involving programs to promote safe and healthy diets and dietary practices should be encouraged at facilities that serve PKU patients and their families.
Acknowledgments
We are grateful to the Graduate Program in Medicine: Medical Sciences at the School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS); the patients at our Center for Diagnosis, Monitoring, and Treatment of Patients with Phenylketonuria in Rio Grande do Sul, Brazil; and the patients at our Center in HCPA.
Financial Disclosure
Financial support was from the Hospital de Clinicas de Porto Alegre Research and Event Incentive Fund (FIPE/HCPA).
Conflict of Interest
All authors declare "no conflicts of interest".
Informed Consent
All the research participants (patients and controls) signed the inform consent form according to the approved project under number 2015-0072 from our Institutional Review Board HCPA.
Author Contributions
Tassia Tonon: study design; data collection and interpretation; manuscript writing. Chenia Martinez: study conception; data interpretation; critical appraisal of manuscript content; final approval. Soraia Poloni: study conception; data interpretation; critical appraisal of manuscript content; final approval. Tatiele Nalin: study conception; data interpretation; critical appraisal of manuscript content; final approval. Anita MacDonald: study conception; data interpretation; critical appraisal of manuscript content; final approval. Ida Vanessa Doederlein Schwartz: study conception; data interpretation; critical appraisal of manuscript content; final approval.
| References | ▴Top |
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376(9750):1417-1427.
doi - van Wegberg AMJ, MacDonald A, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, Burlina A, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12(1):162.
doi pubmed - de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86-89.
doi pubmed - Williams RA, Mamotte CD, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29(1):31-41.
- Carvalho TM. Results of the epidemiological survey of the Brazilian society of neonatal screening (SBSTN). Rev Med Minas Gerais. 2003;13(1 Suppl 2):S109-S135.
- MacDonald A, Rylance GW, Asplin DA, Hall K, Harris G, Booth IW. Feeding problems in young PKU children. Acta Paediatr Suppl. 1994;407:73-74.
doi pubmed - Pliner P, Hobden K. Development of a scale to measure the trait of food neophobia in humans. Appetite. 1992;19(2):105-120.
doi - Ribeiro de Andrade Previato HD, Herman Behrens J. Translation and Validation of the Food Neophobia Scale (Fns) to the Brazilian Portuguese. Nutr Hosp. 2015;32(2):925-930.
- Nalin T, Perry IDS, Refosco LF, et al. Phenylketonuria in the public health system: assessment of adherence to treatment in a medical care center in Rio Grande do Sul. Rev HCPA. 2010;30:225-232.
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660-667.
doi pubmed - Knaapila A, Tuorila H, Silventoinen K, Keskitalo K, Kallela M, Wessman M, Peltonen L, et al. Food neophobia shows heritable variation in humans. Physiol Behav. 2007;91(5):573-578.
doi pubmed - Evans S, Daly A, Chahal S, MacDonald J, MacDonald A. Food acceptance and neophobia in children with phenylketonuria: a prospective controlled study. J Hum Nutr Diet. 2016;29(4):427-433.
doi pubmed - MacDonald A, Harris G, Rylance G, et al. Abnormal feeding behaviours in phenylketonuria. J Hum Nutr Diet. 1997;10:163-170.
doi - Hursti Uk K, Sjoden P. Food and general neophobia and their relationship with self-reported food choice: familial resemblance in Swedish families with children of ages 7-17 years. Appetite. 1997;29(1):89-103.
doi - Rossbach S, Foterek K, Schmidt I, Hilbig A, Alexy U. Food neophobia in German adolescents: Determinants and association with dietary habits. Appetite. 2016;101:184-191.
doi pubmed - Cooke LJ, Wardle J. Age and gender differences in children's food preferences. Br J Nutr. 2005;93(5):741-746.
doi - Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr. 2009;90(3):780S-788S.
doi pubmed - Shim JE, Kim J, Mathai RA, STRONG Kids Research Team. Associations of infant feeding practices and picky eating behaviors of preschool children. J Am Diet Assoc. 2011;111(9):1363-1368.
doi pubmed - Brasil. Ministerio da Saude. Secretaria de Assistencia a Saude. Coordenacao-Geral de Atencao Especializada. Manual de Normas Tecnicas e Rotinas Operacionais do Programa Nacional de Triagem Neonatal / Ministerio da Saude, Secretaria de Assistencia a Saude, Coordenacao-Geral de Atencao Especializada. Brasilia: Ministerio da Saude, 2002.
- World Health Organization. The optimal duration of exclusive breastfeeding. Report of an Expert Consultation. 2001. Geneva, Switzerland.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
