| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 7, Number 5, October 2017, pages 141-145
Obstructive Sleep Apnea Syndrome Is Less Frequent in Patients With Well-Controlled Acromegaly Treated With Somatostatin Analogues, Pegvisomant or in Combination
Burkhard L. Herrmanna, f, Guenter K. Stallab, Katharina Laubnerc, Martin Bidlingmaierd, Dagmar Fuehrer-Sakele
aDivision of Endocrinology, Diabetology and Laboratory Research, Bochum, Germany
bClinical Neuroendocrinology Group, Max Planck Institute of Psychiatry, Munich, Germany
cDivision of Endocrinology and Diabetology, University of Freiburg, Germany
dEndocrine Laboratory, Medical Clinic IV, University of Munich, Germany
eDepartment of Endocrinology and Metabolism, University of Duisburg-Essen, Essen, Germany
fCorresponding Author: Burkhard L. Herrmann, Division of Endocrinology, Diabetology and Laboratory Research, Springorumallee 2, D-44795 Bochum, Germany
Manuscript submitted October 3, 2017, accepted October 12, 2017
Short title: Obstructive Sleep Apnea Syndrome
doi: https://doi.org/10.14740/jem455w
| Abstract | ▴Top |
Background: Obstructive sleep apnea (OSA) often occurs in patients with active acromegaly and improves after treatment. Less is known about the development of OSA in patients after a longer period of control treated with somatostatin analogues (SSA) and pegvisomant.
Methods: Seventy-nine patients (12 females, 17 males; age 49 ± 14 years; body mass index 29.9 ± 5.4 kg/m2; IGF-1 184 ± 73 µg/L; disease duration 13 ± 8 years (mean ± standard deviation)) with well-controlled acromegaly treated with SSA (38%), pegvisomant (38%) or in combination (24%) who underwent ambulatory polygraphy were included in a prospective multicenter cross-sectional study.
Results: Fourteen percent had OSA (range of apnea-hypopnea index (AHI) 5 - 15). Patients with OSA (AHI ≥ 5 vs. < 5) had a longer disease duration (16 ± 1 vs. 12 ± 8 years; P = 0.01) and were older (61 ± 9 vs. 47 ± 13 years; P = 0.037). The AHI of all patients correlated with age (P = 0.01; r = 0.44). No differences were seen in terms of BMI and Epworth sleepiness scale score. Previous transsphenoidal surgery and radiation had no impact of the detection of OSA. The duration of well-controlled acromegaly was 7 ± 3 years.
Conclusion: OSA in patients with well-controlled acromegaly treated with SSA, pegvisomant or in combination is less frequent (14%) than previously described. Early treatment to reduce the active disease period should be aimed to prevent OSA.
Keywords: Acromegaly; Sleep apnea syndrome; Pegvisomant
| Introduction | ▴Top |
Obstructive sleep apnea (OSA) is one of several systemic complications in patients with acromegaly [1]. Soft tissue swelling of the uvula, enlargement of the tongue and prognathism due to the mandibular growth are clinical manifestations of the period of active disease and characterize the OSA which is most prevalent in acromegaly [2-5]. Central apnea is infrequent and is associated with high endogenous somatostatin tonus [6, 7].
It has been shown that 50-87% of patients with active disease have OSA [2]. After sufficient treatment with somatostatin analogues (SSA), pegvisomant or successful resection of the GH-producing adenoma after transsphenoidal surgery, parameters and prevalence of OSA significantly reduced [3, 8].
Beside the fact of high prevalence of OSA in patients with acromegaly, OSA is diagnosed in up to 2-5% of the general population and hence not a rare disease [9]. In this group, OSA is associated with age, body mass index (BMI) and hypertension. Less is known about the prevalence of sleep apnea syndrome in patients with well-controlled acromegaly after a longer period of time. It was mentioned in previous papers that a longer follow-up to detect later potential improvements in OSA will be needed [10, 11]. Therefore, we initiated a multicenter study to evaluate clinical manifestations via Epworth sleepiness scale score and ambulatory polygraphy in patients with well-controlled acromegaly treated with SSA, pegvisomant or in combination.
| Patients and Methods | ▴Top |
Patients
Twenty-nine patients (12 females and 17 males) with a mean age of 49 ± 14 years (range 25 - 73 years) and BMI of 29.9 ± 5.4 kg/m2 (range 20.8 - 29.9 kg/m2) with well-controlled acromegaly (IGF-I 184 ± 73 µg/L (range 74 - 383 µg/L); SSA (38%), pegvisomant (38%) or in combination (24%)) were included in the cross-sectional prospective multicenter study of eight Departments of Endocrinology in Germany. Eleven patients were treated with pegvisomant (10/11 10 - 40 mg daily, 1/11 with 30 mg weekly), six patients with octreotide (10 - 30 mg every 4 weeks), five patients with lanreotide (60 - 120 mg every 4 - 8 weeks), four patients with pegvisomant (10 - 30 mg daily) in combination with lanreotide (60 - 120 mg every 4 - 8 weeks) and three patients with pegvisomant (20 - 30 mg daily) in combination with octreotide (30 mg every 4 weeks). Thirty-four percent of patients had hypertension, 3% diabetes mellitus and 14% both. Twenty-four percent had ACTH deficiency, 41% TSH deficiency, and 7% diabetes insipidus (Table 1). Seventy-six percent underwent previous surgery and 24% surgery and radiation.
 Click to view | Table 1. Patients With Well-Controlled Acromegaly Treated With Somatostatin Analogues, Pegvisomant or in Combination Before Examination of Polygraphy |
The disease duration was estimated from the lag time between the onset of symptoms and signs of disease and the date when the treatment was proven to be effective (well-controlled patients). Moreover, the disease duration was assumed to be the interval between the clinical onset determined by comparison of old photographs and the time of treatment. The duration of well-controlled acromegaly was 7 ± 3 years (range 2 - 11 years). Daytime sleepiness was assessed by the Epworth sleepiness questionnaire.
Ambulatory polygraphy
Ambulatory polygraphy using the Somno-Check (Weinmann Medical Technology, GmbH, Hamburg, Germany) was performed between 10 pm and 7 am. Oronasal airflow was recorded by thermistor; thoracic and abdominal respiratory efforts were measured by impedance plethysmography. Oxygen saturation was measured by finger pulse oximetry (ResMed Model 305A, San Diego, CA, USA). Body position was monitored by a position sensor. Patients had been instructed to behave during the night as “normally” as possible.
Apnea was defined as cessation of airflow or reduction in thermistor signal to less than 10% of the normal flow and lasting for at least 10 s. Apneas shorter than 10 s were counted if they were followed by an oxygen desaturation of 4% or more. Events were classified as obstructive (clear obstructive or mixed with a clear obstructive component in the event) according to the respiratory effort channels. Hyponea was defined as a discernible reduction in airflow of at least 10 s duration followed by a desaturation of 4% or more.
The respiratory events were scored in accordance to the American Academy of Sleep Medicine Task Force recommendations [12]. The apnea-hypopnea index (AHI) was calculated as the number of all respiratory events per hour of sleep. An AHI shorter than 5 was defined as normal. Sleep-related breathing events were considered mild when AHI was between 5 and 15 events per hour, moderate in cases of AHI between 15 and 30 events per hour and severe if AHI was greater than 30 events per hour. Clear oxygen saturation (SaO2) artifacts were excluded manually. Oxygen indices were then calculated by the software from the SaO2 curve with minimal SaO2 being the lowest saturation reached during sleep and with average minimal SaO2 being the mean of all saturation values reached during all respiratory events.
Determination of serum IGF-I concentrations
IGF-I concentrations were measured centrally using the iSYS automated chemiluminescent IGF-I assay (Immunodiagnostic Systems (IDS) Ltd, Boldon, England, UK). The assay employs two monoclonal antibodies and is calibrated against the WHO International Standard 02/254 (National Institute for Biological Standards (NIBSC), Hertfordshire, UK). On board acidification of samples to separate IGF-I from its binding protein IGFBP 3 is followed by incubation in the presence of excess IGF-II to prevent re-aggregation. No interference or cross-reactivity was observed with IGF-II, insulin, proinsulin and the six high affinity IGFBPs. In our hands, intra-assay CVs were between 1.1% and 2.0% at concentrations between 87 and 733 ng/mL, whereas inter-assay CVs ranged from 2.7% to 10.0% at concentrations between 68 and 490 ng/mL. The lower limit of quantification was 10 ng/mL, and the linear range was 10 - 1,200 ng/mL. Up to five freeze/thaw cycles had no detectable influence on measured concentrations. Results of the participants were compared to extensive method specific reference intervals stratified for age and sex [13].
Statistical analyses
The data, if not marked otherwise, represent the mean ± standard deviation. Differences between two groups were tested by Mann-Whitney U-test as a non-parametric procedure. All tests were done two-tailed, and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad InStat version 3.02 (GraphPad Software, San Diego, CA, USA).
Ethics
The trial was conducted in accordance with the Declaration of Helsinki for biomedical research involving human subjects. The study protocol has to be approved by the ethics committee of the medical association of Westfalia-Lippe, Muenster, Germany.
| Results | ▴Top |
Fourteen percent (4/29) had a mild OSA (range of AHI 5 - 15). One patient had an AHI of 5, two 7 and one 15. Patients with an AHI ≥ 5 had a longer disease duration than patients with AHI < 5 (16 ± 1 vs. 12 ± 8 years, P = 0.01, Fig. 1). Age, BMI and the score of the Epworth sleepiness scale were similar in both groups (Table 2). The mean score of the Epworth sleepiness scale of all patients was 5.4 ± 3.5. Age correlated with AHI (P = 0.01, r = 0.44, Fig. 2), but BMI did not correlate with AHI. Previous and current treatment of the two groups did not differ significantly (Table 3). The prevalence values of hypertension and diabetes mellitus were similar in both groups (2/4 patients in group AHI ≥ 5: one hypertension and one hypertension in combination with diabetes mellitus; 13/25 patients in the group AHI < 5: nine hypertension, one diabetes mellitus and three hypertension in combination with diabetes mellitus).
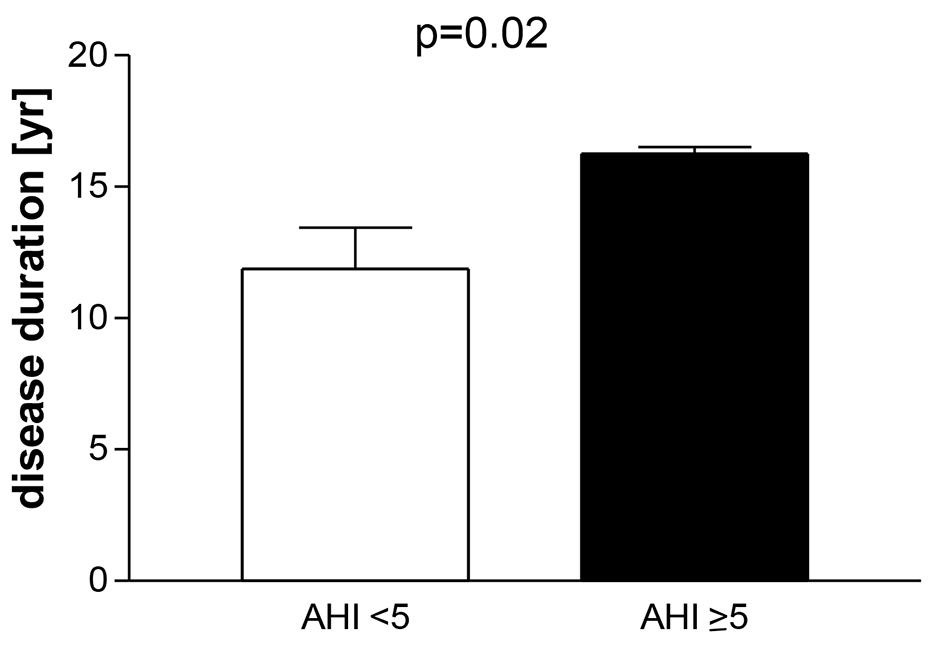 Click for large image | Figure 1. Patients with well-controlled acromegaly treated with somatostatin analogues, pegvisomant or in combination after examination of polygraphy. Division in a group apnea-hypnea index (AHI) < 5 and AHI ≥ 5: mean ± SEM. |
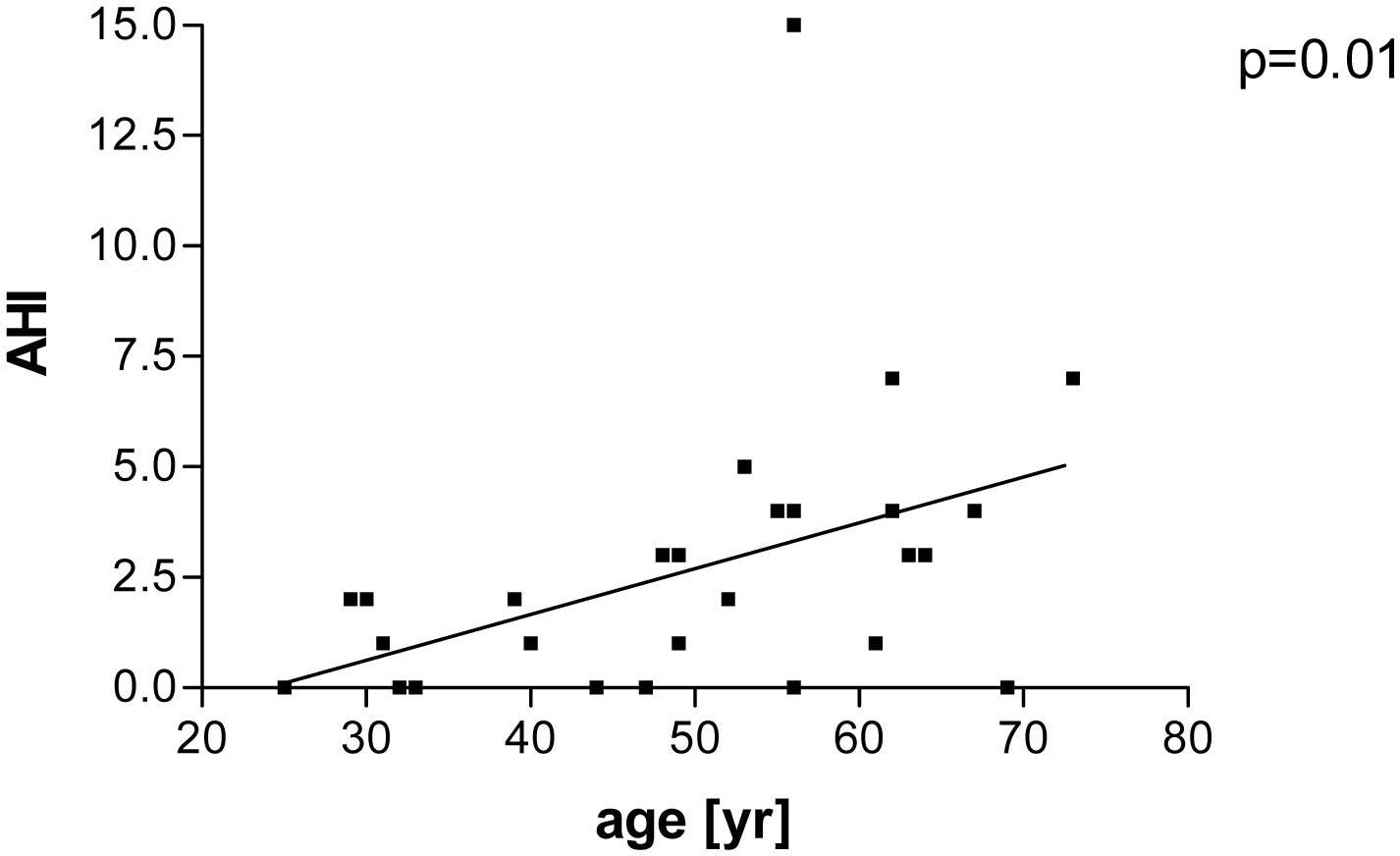 Click for large image | Figure 2. Patients with well-controlled acromegaly treated with somatostatin analogues, pegvisomant or in combination after examination of polygraphy. Correlation between apne-hypnea index (AHI) and age (P = 0.01; r = 0.44). |
 Click to view | Table 2. Patients With Well-Controlled Acromegaly Treated With Somatostatin Analogues, Pegvisomant or in Combination After Examination of Polygraphy |
 Click to view | Table 3. Treatment of Patients With Well-Controlled After Examination of Polygraphy |
| Discussion | ▴Top |
In the present study, it has been shown that patients with well-controlled acromegaly treated with SSA, pegvisomant or in combination had a low frequency (14%) of OSA.
In patients with active acromegaly, prevalence of sleep apnea varies between 50% and 87% [2]. This high range may be due to the different age, BMI and disease duration of the cohorts [14]. Moreover, it has to be considered that sleep apnea can be examined with different procedures: ambulatory polygraphy versus inpatient polysomnography. Furthermore, high prevalence of sleep apnea can be influenced by preselectional bias, if e.g. symptoms and signs of sleep apnea (high Epworth sleepiness scale) are preconditions for polysomnography.
Successful treatments by surgery and with SSA, pegvisomant or in combination improved sleep apneas syndrome [3, 8, 11, 15]. It has been shown that treatment with octreotide can reduce sleep apnea indices (respiratory disturbance index (RDI)) and tongue volume [3, 16]. The same observations could be documented with pegvisomant [8]. The effect of treatment with SSA, pegvisomant and dopaminergic agonists is the result of IGF-I and/or GH-normalization with the consequence of reduced soft tissue swelling of the uvula and the tongue volume, which has a major impact of OSA [17]. The direct effect of these drugs is less relevant. The study from van Haute et al could document that a high prevalence OSA (88%) remained in patients with active acromegaly despite previous treatment with dopaminergic agonist, as well as after surgery and radiotherapy [14].
In a small cohort of 16 patients from Chemla et al, 11 patients were well controlled with SSA (n = 2), pegvisomant (n = 9) or surgery (n = 5) after a period of 11 months. Seven of 16 had SAS, and in 4/7, sleep apnea syndrome improved [15]. Recently, in a retrospective study, 12 patients with OSA were evaluated by polysomnography after a period of 16 months with pegvisomant. Seventy-six percent were pre-treated by surgery or additional radiation (17%). OSA improved in 6/8 patients and disappeared in 4/12 [18].
These and other previous studies examined a very heterogenous cohort with small numbers and a relative short period of well-controlled acromegaly. It was mentioned in previous papers that a longer follow-up to detect later possible improvements in OSA will be needed [10, 11].
For that reason, we performed a cross-sectional multicenter study to examine patients with acromegaly, well-controlled with SSA and/or pegvisomant over a mean period of 7 years. Only 14% had OSA, which was in most of the cases mild to moderate. The low Epworth sleepiness score of 5 confirmed this low prevalence. OSA correlated with age, but not with BMI. This low prevalence is less frequent as in the study of Annamalai et al (2013) [10]. They documented a prevalence of 39% (9/23) after a period of 24 months treatment with lanreotide autogel and well-controlled acromegaly. This higher prevalence may be due to a shorter treatment period of 2 versus 7 years in our study. Moreover, they have included newly diagnosed patients with acromegaly in contrast to our group in the present study, who was pre-treated with surgery (76%) or additional radiation (24%). Another reason for the low prevalence may be due to the high percentage of patients treated with pegvisomant alone or in addition to SSA (59%).
OSA is a disease of the middle age in non-acromegalic population and correlates with age. This fact is similar to our observation that the AHI correlated with the age of the patients with acromegaly, independent of IGF-I levels. Concerning the prevalence of OSA, it may be speculative, whether acromegalics with well-controlled disease assimilate to non-acromegalic over time.
In the present study, patients with OSA had a longer disease duration than patients without OSA. This observation is similar to other systemic complications in acromegaly, such as cardiomyopathy, arrhythmias and cranio-facial alterations [1, 19, 20]. The untreated GH and IGF-I excess induces prognathism, increases tongue volume and enlarges uvula which narrow the upper airway and hence generate signs and symptoms of OSA [5, 21, 22].
Therefore, early detection of acromegaly to reduce the active disease period is the aim to prevent patients with acromegaly of systemic complications such as OSA [23]. Due to the amelioration of treatment by surgery, radiation and sufficient use of SSA and pegvisomant, the prevalence of OSA in patients with well-controlled acromegaly is less frequent.
Acknowledgments
We especially thank our colleagues of the Study Group: C. Berg (EvK Mettmann, Germany), R. Finke (Endocrinology, Medical Practice, Berlin, Germany), H. Etzrodt (Endocrinology, Medical Practice, Ulm, Germany), B. Quadbeck (Endocrinology, Medical Practice, Duesseldorf, Germany), and M. Droste (Endocrinology, Medical Practice, Oldenburg, Germany).
Grant Support
The study was supported by grants from Pfizer GmbH, Germany. We thank Matthias Heinze, Thomas Wittig and Detlev Tippner.
Conflict of Interest
The authors fully declare any financial or other potential conflict of interest.
| References | ▴Top |
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102-152.
doi pubmed - Attal P, Chanson P. Endocrine aspects of obstructive sleep apnea. J Clin Endocrinol Metab. 2010;95(2):483-495.
doi pubmed - Herrmann BL, Wessendorf TE, Ajaj W, Kahlke S, Teschler H, Mann K. Effects of octreotide on sleep apnoea and tongue volume (magnetic resonance imaging) in patients with acromegaly. Eur J Endocrinol. 2004;151(3):309-315.
doi pubmed - Ajaj W, Goyen M, Herrmann B, Massing S, Goehde S, Lauenstein T, Ruehm SG. Measuring tongue volumes and visualizing the chewing and swallowing process using real-time TrueFISP imaging--initial clinical experience in healthy volunteers and patients with acromegaly. Eur Radiol. 2005;15(5):913-918.
doi pubmed - Dostalova S, Sonka K, Smahel Z, Weiss V, Marek J, Horinek D. Craniofacial abnormalities and their relevance for sleep apnoea syndrome aetiopathogenesis in acromegaly. Eur J Endocrinol. 2001;144(5):491-497.
doi pubmed - Grunstein RR, Ho KY, Berthon-Jones M, Stewart D, Sullivan CE. Central sleep apnea is associated with increased ventilatory response to carbon dioxide and hypersecretion of growth hormone in patients with acromegaly. Am J Respir Crit Care Med. 1994;150(2):496-502.
doi pubmed - Hernandez-Gordillo D, Ortega-Gomez Mdel R, Galicia-Polo L, Castorena-Maldonado A, Vergara-Lopez A, Guillen-Gonzalez MA, Torre-Bouscoulet L. Sleep apnea in patients with acromegaly. Frequency, characterization and positive pressure titration. Open Respir Med J. 2012;6:28-33.
doi pubmed - Berg C, Wessendorf TE, Mortsch F, Forsting M, Teschler H, Weischer T, Mann K, et al. Influence of disease control with pegvisomant on sleep apnoea and tongue volume in patients with active acromegaly. Eur J Endocrinol. 2009;161(6):829-835.
doi pubmed - Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479-482.
doi pubmed - Annamalai AK, Webb A, Kandasamy N, Elkhawad M, Moir S, Khan F, Maki-Petaja K, et al. A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J Clin Endocrinol Metab. 2013;98(3):1040-1050.
doi pubmed - Sze L, Schmid C, Bloch KE, Bernays R, Brandle M. Effect of transsphenoidal surgery on sleep apnoea in acromegaly. Eur J Endocrinol. 2007;156(3):321-329.
doi pubmed - Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American academy of sleep medicine task force. Sleep. 1999;22(5):667-689.
doi pubmed - Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Korner A, et al. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712-1721.
doi pubmed - van Haute FR, Taboada GF, Correa LL, Lima GA, Fontes R, Riello AP, Dominici M, et al. Prevalence of sleep apnea and metabolic abnormalities in patients with acromegaly and analysis of cephalometric parameters by magnetic resonance imaging. Eur J Endocrinol. 2008;158(4):459-465.
doi pubmed - Chemla D, Attal P, Maione L, Veyer AS, Mroue G, Baud D, Salenave S, et al. Impact of successful treatment of acromegaly on overnight heart rate variability and sleep apnea. J Clin Endocrinol Metab. 2014;99(8):2925-2931.
doi pubmed - Akkoyunlu ME, Ilhan MM, Bayram M, Tasan E, Yakar F, Ozcelik HK, Karakose F, et al. Does hormonal control obviate positive airway pressure therapy in acromegaly with sleep-disordered breathing? Respir Med. 2013;107(11):1803-1809.
doi pubmed - Davi MV, Dalle Carbonare L, Giustina A, Ferrari M, Frigo A, Lo Cascio V, Francia G. Sleep apnoea syndrome is highly prevalent in acromegaly and only partially reversible after biochemical control of the disease. Eur J Endocrinol. 2008;159(5):533-540.
doi pubmed - Kuhn E, Maione L, Bouchachi A, Roziere M, Salenave S, Brailly-Tabard S, Young J, et al. Long-term effects of pegvisomant on comorbidities in patients with acromegaly: a retrospective single-center study. Eur J Endocrinol. 2015;173(5):693-702.
doi pubmed - Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, Strasburger CJ, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary. 2013;16(3):294-302.
doi pubmed - Herrmann BL, Bruch C, Saller B, Bartel T, Ferdin S, Erbel R, Mann K. Acromegaly: evidence for a direct relation between disease activity and cardiac dysfunction in patients without ventricular hypertrophy. Clin Endocrinol (Oxf). 2002;56(5):595-602.
doi - Herrmann BL, Mortsch F, Berg C, Weischer T, Mohr C, Mann K. Acromegaly: a cross-sectional analysis of the oral and maxillofacial pathologies. Exp Clin Endocrinol Diabetes. 2011;119(1):9-14.
doi pubmed - Kashine S, Kishida K, Funahashi T, Shimomura I. Characteristics of sleep-disordered breathing in Japanese patients with acromegaly. Endocr J. 2012;59(1):31-38.
doi pubmed - Powlson AS, Gurnell M. Cardiovascular disease and sleep-disordered breathing in acromegaly. Neuroendocrinology. 2016;103(1):75-85.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
