| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 7, Number 1, February 2017, pages 5-17
Modified Fine-Needle Aspiration Biopsy for Calcitonin, Procalcitonin and Carcinoembryonic Antigen Levels in the Diagnosis of Thyroid Nodules With Medullary Thyroid Carcinoma
Mohamed K. M. Shakira, b, Thanh D. Hoanga, Diane U. Elegino-Steffensa, Vinh Q. Maia, Patrick W. Clydea
aDepartment of Endocrinology, Walter Reed National Military Medical Center, Bethesda, MD 20889-5600, USA
bCorresponding Author: K.M. Mohamed Shakir, Department of Endocrinology, Walter Reed National Military Medical Center, Bethesda, MD 20889-5600, USA
Manuscript accepted for publication November 28, 2016
Short title: MFNB in Thyroid Nodules With MTC
doi: https://doi.org/10.14740/jem371w
| Abstract | ▴Top |
Background: The diagnostic tests have low sensitivities in detecting medullary thyroid cancer (MTC). We describe a modified fine-needle thyroid nodule biopsy (MFNB) technique that improved the diagnostic accuracy. MFNB was performed in three patients with MTC.
Methods and results: A 41-year-old female presented with thyroid nodules and a family history of MTC. Fine-needle biopsy (FNB) of the thyroid nodules showed non-confirmatory cytology. MFNB established cytology more confirmatory of MTC. Additionally, intravenous administration of calcium-stimulated serum concentrations of procalcitonin (PCt) level, in addition to calcitonin (Ct) and MFNB, also confirmed elevated levels of Ct, PCt, and carcinoembryonic antigen (CEA) in the aspirate samples. The diagnosis of MTC was finally confirmed histologically after thyroidectomy. A 54-year-old female presented to our clinic for evaluation of a right thyroid nodule. An MFNB compared to standard FNB showed a more confirmatory MTC cytology along with markedly elevated levels of Ct, PCt, and CEA in the aspirated samples by the MFNB technique. Additionally, intravenous calcium administration increased PCt serum concentrations. The diagnosis of MTC was confirmed after surgery. A 65-year-old female reported to our clinic with a diagnosis of multinodular goiter and a family history of thyroid cancer. FNB showed atypical cells, although MFNB was more confirmatory for diagnosing MTC. Elevated serum PCt levels were also seen with calcium stimulation and MFNB aspirate samples also confirmed elevated levels of Ct, PCt, and CEA. MFNB of nodules in three patients with surgically confirmed benign thyroid confirmed low levels of Ct, PCt and CEA levels in the aspirate samples. Similar findings were confirmed in a patient with papillary thyroid cancer.
Conclusion: MFNB was more confirmatory for diagnosing MTC than conventional FNB. Determining Ct, PCt, and CEA levels in MFNB aspirate samples may improve the diagnostic accuracy.
Keywords: Fine-needle biopsy; Calcitonin; Procalcitonin; Carcinoembryonic antigen; Thyroid nodules; Medullary thyroid carcinoma; Calcium stimulation
| Introduction | ▴Top |
Medullary thyroid cancer (MTC) originates from parafollicular C cells and accounts for 3-5% of all thyroid malignancies. The majority of cases are sporadic, but 20-25% of patients have the hereditary form. The hereditary form of MTC is usually transmitted via an autosomal dominant inheritance pattern involving the RET proto-oncogene. Early detection and treatment of MTC helps to prevent disease progression and increase the chance of cure. However, the available diagnostic tests are limited due to their low sensitivities and specificities. Elevated basal serum calcitonin (Ct), a marker of MTC, can lead to a consistent number of false positive results [1-9]. Minimally, or even moderately, elevated serum concentrations of Ct may not distinguish MTC from other benign conditions [1-9]. Fine-needle biopsy (FNB) of thyroid nodules is commonly used for malignancy evaluation [9-18]. The majority of previously published reports indicate that FNB has relatively low sensitivity [5, 7, 9, 13-17]. However, some studies have demonstrated a good sensitivity for diagnosing MTC [11, 18] in a patient with moderately elevated serum Ct levels and thyroid nodule. However, mild elevations in serum Ct may be related to a benign disorder [1-10], and several additional options are available to confirm the diagnosis of MTC in these cases, such as FNB of the thyroid nodule, a calcium-pentagastrin stimulation test, a calcium stimulation test and an RET proto-oncogene test [1-23]. Although elevated basal serum Ct level has been used in the preoperative diagnosis of MTC, it is well established that mildly or moderately elevated serum Ct levels may be seen in disorders other than MTC [1-10].
While a study by Mian et al [22] demonstrated preoperative basal serum concentrations of Ct of > 68 pg/mL in males and > 26 pg/mL in females were able to separate non-MTC from MTC cases, it is generally accepted by other investigators that only markedly elevated serum Ct level has high specificity. Although the calcium-pentagastrin stimulation test has a high sensitivity in the diagnosis of MTC, the limited availability of pentagastrin led to the popularity of the calcium stimulation test. Recently, Diazzi et al [24] demonstrated the value of determining Ct in FNB samples (FNBCt) to diagnose MTC, and these investigators have recommended that this test be included in the clinical evaluation of thyroid nodules when MTC is suspected. Thyroid nodules with markedly elevated serum Ct levels and typical features of MTC on ultrasound imaging are relatively easy to diagnose. However, in patients with moderately elevated serum Ct levels and thyroid nodules lacking the characteristic ultrasound features of MTC, the diagnosis of malignancy is often uncertain. We hypothesized that in addition to the intranodular biopsy performed in the traditional way, modified fine-needle biopsy (MFNB) of thyroid lobe parenchyma, particularly the heterogeneous areas surrounding the nodule and the periphery and central areas of the larger thyroid nodules and/or subcentimeter nodules, is more likely to be diagnostic. It is postulated that the technique of biopsy may explain the low confirmatory yields in the diagnosis of thyroid nodules harboring MTC.
Here we report on a patient with a thyroid nodule in which the initial FNB yielded non-confirmatory results. However, biopsies of both thyroid lobe areas surrounding the nodules, plus intranodular areas (MFNB), confirmed atypical cells with suspicious cytology, thus, increasing the probability of malignancy prior to surgery. Similarly, in two additional patients, MFNB yielded more suspicious atypical cells suggestive of MTC compared to the initial FNB of the nodules. We demonstrated that the MFNBCt values were markedly elevated in these biopsy samples. We also measured PCt and CEA levels in MFNB specimens in these patients with MTC, and confirmed the usefulness of measuring these two peptides in aspirate samples. Although previous studies have mainly focused on Ct serum concentrations following calcium stimulation, there are limited studies examining PCt concentrations following calcium stimulation. In this case series, we investigated whether calcium-stimulated serum concentrations of PCt might also be useful.
| Materials and Methods | ▴Top |
Patients
Three patients with surgically confirmed MTC were included in this case series. Initially the FNB of thyroid nodules was carried out in these patients before obtaining any laboratory tests for MTC. Two patients with benign thyroid nodules and mildly elevated serum Ct levels, who were undergoing evaluation for MTC, were also studied, along with one additional patient with a normal basal Ct levels and a final diagnosis of multifocal subcentimeter papillary thyroid cancer. Additionally, we studied a fourth patient with benign thyroid nodules and a normal basal serum calcitonin level.
Calcium stimulation test
Informed and witnessed consent was obtained from all patients. Patients undergoing calcium stimulation test had a detailed evaluation for pro-arrhythmic risks prior to the test [24]. Calcium gluconate (2.5 mg elemental Ca/kg bodyweight) was given intravenously over 60 s under electrocardiographic monitoring. These tests were performed after fasting for 10 - 12 h overnight. Blood samples for Ct, PCt, and CEA were obtained at base line, 2, 5, 10 and 15 min after calcium administration. The blood was immediately centrifuged in a refrigerated centrifuge, and the serum was kept at 4 °C or frozen prior to performing the assays [24].
The basal serum Ct level thresholds for the identification of MTC were defined as > 68 and > 26 pg/mL in males and females, respectively [22]. The thresholds for calcium stimulated Ct levels were > 544 and > 79 pg/mL in males and females, respectively [22]. In this study, the basal serum thresholds for PCt were defined as > 0.5 ng/mL (reference: 0.00 - 0.50 ) and the threshold for calcium stimulated abnormal PCt levels were arbitrarily defined as three times the upper limit of normal plus the value for coefficient of variation ( > 1.65 ng/mL). Similarly, the thresholds for basal serum CEA were arbitrarily defined as CEA > 5.00 ng/mL (reference: 0.2 - 4.7).
Determination of Ct, PCt and CEA in serum samples
The serum Ct, PCt, and CEA assays were performed by clinical laboratories at Walter Reed National Military Medical Center or Quest Diagnostics Nichols Institute, Chantilly, VA. The inter- and intra-assay coefficients of variation (CVs) for Ct were 8.5% and 7.4%, respectively. The inter- and intra-assay CVs for PCt were 5.9% and 4.8%, and for CEA were 7.5% and 6.9%, respectively. We also performed serial dilution studies in these samples to assess for hook effect or interference with any fragments of these peptides (data not shown) and confirmed the absence of interference.
FNB and MFNB
Informed consent was obtained for both FNB and MFNB after detailed counselling. All patients received topical anesthesia with lidocaine 4% cream to the anterior neck skin and sedation with alprazolam prior to performing the biopsy.
We performed ultrasonography-guided (Philips iU22 Matrix Ultrasound System, Andover, MA) FNB and MFNB of the thyroid nodules using probe L12-5. US-guided FNB was carried out with a fine-needle (27-gauge) on a 10-mL plastic syringe. FNB consisted of sampling the central part of the nodule with 3 - 4 biopsies per nodule with 10 - 12 passes on each occasion. The material obtained was quickly smeared on glass slides, air dried and stained with Diff-Quik Stain (Stat Lab Medical Products, McKinney, TX) or alcohol fixed and stained by the Papanicolau method (Richard-Allan Scientific®, Thermo Scientific, Radnor, PA) for cytological examination. Cytological analysis was based on six different diagnostic classes (Bethesda System for reporting thyroid cytopathology (BSRTC)) according to current clinical guidelines [24]. In the patients with bilateral thyroid nodules (patients 1 and 3), FNB was performed on both sides of the thyroid lobes. In the patient (patient 1) with two subcentimeter nodules in one lobe, both nodules were biopsied together and considered as a single aspirate sample. After FNB aspirate samples in the needle have been expelled onto a slide for cytological studies, the used FNB needle was attached to an empty syringe and 0.50 mL of ice cold saline was drawn up through the needle and these needle washings were used for analysis of Ct, PCt, and CEA levels [24]. Samples from the same FNB nodule sites were pooled together and the specimens were then centrifuged at 4 °C, and the supernatants were transferred to plain plastic tubes and immediately frozen, or kept at 4 °C, prior to sending to the laboratory for measurement of Ct, PCt, and CEA levels.
MFNB consisted of sampling the entire intranodular area as well as the surrounding parenchyma of the thyroid lobe with 4 - 5 biopsies per nodule, and 12 - 16 passes on each occasion. In patient (patient 1) with two subcentimeter nodules in the right lobe, both nodule biopsy specimens were processed as one sample. MFNB samples were processed exactly as described for FNB samples for cytological studies and for the determination of Ct, PCt, and CEA levels in aspirate samples.
All biopsies were performed by one investigator to avoid variability in the techniques. The cytological studies were initially reviewed by one of the authors well experienced in FNB of thyroid nodule cytology and later also confirmed by a board certified cytopathologist. Additionally these studies were also peer reviewed.
Assay of FNB and MFNB aspirate samples for Ct, PCt, and CEA levels
The FNB washing samples were analyzed with the Siemens calcitonin reagent assay on the Siemens Immulite 1000, using a solid-phase, two-site chemiluminescence enzyme-labeled immunometric assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA). For samples with calcitonin concentrations > 50 pg/mL, serial dilutions were performed. Linear dilution excluded hook effect and most major interferences. Exogenous calcitonin was added to identify possible interferences that may cause a false-low result in samples that contained calcitonin concentrations < 50 pg/mL (data not shown).
PCt samples from biopsy samples were collected as described for Ct determination. PCt was measured by homogeneous automated immunofluorescent assay on the BRAHMS Kryptor using time resolved amplified cryptate emission technology (Thermo Fisher Scientific BRAHMS LLC, Middletown, VA). CEA measurement from the aspirate samples was performed by using the Bayer Centaur, chemiluminescent method. We also performed serial dilution studies in these aspirate samples to assess for hook effect or interference with any fragments of these two peptides (data not shown) and confirmed the absence of interference.
In order to confirm the stability of Ct, PCt and CEA in normal saline, we used serum with insignificant levels of Ct, PCt, and CEA as diluent. Assays using saline diluent and serum diluent gave identical results for Ct, PCt and CEA (data not shown).
A Ct level of > 1,000 pg/mL in needle biopsy samples was considered diagnostic for MTC [24]. Ct levels in aspirate sample < 17 pg/mL are generally seen in benign thyroid lesions [24]. The upper limits of biopsy aspirate samples for PCt, and CEA were arbitrarily calculated as three times the upper limit of normal serum levels plus the CV value. For PCt aspirate, this level was > 2.0 ng/mL and for CEA, a level of >18 ng/mL was considered to be diagnostic for MTC in aspirate samples.
Genetic testing
RET proto-oncogene testing was performed by Athena Diagnostics, Inc. (Marlborough, MA).
Case history
Patient 1
A 41-year-old female with a family history of MTC presented for evaluation. She was asymptomatic and had no history of hypertension or symptoms of hypercalcemia. Physical examination revealed normal vital signs and thyroid examination revealed a barely palpable thyroid gland without any nodules or cervical lymphadenopathy. Examination of the heart, lungs, and abdomen was normal. Ultrasound of the thyroid showed two well-defined hypoechoic nodules in the right thyroid lobe. Within the superior right lobe, a nodule measuring 0.6 × 0.3 × 0.5 cm without internal calcification or vascularity was seen and, adjacent to this nodule, there was a second poorly defined hypoechoic nodule that measured 0.3 × 0.4 × 0.4 cm without internal calcification or internal vascularity (figure not shown). Within the left lobe, there was a mixed solid and cystic nodule, measuring 1.1 × 0.6 × 0.7 cm without internal vascularity or calcification. There was also a small subcentimeter hypoechoic nodule within the superior pole of left thyroid lobe without increased vascularity or microcalcification (figure not shown). A positive emission (PET) scan was non-diagnostic (figure not shown). FNB of the two right thyroid nodules (Fig. 1a) and left complex nodule (Fig. 1b) revealed only non-confirmatory cytology for MTC. The basal serum calcitonin level in this patient was 41 pg/mL (reference 0.0 - 5.0) and with calcium stimulation increased to 889 pg/mL. Basal serum PCt level was 0.32 ng/mL (reference 0.00 - 0.50) and following calcium stimulation PCt increased to a peak level of 3.99 ng/mL (Table 1). The basal serum CEA was normal and did not change with calcium stimulation (Table 1). Additionally FNB of the thyroid nodules revealed moderately elevated levels of Ct, PCt and CEA levels (Table 2). The patient wanted a positive diagnosis of cancer prior to surgery in order to assist in the extent of the surgery, and she requested additional diagnostic tests, including a repeat biopsy. In order to facilitate the extent of thyroidectomy and lymph node dissection, given the history of patient’s mother with MTC and elevated serum Ct levels, MFNB of the right thyroid nodules and left thyroid complex nodule were performed. Additionally, the MFNB included the parenchyma surrounding these nodules as described in “Methods”. The MFNB samples were processed for cytology as well as Ct, PCt and CEA. MFNB of the right lobe nodule showed cytology suggesting MTC (Fig. 1c) and permanent sections of the right lobe confirmed MTC (figure not shown). Interestingly MFNB of the left nodule revealed a clump of amorphous material within a background of colloid which may represent amyloid (Fig. 1d). MFNB of this nodule also showed atypical cells more confirmatory for MTC (Fig. 1e and f) and final histology confirmed MTC (figure not shown). MFNB samples showed a Ct level of 1,036 pg/mL from the right lobe nodules and 1,870 pg/mL from the left thyroid nodule. PCt and CEA levels from the right lobe nodules MFNB aspirates were 3.2 and 21.9 ng/mL. Similarly the Ct, PCt and CEA levels in MFNB aspirates from left nodule were significantly higher than those obtained by FNB (Tables 2 and 3).
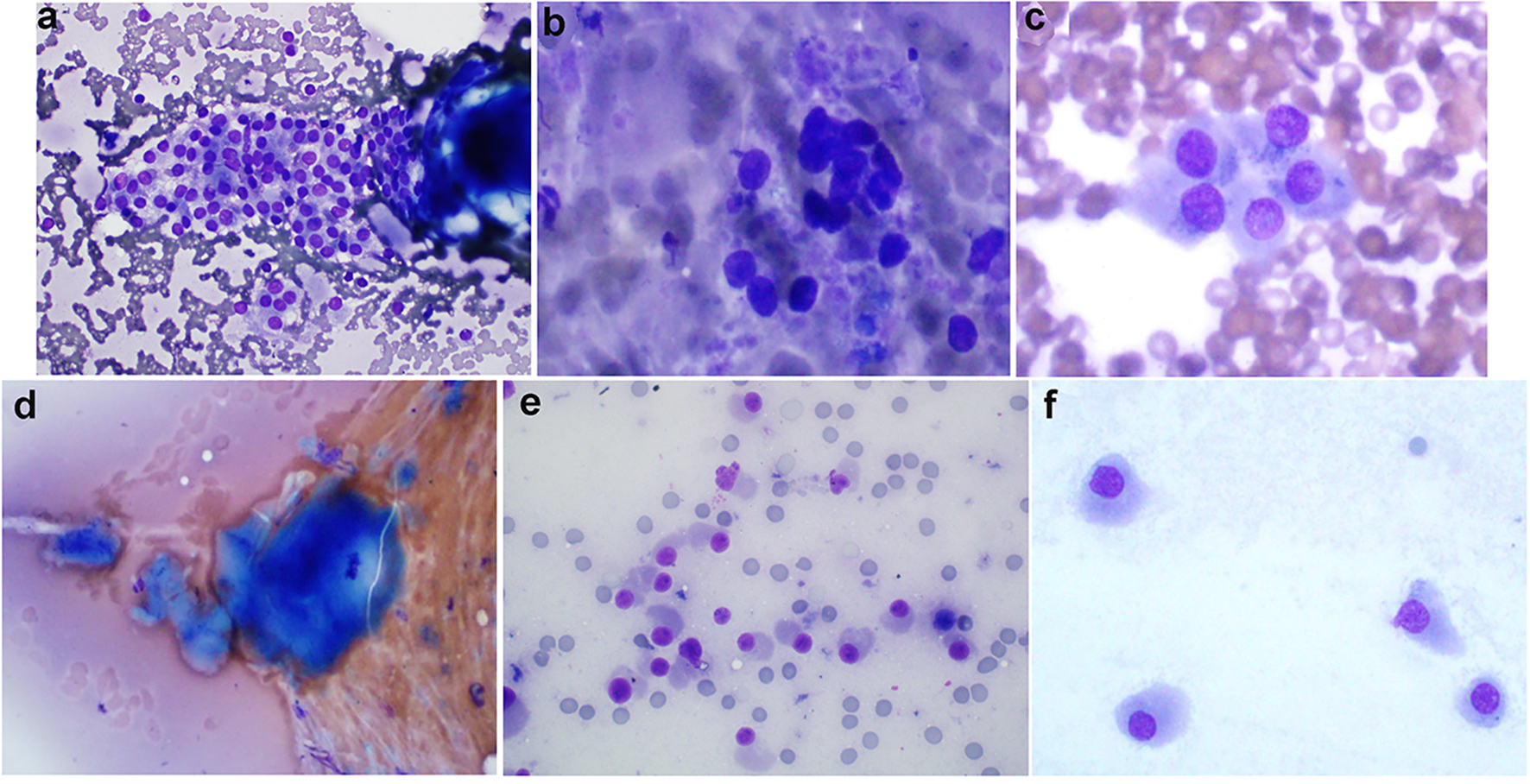 Click for large image | Figure 1. (Patient 1) (a) Results of FNB biopsy of the right thyroid nodules (two nodules, 1a and 1b biopsied together): follicular cells trying to form microfollicles. Follicular cells show some degree of atypia. These features suggest follicular lesions of undetermined significance (Diff-Quik, × 600). (b) Results of FNB biopsy of the left thyroid nodule (nodule 2a): follicular cells forming microfollicles. Follicular cells show some degree of atypia. These features suggest follicular lesions of undetermined significance (Diff-Quik, × 600). (c) Results of MFNB biopsy of the right thyroid nodules (two nodules, 1a and 1b biopsied together): cellular smears consisting of discohesive cells with abundant amphophillic cytoplasm, and eccentrically placed nuclei with prominent nucleoli. These features along with elevated serum calcitonin suggest medullary thyroid cancer (Diff-Quik, × 600). (d) MFNB of the left thyroid nodule (nodule 2a): amorphous material suggestive of amyloid material (Congo red stain not done) (Diff-Quik, × 600). (e) MFNB of the left thyroid nodule (nodule 2a): crowded groups of mildly enlarged cells with oval to focally spindled nuclei, moderate to abundant cytoplasm and finely stippled chromatin. While not classic for medullary carcinoma, the concurrent finding of elevated serum calcitonin makes the diagnosis more likely (Diff-Quik, × 600). (f) MFNB of the left thyroid nodule (nodule 2a): cells with oval to focally spindled nuclei, abundant cytoplasm and finely stippled chromatin. While not classic for medullary carcinoma, the concurrent finding of elevated serum calcitonin makes the diagnosis more likely (Diff-Quik, × 600). FNB: fine-needle biopsy; MFNB: modified fine-needle biopsy; Diff. quick: Diff quick stain. |
 Click to view | Table 1. Serum Levels of Calcium, Calcitonin (Ct), Procalcitonin (PCt) and Carcinoembryonic Antigen (CEA) in Patients With Medullary Thyroid Cancer Following Intravenous Administration of Calcium Gluconate |
 Click to view | Table 2. Calcitonin (Ct), Procalcitonin (PCt) and Carcinoembryonic Antigen (CEA) Levels in Serum and Fine-Needle Aspiration Biopsy (FNB) Samples in Patients With Medullary Thyroid Cancer |
 Click to view | Table 3. Calcitonin (Ct), Procalcitonin (PCt) and Carcinoembryonic Antigen (CEA) Levels in Serum and Modified Fine-Needle Aspiration Biopsy (MFNB) Samples in Patients With Medullary Thyroid Cancer |
A presumptive diagnosis of MTC involving both thyroid lobes was made from these findings. The patient consented to a total thyroidectomy with central neck lymph node dissection. Histopathological examination of the resected thyroid gland revealed a 7 mm MTC in the right lobe (figure not shown) and an 8 mm MTC in left lobe (figure not shown), and areas of C-cell hyperplasia. The central lymph nodes were negative for metastatic MTC. Genetic testing for proto-oncogene studies revealed MEN2 and FMTC mutations, exon 10, 11, 13-16, confirming a heterozygous RET mutation. (Nucleotide sequence analysis of the RET proto-oncogene (exons 10, 11, 13, 14, 15, and 16) indicated a mutation at p.C620Y. This mutation changes the normal cysteine at amino acid position 620 of the RET protein to tyrosine. It is caused by a G>A change at nucleotide c.1859 (c.1859G>A) in exon 10 of the RET gene). Twelve months later, the patient remained asymptomatic and the ultrasound of the neck showed no residual thyroid or nodules and no abnormal cervical lymphadenopathy. Basal serum Ct was < 2.0 pg/mL with normal serum PCt and CEA levels and ultrasound of neck revealed no residual tumors in thyroid bed or abnormal cervical lymph nodes.
Patient 2
A 54-year-old female presented for evaluation of a thyroid nodule. The patient had no history of neck radiation or family history of thyroid cancer. Past history was negative for hypertension and hyperparathyroidism. Physical examination revealed a 2 cm right thyroid nodule with no cervical lymphadenopathy. The rest of the physical examination was normal. Laboratory tests demonstrated normal complete blood count, serum calcium, alkaline phosphatase and liver-associated enzymes. Thyroid function tests showed serum TSH 1.61 mIU/mL (reference 0.27 - 4.20), serum free T4 0.94 ng/dL (reference 0.93 - 1.7), anti-thyroid peroxidase antibody 329 IU/mL (reference 0 - 340), and anti-thyroglobulin antibody 3.3 mIU/mL (reference < -1.0). A thyroid ultrasound showed a hypervascular solid isoechoic nodule within the inferior aspect of the right lob measuring 2.1 × 1.3 × 1.5 cm with no evidence of microcalcification (figure not shown). A PET scan revealed an intense focus of hypermetabolism associated with the right thyroid lobe, correlating with the 2.1 cm right thyroid lobe nodule suspicious for thyroid malignancy (figure not shown). Basal serum concentration of calcitonin was 58 pg/mL and, following calcium stimulation, the level rose up to a peak level of 142 pg/mL. The peak PCt level following calcium stimulation was 7.11 ng/mL. There were no changes in CEA levels following calcium stimulation (Table 1). An FNB of the right thyroid nodule demonstrated a follicular lesion of unknown significance (Fig. 2a). Additionally, FNB aspirate samples showed elevated level of Ct and moderately elevated levels of PCt and CEA (Table 2). Because of the elevated basal serum Ct and abnormal positive emission tomographic findings, the patient consented to an MFNB of the right thyroid nodule, which showed more confirmatory cytology suggesting MTC (Fig. 2b). MFNB samples also showed markedly elevated Ct (1,440 pg/mL), PCt (7.8 ng/mL) and CEA (29.9 ng/mL) (Table 3). However MFNBCt value was lower than that obtained by FNB although the level was still higher than 1,000 pg/mL [24]. Both PCt and CEA levels in MFNB aspirates were much higher compared to FNB values (Tables 2 and 3). Based on this information patient consented for surgery. The patient underwent a total thyroidectomy with central and right neck lymph node dissection. Pathology confirmed a 2.4 cm MTC in the right lobe (figure not shown) with metastases to the lymph nodes in the central compartment but no metastases in lateral cervical lymph nodes. Genetic testing confirmed no mutations in the RET proto-oncogene to suggest familial MTC. The patient had no symptoms 18 months later, and a neck ultrasound demonstrated no thyroid tissue or cervical lymphadenopathy. Post-operative basal and stimulated serum Ct, PCt, CEA levels were normal.
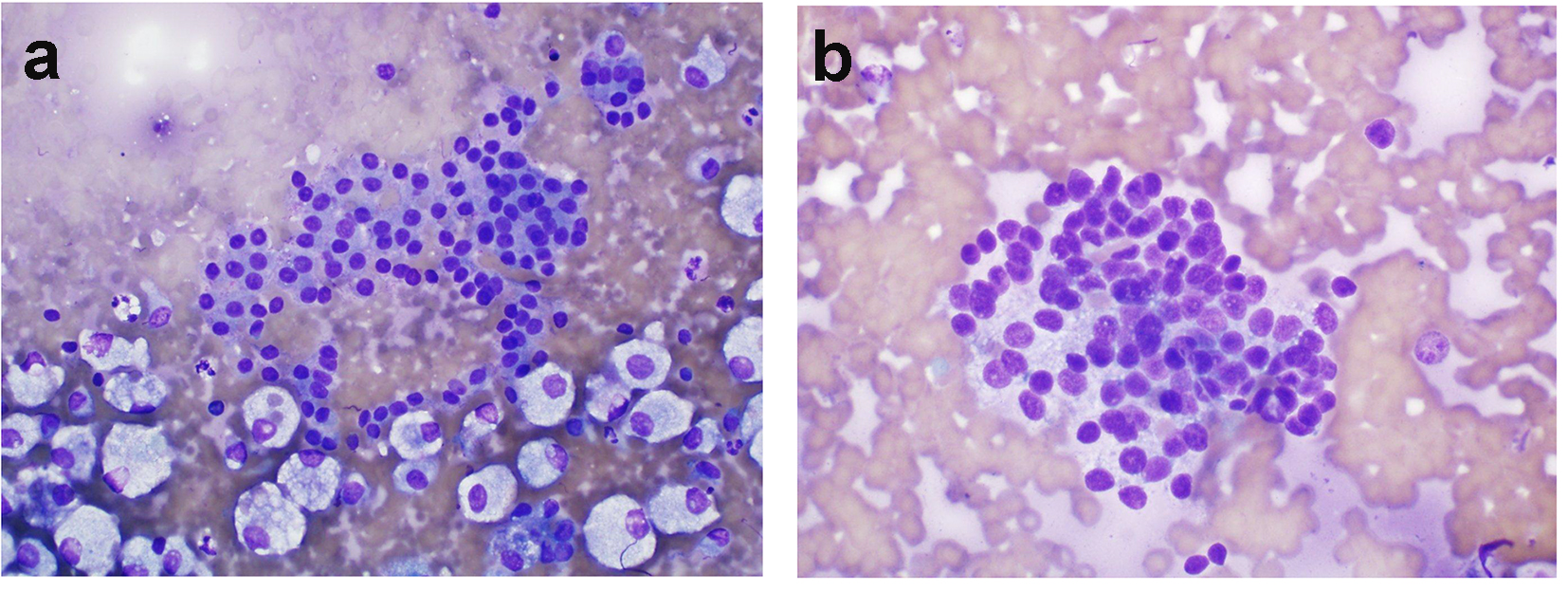 Click for large image | Figure 2. (Patient 2) (a) Results of FNB biopsy of the right thyroid nodule: follicular cells forming microfollicles and showing some degree of atypia. These features suggest follicular lesions of undetermined significance (Diff-Quik, × 600). (b) Results of MFNB of right thyroid nodule: crowded groups of mildly enlarged cells with oval to focally spindled nuclei, moderate cytoplasm and finely stippled chromatin. While not classic for medullary carcinoma, the concurrent finding of elevated serum calcitonin makes this diagnosis more likely (Diff-Quik, × 600). FNB: fine-needle biopsy; MFNB: modified fine-needle biopsy; Diff. quick: Diff quick stain. |
Patient 3
A 65-year-old female presented for evaluation of osteoporosis. The patient also noted fullness on the anterior aspect of her neck. Review of systems was unremarkable. She reported a history of thyroid cancer in her brother, diagnosed 14 years earlier, but she did not have any additional details. Physical examination revealed normal vital signs but an abnormal thyroid examination of a 45-g multinodular goiter with a 3 cm left thyroid nodule and 2 cm right thyroid nodule. There were no palpable cervical lymph nodes. Routine laboratory studies showed normal complete blood count, BUN, creatinine, alkaline phosphatase, and liver-associated enzymes. Serum calcium was 11.6 mg/dL (reference 8.6 - 10.2), serum phosphorous was 2.6 mg/dL (reference 2.5 - 4.5), serum parathyroid hormone was 108 pg/mL (reference 15 - 65), 24-h urine calcium was 390 mg (reference 120 - 250), plasma metanephrine was < 10 pg/mL (reference 0 - 62) and normetanephrine was 49 pg/mL (reference 0 - 145).
Ultrasound of the thyroid demonstrated a right middle lobe thyroid nodule measuring 1.1 × 1.0 × 1.1 cm, hypoechoic in texture with no microcalcification and no significant internal or peripheral color Doppler blood flow (figure not shown). The left lobe showed a 3.7 × 2.3 × 2.7 cm hypoechoic nodule, with coarse calcifications and mildly increased peripheral and central blood flow (figure not shown). A PET scan showed a hypermetabolic nodule (2.04 SUV-m) in the right thyroid lobe measuring approximately 1.0 cm in maximal axial diameter and a hypermetabolic (3.59 SUV-m) nodule in the left thyroid lobe measuring 2.7 × 2.2 cm, additionally showing extensive coarse calcifications (figure not shown). Basal serum calcitonin was 69 pg/mL and intravenous calcium stimulation caused the level to rise to a peak level of 439 pg/mL. Similarly, the stimulated serum PCt level was 3.94 ng/mL, whereas there were no changes in serum CEA level with calcium stimulation (Table 1). An FNB of the right thyroid nodules showed atypical thyroid cells interpreted as possibly MTC (figure not shown). An MFNB was performed using a 27 g needle sampling the central and peripheral parts of the nodules as well as the thyroid parenchyma around the nodules. This MFNB of the right nodule was more confirmatory (Fig. 3a). Final histology of the right lobe confirmed MTC (figure not shown). FNB of the left nodule cytology was interpreted as follicular lesion of unknown significance (figure not shown). However MFNB aspirate cytology of the left nodule was more confirmatory for MTC (Fig. 3b) and MFNB aspirate samples revealed higher levels of Ct, PCt, and CEA levels compared to FNB samples (Tables 2 and 3). Calcium stimulation was performed, and the basal and post-stimulation serum samples revealed markedly elevated Ct and PCt levels, whereas there were no changes in serum CEA levels (Table 1).
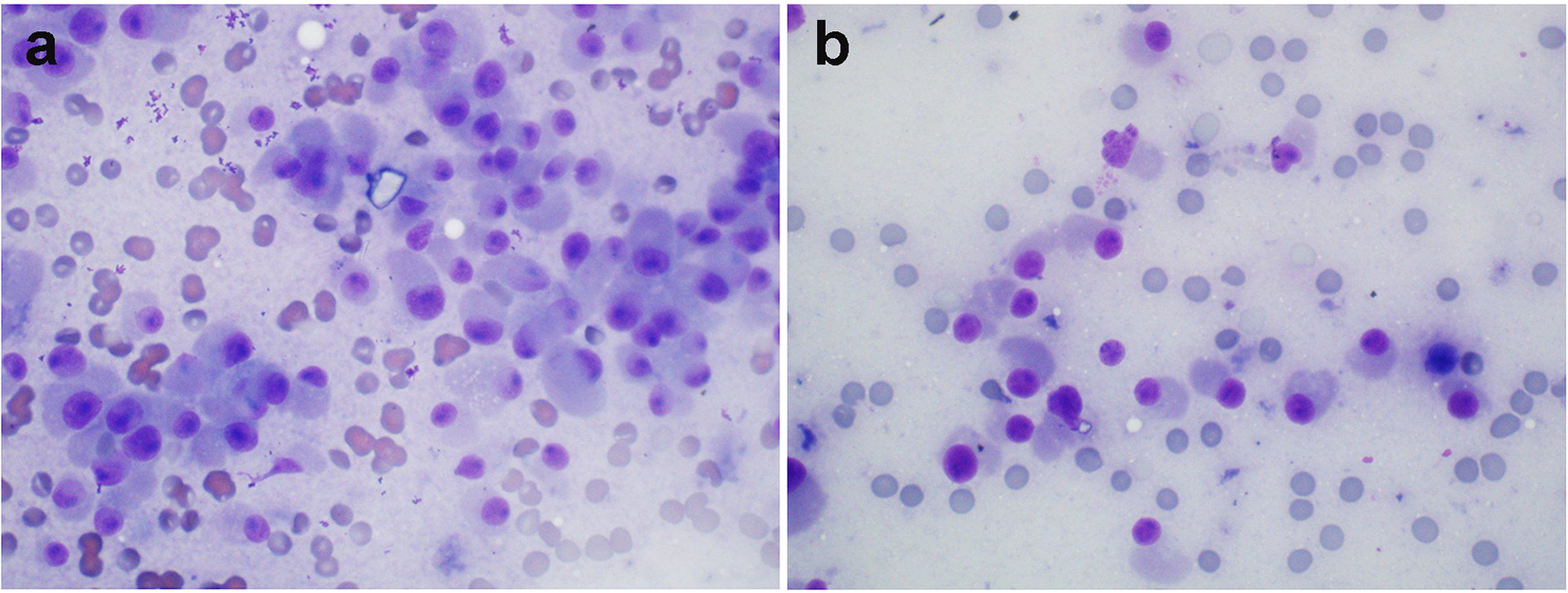 Click for large image | Figure 3. (Patient 3) (a) MFNB of the right thyroid nodule showing: cellular smears consisting of discohesive cells with abundant amphophillic cytoplasm, and eccentrically placed nuclei with prominent nucleoli (oncocytic features) (Diff-Quik, × 600). (b) MFNB of the left thyroid nodule: smears consisting of discohesive, focally enlarged cells with eccentric nuclei with occasional nucleoli, coarse chromatin and abundant cytoplasm. These features along with elevated serum calcitonin suggest medullary thyroid cancer (Diff-Quik, × 600). FNB: fine-needle biopsy; MFNB: modified fine-needle biopsy; Diff. quick: Diff quick stain. |
With this information, the patient underwent total thyroidectomy. Histopathological examination showed multifocal bilateral MTC with a 0.9 cm right lobe MTC lesion (figure not shown) and a 4.3 cm, maximum diameter MTC lesion in the left lobe (figure not shown). Central lymph node compartment dissection was positive for metastases and focal lymphovascular invasion. In addition, multiple foci of C-cell hyperplasia and a parathyroid adenoma weighing 960 mg were confirmed. Genetic studies revealed a DNA sequence change from G to A, detected at nucleotide position c.1832 in exon 10 of the RET gene (c.1832G>A), resulting in an amino acid change from cysteine (C) to tyrosine (Y) at position 611 in the RET protein (p. C 611Y). Six months later basal and stimulated serum concentrations of Ct, PCt and CEA were normal.
Patients without MTC
Only female patients (n = 4) were included in these series. Two patients (patients 1 and 2) with surgically confirmed benign thyroid nodules and moderately elevated basal serum Ct levels (Table 4) were evaluated. Interestingly these two patients (patients 1 and 2) showed stimulated Ct levels consistent with a diagnosis of MCT [22] although the final histological studies of the resected thyroid glands were benign. In a third patient (patient 3) with a remote family history of thyroid cancer and normal basal Ct levels intravenous administration of calcium gluconate stimulated serum Ct to a peak level of 36 pg/mL and this patient also had benign histology. These three patients had no evidence of C-cell hyperplasia. Interestingly none of these three patients had abnormal basal or stimulated levels of PCt or CEA. MFNBs in these three patients showed benign cytology and MFNB samples also had low levels of Ct, PCt and CEA (Table 5). In patient 4 with mildly elevated basal serum calcitonin level (Table 4), an initial FNB showed abnormal cells, suggestive of follicular neoplasm. However, an MFNB confirmed papillary thyroid cancer and surgical pathology confirmed multicentric subcentimeter papillary thyroid cancer involving both lobes. In this patient both FNB and MFNB samples also showed low levels of Ct, PCt and CEA (Table 5) and a calcium stimulation test also showed no significant changes in serum Ct, PCt and CEA levels in patient 4 (Table 4).
 Click to view | Table 4. Serum Levels of Calcium, Calcitonin (Ct), Procalcitonin (PCt) and Carcinoembryonic Antigen (CEA) in Patients With Benign Thyroid Nodules (n = 3, Patients 1, 2 and 4) and Multifocal Papillary Thyroid Cancer (n = 1, Patient 3) Following Intravenous Administration of Calcium Gluconate |
 Click to view | Table 5. Calcitonin (Ct), Procalcitonin (PCt), and Carcinoembryonic Antigen (CEA) in Serum and Modified Find-Needle Aspiration Biopsy (MFNB) Samples in Benign Thyroid Nodules (n = 3, Patients 1 - 3) and in Another Patient (Patient 4) With Subcentimeter Multicentric Papillary Thyroid Cancer |
| Discussion | ▴Top |
MTC can follow an aggressive course, especially if not diagnosed at an early stage. Traditionally, in addition to FNB of thyroid nodules, basal serum concentrations of Ct, along with pentagastrin-stimulated or calcium-stimulated serum Ct, have been used to diagnose MTC [1-12]. RET proto-oncogene testing for identifying familial forms may also aid in case identification [20, 21]. However, pentagastrin is currently not available in United States or in Europe. C-cell hyperplasia, the earliest manifestation of MTC, may occur in other benign conditions such as autoimmune thyroiditis, benign thyroid nodules, renal insufficiency, hyperparathyroidism, or hypergastrinemia, including after the administration of proton pump inhibitors [1-8]. Despite the availability of various diagnostic tests, establishing an early diagnosis of MTC remains challenging. Sometimes the basal Ct levels cannot distinguish between benign disorders and MTC. Mian et al [22] recently reported that basal Ct levels higher than 58 pg/mL in males and higher than 26 pg/mL in females can distinguish patients with C-cell hyperplasia from patients with MTC. These thresholds for basal calcitonin are comparable to those found in other reports. The basal Ct levels in all three patients with MTC reported in our case series were above the threshold of diagnostic levels reported in the literature, but false positive results may still occur as seen in our control patients. Since the FNB results were not confirmatory, our patients requested additional confirmatory testing prior to undergoing aggressive surgical treatment.
Serum Ct is an unstable hormone, decreasing by 50% when the serum samples are stored at room temperature. Even when stored in the refrigerator or frozen, there is a decrease in concentration [25]. Additionally, the measurement of serum Ct may yield inaccurate results due to assay problems, the presence of heterogeneous antibodies, the high-dose hook effect, or the effects of high levels of ascorbic acid, creatine, and urea [25-28]. Patients with neuroendocrine tumors or follicular thyroid carcinoma with metastasis to liver, lung, and lymph nodes, or even multinodular goiter, may also demonstrate elevated serum Ct [29]. PCt, a precursor of Ct, is generally elevated in the presence of bacterial infection or sepsis [29]. Serum concentrations of PCt show much better stability under all conditions, with concentrations decreasing to no more than 10% when stored at room temperature [30]. Thus, it was suggested that basal serum PCt could be a promising additional diagnostic test to exclude MTC [29-34]. The other potential advantage of measuring PCt is its lack of susceptibility to interfering isoforms or fragments, which cause falsely low results and in vitro pre-analytical instability [30]. A recent study by Machens et al has shown that serum PCt measurement has comparable diagnostic accuracy and the potential to replace serum Ct measurement because of its stability at room temperature [31]. As shown in our study, measurement of basal PCt may serve as an additional test useful in diagnosing MTC. Giovanella et al [34] have also confirmed that serum PCt measurements show significantly fewer false positives than Ct in patients with non-medullary benign thyroid disorders. Giovanella [35] also demonstrated that the intravenous administration of calcium gluconate has minimal to no effect on serum PCt levels in normal subjects. Thus, calcium-stimulated PCt may also be useful for further confirmation of the diagnosis of MTC. Kratzsch et al demonstrated that by performing pentagastrin stimulation and measuring PCt, a clear distinction between patients with chronic renal disease and MTC can be made [25]. Our present study reconfirms the usefulness of measuring both basal and calcium stimulated PCt in diagnosing MTC. Since Ishikawa and Hamada described elevated serum CEA in patients with MTC, several subsequent studies have confirmed the usefulness of measuring CEA in serum, especially in patients with advanced MTC [36-38]. Ct and PCt are stored in dense-core secretory granules, and these hormones can be released into the blood stream by intravenous administration of pentagastrin or calcium [36-38]. In contrast, CEA is integrated into the cell membrane [37, 38], so it is not surprising that serum levels of CEA remain stable with calcium stimulation as occurred in our study.
Generally, FNB cytology is regarded as the gold standard procedure to distinguish between benign and malignant thyroid nodules. However, in contrast to papillary thyroid cancer, the sensitivity of FNB cytology for diagnosing MTC is low. The reported sensitivity for FNB cytology in diagnosing MTC varies from 53% to 75% [5, 7, 9, 13-16]. Kudo et al [13] could not identify MTC in five patients by FNB cytology. Elisei et al [8] demonstrated that serum Ct had higher sensitivity to diagnose MTC in comparison with FNB cytology. Bugalho et al [9] reported FNB cytology was able to diagnose MTC in 42 of 67 patients with a sensitivity of 63%. Papaparaskeva et al [11] and Forrest et al [12] reviewed FNB cytology in patients with MTC, and found a positive predictive value of 89% and 81%, respectively. Trimboli et al [39] detected MTC in 21 of 37 thyroid nodules with a sensitivity of 58.8%. These investigators also suggested that FNBCt measurement is superior to diagnose MTC compared to cytology [40, 41]. Thus, the sensitivity of FNB alone for diagnosing MTC is reportedly poor, and false negative findings may often occur with this diagnostic approach. Even when combining FNB cytology with calcitonin determination in the FNB needle washout, the sensitivity remains low.
Since multinodular goiter is a common disorder encountered especially in the elderly, the presence of several nodules may necessitate biopsy of all the nodules. However, ultrasound characteristics, such as coarse calcification and other features, may help to identify the most suspicious nodules. In a recent study by Essig et al [17] involving 313 patients, FNB confirmed MTC in only 43.7% and possible MTC in an additional 2.4%. These authors also confirmed the low sensitivity of FNB cytological evaluation and confirmed the limitations of using only FNB for the preoperative diagnosis of MTC. We hypothesized that MFNB of the thyroid nodules involving both central and peripheral part of the nodule and surrounding thyroid parenchyma in patients suspected with MTC, especially with those with C-cell hyperplasia, may yield more positive results and thus improve sensitivity. Out of the three patients with MTC, two patients had prior non-confirmatory FNB cytology and in the third patient FNB was suspicious for MTC. However, when we performed an MFNB in these patients the cytology results in all three patients were more specific of MTC, thus confirming the validity of this technique. Since all biopsies were performed by one investigator, the variability in the biopsy techniques cannot explain this outcome. The advantage of MFNB was also demonstrated in one patient with multifocal papillary thyroid cancer.
The MFNB biopsy as we performed it may sample more areas, especially in patients with subcentimeter thyroid nodules and it is also possible that the biopsy materials may include more C-cells from C-cell hyperplasia areas in addition to malignant cells and this may explain the more positive yield by our technique. It is to be noted that one of the three patients despite not showing C-cell hyperplasia in final pathological specimen demonstrated superior cytological yield with MFNB confirming MTC. The superiority of MFNB was further confirmed when Ct, PCt and CEA levels in MFNB aspirates were compared with FNB technique.
Recently, the measurement of Ct levels in washout fluids of needle after aspiration (FNBCt) has been reported to be useful in the diagnosis of MTC [39-46]. Trimboli et al [41, 42] suggested that FNBCt cutoff should be calculated by serum cutoff plus the highest value of interlaboratory CV of serum Ct and FNB Ct. These authors reported an FNBCt threshold of 39.6 pg/mL provided 100% in detecting MTC. Boi et al [43] examined 23 patients with MTC and proposed an arbitrary FNBCt cutoff of 36 pg/mL corresponding to three times the value found in controls. However, Kudo et al [13] reported high FNB Ct levels ranging from 17,000 to 560,000 pg/mL in MTC patients although in this study the FNB cytology was only positive in one of five patients. In this study control patients had calcitonin values ranging from < 10 to 67 pg/mL in FNB aspirate samples. Abraham et al [43] reported five patients with MTC where they had been able to localize the lesions harboring MTC by FNBCt and in this report the FNBCt levels ranged from 237 to 79,118 pg/mL. A recent study by Diazzi et al [24], along with these previous studies, has thus confirmed the reliability of diagnosing of MTC by FNB aspirate sample assays for Ct and Diazzi et al even suggested that FNBCt levels in aspirate samples be determined in addition to basal serum Ct levels and pentagastrin or calcium stimulated Ct levels. It is generally considered that FNBCt levels above 1,000 pg/mL are diagnostic for confirming MTC [24], and we have used these criteria in our patients, although this may need for further confirmation. All our three patients with MTC met these criteria when MFNB was performed. In contrast, in these patients relatively lower levels of Ct were seen in FNB aspirate samples, thus further suggesting the superiority of MFNB as a technique. However, we did not study any patients with C-cell hyperplasia alone. In comparison, in our patients with benign thyroid nodules the MFNBCt levels were less than 3.2 pg/mL. We have also confirmed that measuring PCt and CEA levels in MFNB aspirate samples is also useful in diagnosing thyroid nodules harboring MTC, although further studies are needed to confirm this. Immunostaining for calcitonin is useful in diagnosing MTC in needle aspirate samples although we did not perform this. The recent availability of gene testing in FNB samples has advanced the diagnostic accuracy for confirming papillary and follicular cancer and even MTC [48], although further studies are needed to verify the utility of this technique to diagnose MTC. MFNBs, along with determining Ct, PCt and CEA in aspirate samples, are easy to perform and relatively inexpensive especially compared with determining molecular markers.
It may be said that FNB of thyroid nodules along with determination of serum calcitonin may have confirmed the diagnosis of MTC in our patients. The recently published guidelines do not advocate performing routine serum calcitonin measurement in patients with thyroid nodules [49]. In this small case series, we have investigated whether the techniques in performing biopsy may be involved in false negative biopsy results in patients with thyroid nodules and MTC. In conclusion, we have demonstrated that MFNB, along with determination of Ct, PCt, and CEA levels in aspirate samples, is useful in confirming the diagnosis of MTC in patients with suspected MTC and thyroid nodules. Additionally, calcium-stimulated serum PCt levels may also be useful. However, studies involving larger numbers of patients with MTC and with benign thyroid nodules are required.
Acknowledgments
We would like to express our sincere thanks to Dr. David Larson, Pathology Department for reviewing the pathology slides. We would also like to thank Mr. Asif Ismail, Rutgers University, New Brunswick, NJ for editorial assistance.
Disclosure
The authors have no multiplicity of interest to disclose. The views expressed are those of the authors and do not reflect the official policy of the Department of Army, Navy or the Department of Defense or the United States Government. This manuscript was reviewed and cleared for publication by the Institutional Review Board of Walter Reed National Military Medical Center.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
| References | ▴Top |
- Hu MI, Jimenez CO, Cote GJ , Gagel RF. Medullary thyroid carcinoma. In: Braverman LE, Cooper DS, (eds). Werner & Ingbar’s the Thyroid a Fundamental and Clinical Text, 10th edition. Philadelphia, PA: Wolters /Kluwer Lippincott Williams & Wilkins. 2013. p. 744-765.
- Hodak SP, Burman KD. The calcitonin conundrum - is it time for routine measurement of serum calcitonin in patients with thyroid nodules? J Clin Endocrinol Metab. 2004;89(2):511-514.
doi pubmed - Pacini F, Fontanelli M, Fugazzola L, Elisei R, Romei C, Di Coscio G, Miccoli P, et al. Routine measurement of serum calcitonin in nodular thyroid diseases allows the preoperative diagnosis of unsuspected sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab. 1994;78(4):826-829.
pubmed - Niccoli P, Wion-Barbot N, Caron P, Henry JF, de Micco C, Saint Andre JP, Bigorgne JC, et al. Interest of routine measurement of serum calcitonin: study in a large series of thyroidectomized patients. The French Medullary Study Group. J Clin Endocrinol Metab. 1997;82(2):338-341.
doi pubmed - Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, Miccoli P, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89(1):163-168.
doi pubmed - Borget I, De Pouvourville G, Schlumberger M. Editorial: Calcitonin determination in patients with nodular thyroid disease. J Clin Endocrinol Metab. 2007;92(2):425-427.
doi pubmed - Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, Crocetti U, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92(2):450-455.
doi pubmed - Elisei R. Routine serum calcitonin measurement in the evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22(6):941-953.
doi pubmed - Bugalho MJ, Santos JR, Sobrinho L. Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. J Surg Oncol. 2005;91(1):56-60.
doi pubmed - Ahmed SR, Ball DW. Clinical review: Incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab. 2011;96(5):1237-1245.
doi pubmed - Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol. 2000;22(6):351-358.
doi - Forrest CH, Frost FA, de Boer WB, Spagnolo DV, Whitaker D, Sterrett BF. Medullary carcinoma of the thyroid: accuracy of diagnosis of fine-needle aspiration cytology. Cancer. 1998;84(5):295-302.
doi - Kudo T, Miyauchi A, Ito Y, Takamura Y, Amino N, Hirokawa M. Diagnosis of medullary thyroid carcinoma by calcitonin measurement in fine-needle aspiration biopsy specimens. Thyroid. 2007;17(7):635-638.
doi pubmed - Dustin SM, Jo VY, Hanley KZ, Stelow EB. High sensitivity and positive predictive value of fine-needle aspiration for uncommon thyroid malignancies. Diagn Cytopathol. 2012;40(5):416-421.
doi pubmed - Lew JI, Snyder RA, Sanchez YM, Solorzano CC. Fine needle aspiration of the thyroid: correlation with final histopathology in a surgical series of 797 patients. J Am Coll Surg. 2011;213(1):188-194; discussion 194-185.
- Choi N, Moon WJ, Lee JH, Baek JH, Kim DW, Park SW. Ultrasonographic findings of medullary thyroid cancer: differences according to tumor size and correlation with fine needle aspiration results. Acta Radiol. 2011;52(3):312-316.
doi pubmed - Essig GF, Jr., Porter K, Schneider D, Debora A, Lindsey SC, Busonero G, Fineberg D, et al. Fine needle aspiration and medullary thyroid carcinoma: the risk of inadequate preoperative evaluation and initial surgery when relying upon FNAB cytology alone. Endocr Pract. 2013;19(6):920-927.
doi pubmed - Chang TC, Wu SL, Hsiao YL. Medullary thyroid carcinoma: pitfalls in diagnosis by fine needle aspiration cytology and relationship of cytomorphology to RET proto-oncogene mutations. Acta Cytol. 2005;49(5):477-482.
doi pubmed - Colombo C, Verga U, Mian C, Ferrero S, Perrino M, Vicentini L, Dazzi D, et al. Comparison of calcium and pentagastrin tests for the diagnosis and follow-up of medullary thyroid cancer. J Clin Endocrinol Metab. 2012;97(3):905-913.
doi pubmed - Verga U, Ferrero S, Vicentini L, Brambilla T, Cirello V, Muzza M, Beck-Peccoz P, et al. Histopathological and molecular studies in patients with goiter and hypercalcitoninemia: reactive or neoplastic C-cell hyperplasia? Endocr Relat Cancer. 2007;14(2):393-403.
doi pubmed - Takano T, Miyauchi A, Matsuzuka F, Liu G, Higashiyama T, Yokozawa T, Kuma K, et al. Preoperative diagnosis of medullary thyroid carcinoma by RT-PCR using RNA extracted from leftover cells within a needle used for fine needle aspiration biopsy. J Clin Endocrinol Metab. 1999;84(3):951-955.
doi - Mian C, Perrino M, Colombo C, Cavedon E, Pennelli G, Ferrero S, De Leo S, et al. Refining calcium test for the diagnosis of medullary thyroid cancer: cutoffs, procedures, and safety. J Clin Endocrinol Metab. 2014;99(5):1656-1664.
doi pubmed - Elisei R, Romei C, Cosci B, Agate L, Bottici V, Molinaro E, Sculli M, et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J Clin Endocrinol Metab. 2007;92(12):4725-4729.
doi pubmed - Diazzi C, Madeo B, Taliani E, Zirilli L, Romano S, Granata AR, De Santis MC, et al. The diagnostic value of calcitonin measurement in wash-out fluid from fine-needle aspiration of thyroid nodules in the diagnosis of medullary thyroid cancer. Endocr Pract. 2013;19(5):769-779.
doi pubmed - Kratzsch J, Petzold A, Raue F, Reinhardt W, Brocker-Preuss M, Gorges R, Mann K, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin Chem. 2011;57(3):467-474.
doi pubmed - Becker KL, Snider RH, Silva OL, Moore CF. Calcitonin heterogeneity in lung cancer and medullary thyroid cancer. Acta Endocrinol (Copenh). 1978;89(1):89-99.
doi - Goltzman D, Tischler AS. Characterization of the immunochemical forms of calcitonin released by a medullary thyroid carcinoma in tissue culture. J Clin Invest. 1978;61(2):449-458.
doi pubmed - Leboeuf R, Langlois MF, Martin M, Ahnadi CE, Fink GD. "Hook effect" in calcitonin immunoradiometric assay in patients with metastatic medullary thyroid carcinoma: case report and review of the literature. J Clin Endocrinol Metab. 2006;91(2):361-364.
doi pubmed - Novotny AR, Luppa P, Rosenberg R, Schneider H, Maak M, Bartels H, Holzmann B, et al. Procalcitonin can be used for monitoring sepsis in patients with medullary thyroid carcinoma. Thyroid. 2009;19(11):1287-1289.
doi pubmed - Algeciras-Schimnich A, Preissner CM, Theobald JP, Finseth MS, Grebe SK. Procalcitonin: a marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2009;94(3):861-868.
doi pubmed - Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab. 2014;99(8):2986-2994.
doi pubmed - Walter MA, Meier C, Radimerski T, Iten F, Kranzlin M, Muller-Brand J, de Groot JW, et al. Procalcitonin levels predict clinical course and progression-free survival in patients with medullary thyroid cancer. Cancer. 2010;116(1):31-40.
pubmed - Bolko P, Manuszewska-Jopek E, Michalek K, Wasko R, Jaskula M, Sowinski J. Efficacy of procalcitonin measurement in patients after total thyroidectomy due to medullary thyroid carcinoma. Arch Immunol Ther Exp (Warsz). 2003;51(6):415-419.
- Giovanella L, Verburg FA, Imperiali M, Valabrega S, Trimboli P, Ceriani L. Comparison of serum calcitonin and procalcitonin in detecting medullary thyroid carcinoma among patients with thyroid nodules. Clin Chem Lab Med. 2013;51(7):1477-1481.
doi pubmed - Giovanella L. Serum procalcitonin and calcitonin normal values before and after calcium gluconate infusion. Exp Clin Endocrinol Diabetes. 2012;120(3):169-170.
doi pubmed - Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Arch Surg. 2007;142(3):289-293; discussion 294.
doi pubmed - Osamura RY, Yasuda O, Kawakami T, Itoh Y, Inada K, Kakudo K. Immunoelectron microscopic demonstration of regulated pathway for calcitonin and constitutive pathway for carcinoembryonic antigen in the same cells of human medullary carcinomas of thyroid glands. Mod Pathol. 1997;10(1):7-11.
pubmed - Fischer S, Asa SL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008;132(3):359-372.
pubmed - Trimboli P, Cremonini N, Ceriani L, Saggiorato E, Guidobaldi L, Romanelli F, Ventura C, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol (Oxf). 2014;80(1):135-140.
doi pubmed - Trimboli P, Rossi F, Baldelli R, Laurenti O, Nigri G, Ventura C, Appetecchia M, et al. Measuring calcitonin in washout of the needle in patients undergoing fine needle aspiration with suspicious medullary thyroid cancer. Diagn Cytopathol. 2012;40(5):394-398.
doi pubmed - Trimboli P, Nigri G, Romanelli F, Cicciarella Modica DD, Crescenzi A, Valabrega S, Giovanella L. Medullary thyroid nodules by measurement of calcitonin (Ct) in aspiration needle washout in patients with multinodular goiter and moderately elevated serum Ct. Exp Clin Endocrinol Diabetes. 2012;120(4):234-237.
doi pubmed - Boi F, Maurelli I, Pinna G, Atzeni F, Piga M, Lai ML, Mariotti S. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(6):2115-2118.
doi pubmed - Abraham D, Gault PM, Hunt J, Bentz J. Calcitonin estimation in neck lymph node fine-needle aspirate fluid prevents misinterpretation of cytology in patients with metastatic medullary thyroid cancer. Thyroid. 2009;19(9):1015-1016.
doi pubmed - Massaro F, Dolcino M, Degrandi R, Ferone D, Mussap M, Minuto F, Giusti M. Calcitonin assay in wash-out fluid after fine-needle aspiration biopsy in patients with a thyroid nodule and border-line value of the hormone. J Endocrinol Invest. 2009;32(4):308-312.
doi pubmed - Bihan H, Becker KL, Snider RH, Nylen E, Vittaz L, Lauret C, Modigliani E, et al. Calcitonin precursor levels in human medullary thyroid carcinoma. Thyroid. 2003;13(8):819-822.
doi pubmed - Giovanella L, Maffioli M, Suriano S, Dorizzi RM. Elevated calcitonin and procalcitonin levels in nonmedullary benign and malignant thyroid nodules. Clin Endocrinol (Oxf). 2010;72(6):852-853.
doi pubmed - de Crea C, Raffaelli M, Maccora D, Carrozza C, Canu G, Fadda G, Bellantone R, et al. Calcitonin measurement in fine-needle aspirate washouts vs. cytologic examination for diagnosis of primary or metastatic medullary thyroid carcinoma. Acta Otorhinolaryngol Ital. 2014;34(6):399-405.
pubmed - Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin Cancer Res. 2013;19(9):2283-2288.
doi pubmed - Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
