| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 1, Number 3, August 2011, pages 113-124
Influence of Efavirenz and Nevirapine on the Pharmacodynamics and Pharmacokinetics of Gliclazide in Rabbits
Kilari Eswar Kumara, Shaik Mastanb, c, d
aPharmacology Division, AU College of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, Andhra Pradesh, India
bResearch Scholar, Research and Development Cell, Jawaharlal Nehru Technological University, Hyderabad-500 085, Andhra Pradesh, India
cCytel Statistical Software and Services Pvt Ltd, Pune-411029, Maharashtra, India
dCorresponding author: Shaik Mastan, Cytel Statistical Software and Services Pvt Ltd, (Subsidiary of Cytel Inc., USA), 8th Floor, Siddharth Towers, Off Karve Road, Kothrud, Pune-411029, Maharashtra, India
Manuscript accepted for publication August 8, 2011
Short title: NNRTIs on Gliclazide Activity
doi: https://doi.org/10.4021/jem34w
| Abstract | ▴Top |
Background: Since polypharmacy is very common practice in diabetic patients, the study of drug-drug interactions is an imperative rational approach with respect to safety and efficacy determination. Gliclazide is a widely used drug for the treatment of type 2 diabetes. Efavirenz and nevirapine are widely used non-nucleoside reverse transcriptase inhibitors (NNRTIs) with fewer side effects concerning diabetic complications.The objective of the study is to investigate the effect of selected NNRTIs on the pharmacodynamics and pharmacokinetics of gliclazide in rabbits with respect to safety and efficacy of the combination.
Methods: Influence of selected NNRTIs on the activity of gliclazide was determined by conducting a single dose interaction followed by multiple dose interaction study with two groups consisting of 6 normal rabbits each. Each group was treated with an oral dose of 5.6 mg/1.5 kg bd. wt. of gliclazide, interacting drug treatment (42mg/1.5 kg bd. wt. of efavirenz or 14 mg/1.5 kg bd. wt. of nevirapine) and their combination with a one week washout period between each treatment. One week washout after this single dose interaction study, each group was continued with the daily treatment of interacting drug for the next eight days and then the combined treatment on the ninth day. Blood samples were collected at regular intervals by marginal ear vein puncture in rabbits and were analyzed for blood glucose by GOD/POD method and insulin by radioimmunoassay method. The serum gliclazide levels were estimated by HPLC method and pharmacokinetic analysis was conducted by noncompartmental analysis using WinNonlin software.
Results: In combination, efavirenz significantly (P < 0.05) reduced the pharmacodynamic activity and serum levels of gliclazide. The pharmacokinetic parameters of gliclazide were significantly (P < 0.05) altered. The percent decrease of serum gliclazide concentration level is 24.23% and 15.20% following single dose and multiple dose administration of efavirenz, respectively. In combination, nevirapine has no significant effect on the pharmacodynamics and pharmacokinetics of gliclazide in rabbits.
Conclusions: The significant pharmacokinetic interaction of efavirenz at metabolic level by CYP3A4 induction results in decreased serum gliclazide levels and pharmacodynamic activity of gliclazide. The combination of nevirapine and gliclazide was proved to be safe.
Keywords: Gliclazide; Efavirenz; Nevirapine; Diabetes; HIV infection; Pharmacokinetics
| Introduction | ▴Top |
Polypharmacy is very common practice for the patients suffering with chronic diseases such as diabetes mellitus and HIV infection, and thus leads to the undesirable potent drug-drug interactions (pharmacodynamic and/orpharmacokinetic) which can alter the safety and efficacy profile of a drug in many ways. Recent reports [1, 2] reveals that drug interactions played a vital role in reported adverse events and that majority of the drugs withdrawn for safety reasons from the US market were related with significant drug-drug interactions. The importance of this fact is further emphasized by increased post marketing adverse event reports by 240% over the last decade [3].
There is a propensity for drug-drug interactions in patients with concurrent type 2 diabetes mellitus and HIV infection that are likely to be treated with antiretroviral and antidiabetic therapy. Diabetes mellitus is a metabolic disorder that needs treatment for prolonged periods and maintenance of normal blood glucose level is very important in this condition, since both hyperglycemia as well as hypoglycemia is unwanted phenomenon [4, 5]. Since many studies suggested that PI therapy [6, 7] is linked to the development of diabetic complications, it is of importance to propose therapeutic strategies with fewer side effects, such as the use of the non-nucleoside reverse transcriptase inhibitors (NNRTIs) and this approach appear successful to control HIV infection [8-10]. In this contest, there are more chances of co-administration of NNRTIs with oral hypoglycemic drugs in patients with concurrent type 2 diabetes mellitus and HIV infection which may leads to potent drug-drug interactions. However, there is no much information available to elucidate the mechanisms of drug interactions between NNRTIs and oral hypoglycemic drugs which are essential to the clinicians to prescribe the rational drug combinations with respect to safety and efficacy.
Oral hypoglycemic agents are used in the treatment of type 2 diabetes, among which gliclazide; a second generation sulphonylurea derivative is preferred in therapy because of its antidiabetic activity and other beneficial effects include antioxidant property, low incidence of hypoglycemia and other haemobiological effects [11-14]. Efavirenz and nevirapine are widely used NNRTIs to treat HIV infection. Based on this background, formerly we have conducted a preliminary study [4] to investigate the effect of efavirenz and nevirapine on the pharmacodynamic activity of gliclazide in rats (normal and diabetic) and rabbits with respect to blood glucose levels only. However, determination of insulin along with blood glucose levels would be a more precious and dependent approach to conclude a clear pharmacodynamic interaction scenario in the view of clinical and scientific stand-point. Since the pharmacodynamic (PD) activity of a drug depends on its pharmacokinetics (PK), the PK-PD interaction should be concurrently performed in same group in order to undoubtedly conclude the effect/relation of PK interaction on the pharmacodynamics as a consequence. So the present study was planned to investigate the effect of selected NNRTIs (efavirenz and nevirapine) on the pharmacodynamics (glucose and insulin levels) and pharmacokinetics of gliclazide in rabbits with respect to safety and efficacy of the combination.
| Materials and Methods | ▴Top |
Drugs and chemicals
Gliclazide and NNRTIs are the gift samples from Micro Labs (Bangalore, India) and Aurobindo Pharma Ltd (Hyderabad, India), respectively. Glucose kits (Span diagnostics) were purchased from local pharmacy. Acetonitrile (HPLC grade), orthophosphoric acid (AR grade) and dichloromethane (AR grade) were obtained from Qualigens Chemicals (Mumbai, India), SD Fine Chemicals (Mumbai, India) and Loba Chemie Pvt. Ltd., (Mumbai, India), respectively. All other reagents or chemicals used were of analytical grade.
Animals
Normal albino rabbits of either sex of 3 months of age, weighing between 1.5-2 Kg were procured from National Institute of Nutrition, Hyderabad, India. Animals were fed with a commercial pellet diet (Rayan’s Biotechnologies Pvt Ltd., Hyderabad, India) and water ad libitum. The animal experiments were performed after prior approval of the study protocol by the Institutional Animal Ethics Committee. The animals were maintained under standard laboratory conditions and the study was conducted in accordance with the guidelines provided by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Study design
Concerning the use of NNRTIs and gliclazide in clinical practice, human therapeutic oral doses were extrapolated to rabbit based on body surface area [15], and these doses were administered orally [4, 16, 17]. The study design is shown in Figure 1 for more information.
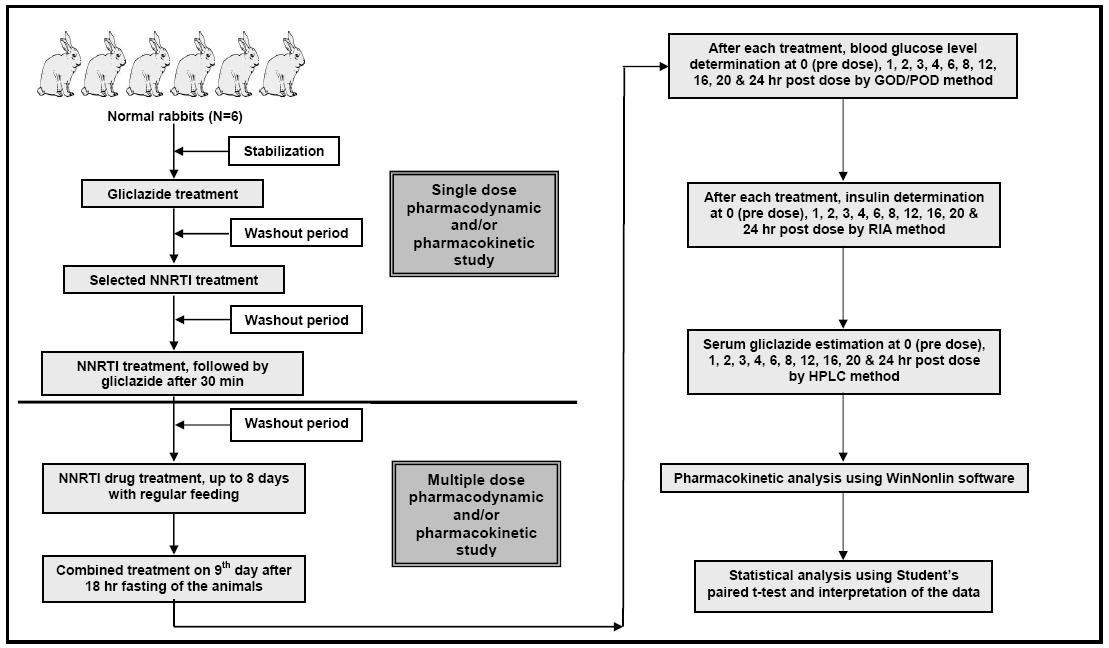 Click for large image | Figure 1. Study design for conducting pharmacodynamic and pharmacokinetic interaction studies of selected non-nucleoside reverse transcriptase inhibitors (NNRTIs) with gliclazide in rabbits. |
This study consists of two groups:
Group 1: Interaction study of efavirenz and gliclazide in rabbits (n = 6);
Group 2: Interaction study of nevirapine and gliclazide in rabbits (n = 6).
Each group of six rabbits was administered with 5.6 mg/1.5 kg bd. wt. of gliclazide, orally. The same group was administered with interacting drug (efavirenz 42mg/1.5 kg bd. wt. or nevirapine 14 mg/1.5 kg bd. wt., orally) and the combination of interacting drug and gliclazide. One week washout period was maintained between each treatment. One week washout after this single dose interaction study, the respective group was continued with the daily treatment of interacting drug (efavirenz/nevirapine) for the next eight days with regular feeding. Later after 18 h fasting they were again given the combined treatment on the ninth day.
Blood sampling and determination of blood glucose and insulin
Blood samples were withdrawn from the marginal ear vein of each rabbit at 0, 1, 2, 3, 4, 6, 8, 12, 16, 20 and 24 h. These blood samples were analyzed for blood glucose by GOD/POD method [18] using commercial glucose kits and insulin by Radioimmunoassay method [19] using a commercially available kit (Biomedica, Saluggia, Italy) as per the instructions provided by the manufacturers.
Chromatography
The serum gliclazide concentrations were determined by HPLC method [20], briefly, a gradient High Pressure Liquid Chromatograph (Shimadzu HPLC Class VP series) equipped with the software "Class-VP series version 6.12 SP2 (Shimadzu) was used. Nicorandil was used as internal standard and the mobile phase consisted of acetonitrile and triple distilled water in the ratio of 30:70 (Acetonitrile: triple distilled water containing 0.5% triethylamine). The mobile phase was eluted at a flow rate 0.8 mL/min and the effluent was monitored at a wavelength of 230 nm. The ratio of peak area of gliclazide to that of internal standard was used for the quantification of gliclazide in serum samples. The HPLC method was validated in terms of reproducibility, system suitability, recovery, accuracy and precision and then applied for the estimation of gliclazide in rabbit serum.
Pharmacokinetic analysis
The peak concentration in plasma (Cmax) and concentration peak time (Tmax) were directly read from the concentration-time data. Other pharmacokinetic parameters were determined on subjecting the concentration-time data to non-compartmental analysis using WinNonlin (version 5.0.1) software. The elimination rate constant (Kel) was determined by linear regression analysis of the log-linear part of the plasma drug concentration-time curve. A minimum of three data points were used to calculate the terminal half-life (T½ = ln2/Kel). Area under the concentration-time curve (AUC) was calculated by use of the linear trapezoidal rule with extrapolation to infinity by dividing the last measured concentration by Kel. The mean residence time (MRT) was calculated using formula, MRT = AUMC0-Inf/AUC0-Inf.
Data and statistical analysis
Data are presented as mean ± SD. P < 0.05 was considered significant and it was determined by applying Student’s paired t-test.
| Results | ▴Top |
Chromatography
The calibration curve in the rabbit serum for gliclazide was linear within the concentration range of 20-1000 ng/mL. The intra-day % accuracy (% CV) for 20, 100, 500 and 1000 ng/mL of gliclazide was 100.20 (0.20), 99.92 (1.72), 99.25 (1.25), 99.29 (1.26), respectively. The inter-day % accuracy (% CV) for 20, 100, 500 and 1000 ng/mL of gliclazide was 100.55 (0.15), 99.86 (1.32), 99.25 (1.20), 98.92 (1.28), respectively. The system suitability parameters of gliclazide were determined as limit of quantification (ng/mL)-20, theoretical plates-12 248, tailing factor-1.14, retention time of gliclazide-7.82 to 8.32 minutes, retention time of internal standard (I.S.)-6.02 to 6.44 minutes, and resolution between drug peak and I.S. peak-2.316. The standard graph and chromatogram are shown in Figures 2, 3.
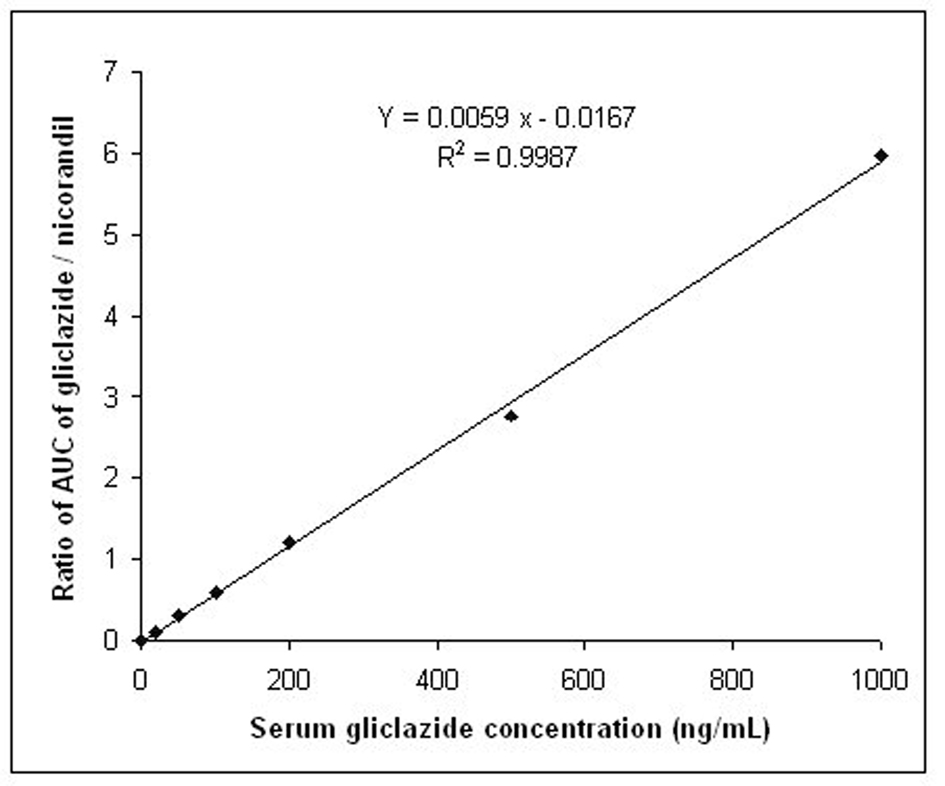 Click for large image | Figure 2. Standard graph for the estimation of gliclazide levels in rabbit serum. |
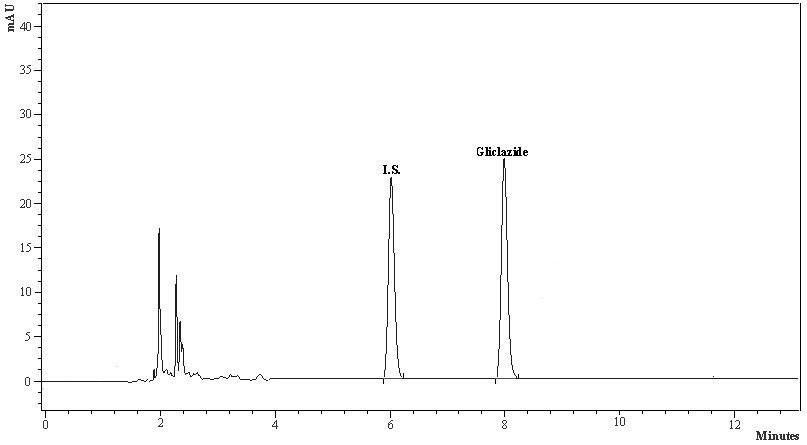 Click for large image | Figure 3. The typical HPLC chromatogram of gliclazide and internal standard. |
Pharmacodynamic interaction study of efavirenz with gliclazide
The percent blood glucose reduction and insulin levels of gliclazide in presence and absence of efavirenz are shown in Table 1 and Figure 4, respectively. Gliclazide produced hypoglycemic activity with maximum reduction of 32.82 ± 5.34% and maximum insulin level of 22.32 ± 1.29 at 3 h in normal rabbits. Efavirenz alone has not produced any significant effect on the blood glucose and insulin levels. Where as in combination, efavirenz significantly (P < 0.05) reduced the gliclazide activity (by decreasing its hypoglycemic activity and insulin levels) and the reduction was more significant with the single dose treatment of efavirenz than multiple dose treatment.
 Click to view | Table 1. Mean Percent Blood Glucose Reduction of Gliclazide in Presence and Absence of Efavirenz in Rabbits (n = 6) |
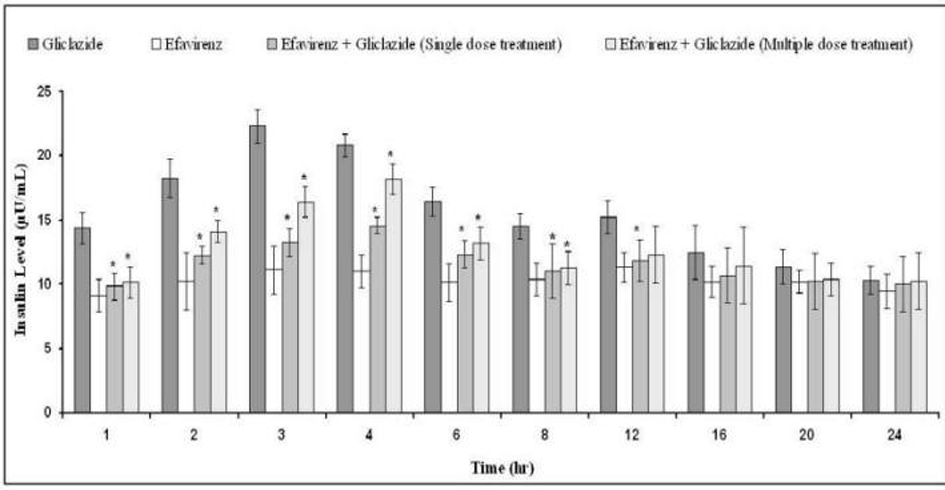 Click for large image | Figure 4. Mean insulin levels (µU/mL) of gliclazide before and after treatment with efavirenz in normal rabbits (n = 6) (Data are expressed as mean ± SD; *Significant difference at P < 0.05 compared to gliclazide control). |
Pharmacokinetic interaction study of efavirenz with gliclazide
The serum concentration-time profiles of gliclazide following single dose and multiple dose administration of efavirenz are shown in Figure 5. Mean pharmacokinetic data of gliclazide in presence and absence of efavirenz is summarized in Table 2. The serum gliclazide concentrations were decreased and pharmacokinetic parameters of gliclazide like Cmax, Tmax, AUC, AUMC, MRT, Kel, Cl, and T½ were significantly altered (Table 2) with single- and multiple-dose treatment of efavirenz. Efavirenz significantly decreased the serum gliclazide concentration level by 24.23% and 15.20% following single dose and multiple dose administration of efavirenz, respectively. Efavirenz significantly decreased overall plasma exposure (AUC) and peak concentration (Cmax) while increasing the clearance and apparent elimination half-life of gliclazide following single- and multiple-dose treatment of efavirenz.
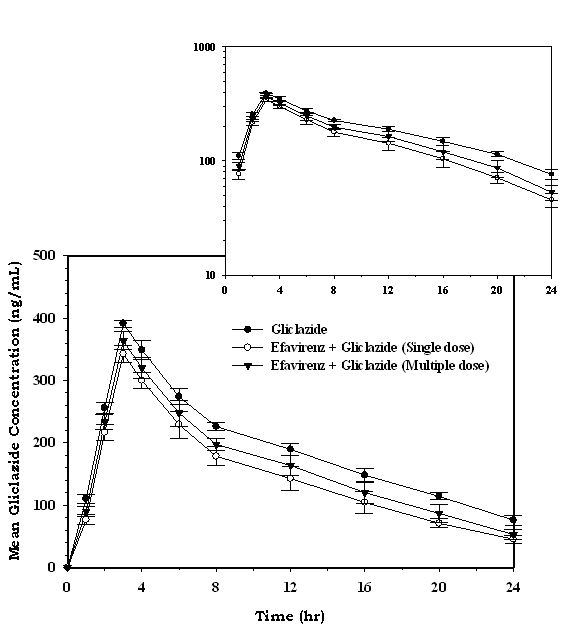 Click for large image | Figure 5. Mean serum gliclazide concentration (ng/mL) before and after treatment with efavirenz in rabbits (n = 6; inset = semi log scale) (Data are expressed as mean ± SD). |
 Click to view | Table 2. Mean Pharmacokinetic Parameters of Gliclazide Before and After Efavirenz Administration in Rabbits (n = 6) |
Pharmacodynamic interaction study of nevirapine with gliclazide
The percent blood glucose reduction and insulin levels of gliclazide in presence and absence of nevirapine are shown in Table 3 and Figure 6 respectively. Gliclazide produced hypoglycemic activity with maximum reduction of 34.03 ± 2.11% and maximum insulin level of 23.26 ± 0.65 at 3 h in normal rabbits. Nevirapine alone has not produced any significant effect on the blood glucose and insulin levels. In combination also, nevirapine has no impact on the pharmacodynamic activity of gliclazide.
 Click to view | Table 3. Mean Percent Blood Glucose Reduction of Gliclazide in Presence and Absence of Nevirapine in Rabbits (n = 6) |
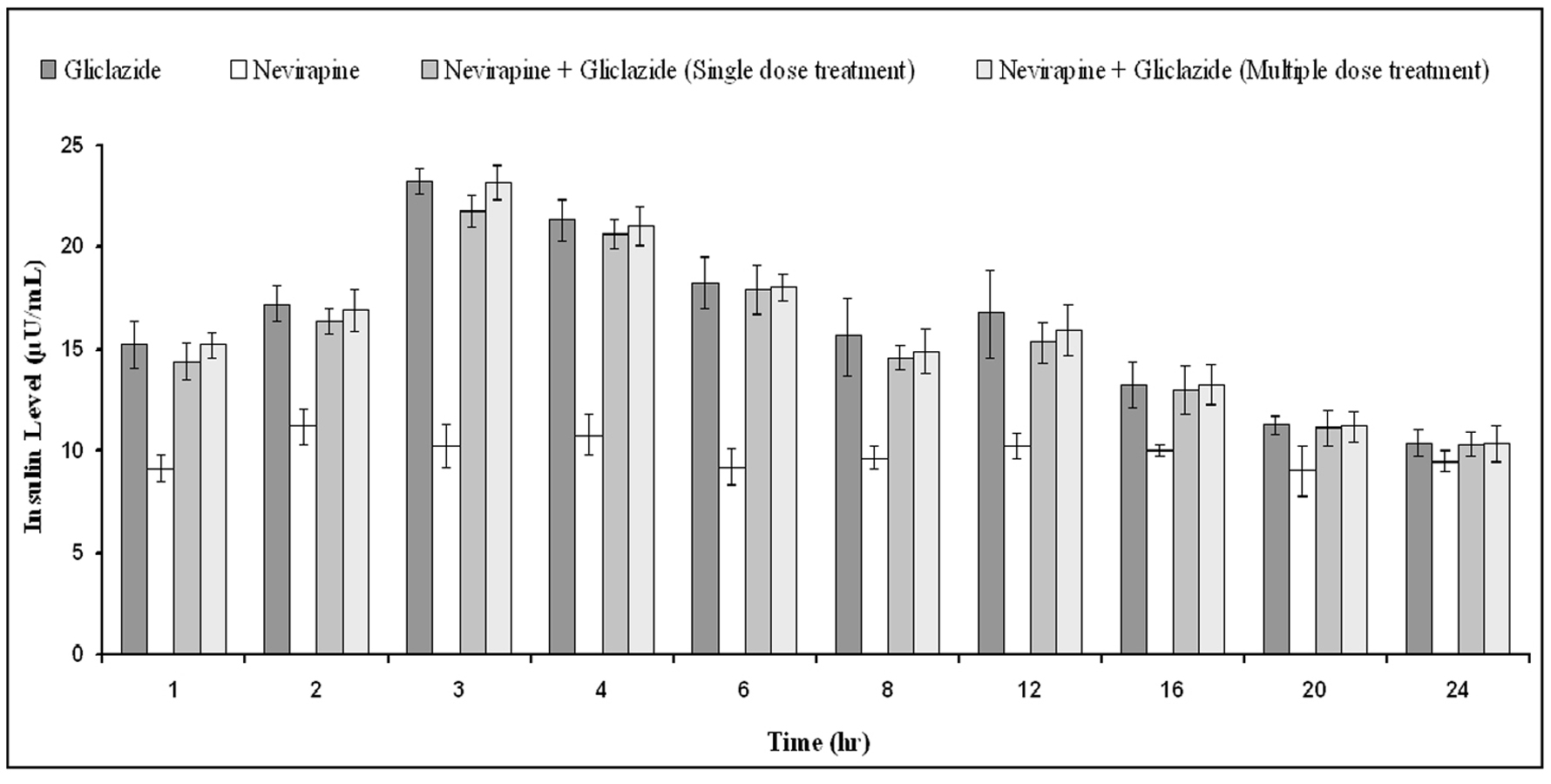 Click for large image | Figure 6. Mean insulin levels (µU/mL) of gliclazide before and after treatment with nevirapine in normal rabbits (n = 6) (Data are expressed as mean ± SD; No significant difference at P < 0.05 compared to gliclazide control). |
Pharmacokinetic interaction study of nevirapine with gliclazide
The serum concentration-time profiles of gliclazide following single dose and multiple dose administration of nevirapine are shown in Figure 7. Mean pharmacokinetic data of gliclazide in presence and absence of nevirapine is summarized in Table 4. The serum concentration levels and pharmacokinetic parameters of gliclazide were not altered significantly with nevirapine following single and multiple dose administration.
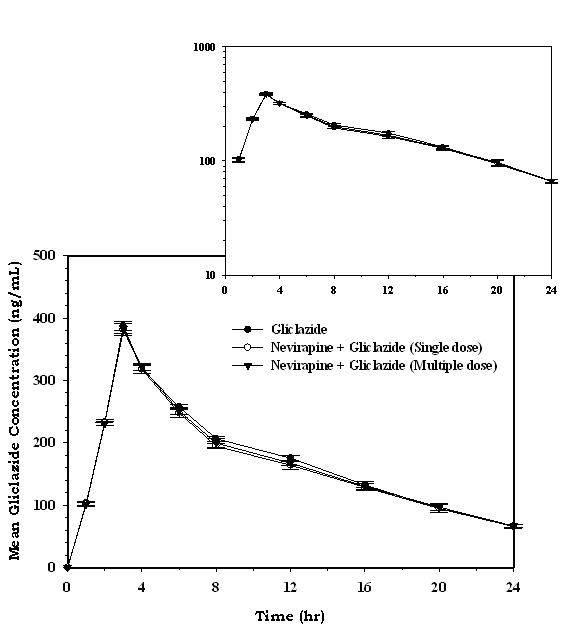 Click for large image | Figure 7. Mean serum gliclazide concentration (ng/mL) before and after treatment with nevirapine in rabbits (n = 6; inset = semi log scale) (Data are expressed as mean ± SD). |
 Click to view | Table 4. Mean Pharmacokinetic Parameters of Gliclazide Before and After Nevirapine Administration in Rabbits (n = 6) |
| Discussion | ▴Top |
Drug-drug interaction studies are an important aspect of pharmacology research and can be a critical step to optimize the use of selected drugs, especially in the treatment of chronic diseases such as HIV infection and diabetes in which the polypharmacy is very common. This study was planed to evaluate the pharmacodynamic and pharmacokinetic interactions of selected NNRTIs (efavirenz and nevirapine) with gliclazide. It is the first to evaluate efavirenz and nevirapine effects on the pharmacodynamics and pharmacokinetics of gliclazide to explore the possible mechanism of interaction, as well as the first to compare single dose and multiple dose NNRTIs influence on gliclazide disposition.
This study was designed 1) by extrapolating the human therapeutic doses (gliclazide-80 mg; efavirenz-600 mg and nevirapine-200 mg) [21-23] based on body surface area which underscores the clinical relevance of the current investigation 2) to conduct pharmacodynamic and pharmacokinetic studies concurrently in a same group in order to establish a clear association between PD-PK to explore the possible mechanism of drug interaction 3) to determine glucose and insulin levels at regular time intervals up to 24 hours to evaluate an antidiabetic drug (gliclazide) activity in order to emphasize the clinical relevance considering the strong relation between glucose and insulin levels to regulate metabolic homeostasis [24] in diabetic patients 4) to conduct single- and multiple-dose PK/PD interaction studies in order to provide valuable information on the time course and magnitude of NNRTIs interaction with gliclazide with respect to clinical prospective.
Drug interactions are generally evaluated in animal models. Although animal models can never replace the need for comprehensive studies in human subjects, their use can provide important insights to understand and to evaluate the mechanism of potent drug interactions. It is worth noting that several findings have confirmed the functional similarity of CYP forms in rabbits and humans apart from convenience in serial blood sampling design suggesting that the rabbit is a valuable in vivo model for the assessment of drug interactions [5, 19, 25-28].
Gliclazide is known to produce hypoglycaemic/antihyperglycemic activity by pancreatic (stimulating insulin secretion by blocking K+ channels in the pancreatic βcells) and extra pancreatic (increasing tissue uptake of glucose) mechanisms [11-14]. Gliclazide produced maximum blood glucose reduction, maximum insulin level and maximum serum concentration at 3 h representing the consistency between pharmacodynamic and pharmacokinetic results. Our study revealed the safety profile of efavirenz and nevirapine with respect to glucose-insulin homeostasis.These results are also consistent with the available literature [5, 19, 25, 28] and our former preliminary study [4]. But in combination, efavirenz significantly decreased the pharmacodynamic activity (hypoglycemic effect and insulin levels) of gliclazide and it confirms the presence of potent interaction between efavirenz and gliclazide. These pharmacodynamic observations are in agreement with our pharmacokinetic findings in which significant decrease in serum concentration levels and alteration in pharmacokinetic parameters of gliclazide in the presence of efavirenz. Therefore, these experimental findings explicitly convince that there is a significant pharmacokinetic interaction between efavirenz and gliclazide which has resulted in decreased serum gliclazide levels and subsequently decreased pharmacodynamic activity.
The pharmacokinetic data clearly suggest that efavirenz has not altered the onset of action (Tmax) of gliclazide, but significantly decreased the overall plasma exposure (AUC and AUMC) and peak concentration (Cmax) of gliclazide indicating overall decreased availability of gliclazide. There might not be an interaction at absorption level since oral bioavailability of efavirenz in animals is 16% [29] while gliclazide is more than 50% [30, 31], further this is emphasized by no alteration in gliclazide Tmax. Gliclazide and efavirenz are highly protein bound drugs (gliclazide: 89%; efavirenz: 99.5-99.75%) [21, 22] and hence there is every possibility for displacement of gliclazide from the protein binding sites by efavirenz and may lead to increased free gliclazide concentration levels. But in fact, the volume of distribution (Vd) of gliclazide was not significantly altered and gliclazide concentration levels were decreased in presence of efavirenz which explicitly convince that the protein displacement is not involving in this interaction. Hence, the decreased gliclazide concentration levels in the presence of efavirenz might be either in metabolism or excretion process as suggested by increased clearance andKel, and decreased MRT and T½.
Efavirenz is converted to inactive metabolites by the CYP system, primarily by CYP2B6 and CYP3A4. In vitro and in vivo studies demonstrated that efavirenz is a potent inducer of CYP3A4 in a concentration- and time-dependent manner [22, 32, 33]. Clinical drug-drug interaction studies showed that efavirenz significantly induced CYP enzymes and decreased the concentration levels of several CYP3A4 [34, 35] substrates predominantly and, CYP2C9 and CYP2C19 substrates partly [36]. Enzyme induction has important clinical implications when enhanced drug metabolism results in lower drug concentrations, which leads to a suboptimal efficacious response or, even worse, the development of drug resistance. Enzyme induction can be due to (i) a drug affecting its own metabolism (autoinduction), (ii) co-medication(s) with induction capability, or (iii) both. Gliclazide is known to be metabolized by CYP2C9 primarily and partly by CYP3A4 [5, 25, 28]. Gliclazide is primarily eliminated via renal (60-70%) [21] while efavirenz is primarily eliminatedthrough feces (16-61%) [22]. Based on this context, pharmacokinetic results such as increased Cl and Kel and decreased MRT and T½ suggest that efavirenz causes time-dependent induction of gliclazide metabolism which in turn results induction of clearance and subsequently decreased gliclazide serum concentrations. This type of efavirenz induced metabolism and clearance was reported in other preclinical [29] and clinical studies [33, 34] also.
In this study, interestingly, the multiple dose treatment of efavirenz on gliclazide activity (pharmacodynamics, serum concentration level and pharmacokinetics) is relatively less compared to single dose treatment. As per the literature, administration of multiple doses of efavirenz results in decreased exposure in humans and animals, suggesting an autoinduction of efavirenz metabolism [29, 32, 33, 36, 37]. The plasma half-life of efavirenz in animals is approximately 0.8 to 1.9 h compared to more than 40 h in humans [38, 39]. In this study, as a part of multiple dose interaction, efavirenz was administered daily up to 9 days which was estimated to be enough time to achieve steady state of efavirenz considering its half life of 0.8 to 1.9 h in animals, representing the clinical relevance. So autoinduction of efavirenz might be the reason behind the less impact of multiple dose treatment of efavirenz on the activity of gliclazide in rabbits.
On other hand, minor and non-significant decrease in gliclazide activity was observed following single and multiple dose administration of nevirapine.Nevirapine is known to be an inducer of CYP3A4 and CYP2B6 [23] and thus leads to this minor and non-significant decreased gliclazide activity. These results are consistent with the literature and our former preliminary study. However, there was no significant interaction (either pharmacodynamic or pharmacokinetic) between nevirapine and gliclazide and thus proved to be a safe combination.
There are recognized potential limitations with this investigation. First, the preclinical results may not be correlated directly to human beings considering inter-species metabolic variations. Second, NNRTIs effects were evaluated in healthy animal models rather than diabetic and HIV-infected validated models. Third, the role of P-glycoprotein or other efflux membrane proteins in this interaction has to be determined in further study. Nevertheless, the consistency observed in the pharmacodynamics, serum concentration levels and pharmacokinetics in this study underscore the clinical relevance of the current investigation.
Conclusion
The results confirmed the presence of pharmacokinetic interaction of efavirenz with gliclazide at metabolic level by CYP3A4 induction and thus result in decrease in pharmacodynamic activity of gliclazide in normal rabbits. This combination needs dose adjustment and care should be taken when this combination is prescribed for the clinical benefit. The combination of nevirapine and gliclazide was proved to be safe.
Acknowledgments
The authors are thankful to M/s. Aurobindo Pharma Ltd, Hyderabad and M/s. Micro Labs, Bangalore for supplying gift samples of NNRTIs and gliclazide, respectively. The authors are grateful to Cytel Management, Pune for the kind help and support during the pharmacokinetic analysis.
Conflict of interests
The authors declare that they have no competing interests.
| References | ▴Top |
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15-19.
pubmed doi - Huang SM, Lesko LJ. Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J Clin Pharmacol. 2004;44(6):559-569.
pubmed doi - Huang SM, Strong JM, Zhang L, Reynolds KS, Nallani S, Temple R, Abraham S, et al. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol. 2008;48(6):662-670.
pubmed doi - Mastan S, Kumar KE. Influence of non-nucleoside reverse transcriptase inhibitors (efavirenz and nevirapine) on the pharmacodynamic activity of gliclazide in animal models. Diabetol Metab Syndr. 2009;1(1):15.
pubmed - Satyanarayana S, Kilari EK. Influence of nicorandil on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. Mol Cell Biochem. 2006;291(1-2):101-105.
pubmed doi - Hruz PW. Molecular Mechanisms for Altered Glucose Homeostasis in HIV Infection. Am J Infect Dis. 2006;2(3):187-192.
pubmed doi - Dube MP. Disorders of glucose metabolism in patients infected with human immunodeficiency virus. Clin Infect Dis. 2000;31(6):1467-1475.
pubmed doi - Martinez E, Arnaiz JA, Podzamczer D, Dalmau D, Ribera E, Domingo P, Knobel H, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med. 2003;349(11):1036-1046.
pubmed doi - Saag MS, Powderly WG, Schambelan M, Benson CA, Carr A, Cirrier JS. Switching antiretroviral drugs for treatment of metabolic complications in HIV-1 infection: summary of selected trials. Topics in HIV Med. 2002;10(1):47-51.
- Martinez E, Conget I, Lozano L, Casamitjana R, Gatell JM. Reversion of metabolic abnormalities after switching from HIV-1 protease inhibitors to nevirapine. AIDS. 1999;13(7):805-810.
pubmed doi - Schernthaner G. Gliclazide modified release: A critical review of pharmacodynamic, metabolic, and vasoprotective effects. Metabolism. 2003;52(8 Suppl 1):29-34.
pubmed doi - O'Brien RC, Luo M, Balazs N, Mercuri J. In vitro and in vivo antioxidant properties of gliclazide. J Diabetes Complications. 2000;14(4):201-206.
pubmed doi - Fava D, Cassone-Faldetta M, Laurenti O, De Luca O, Ghiselli A, De Mattia G. Gliclazide improves anti-oxidant status and nitric oxide-mediated vasodilation in Type 2 diabetes. Diabet Med. 2002;19(9):752-757.
pubmed doi - Ziegler O, Drouin P. Hemobiological properties of gliclazide. J Diabetes Complications. 1994;8(4):235-239.
pubmed doi - Paget GE, Barnes JM. From toxicity tests. In evaluation of drug activities: Pharmacometrics. Volume 1. Edited by Laurence DR and Bacharach AL. London: Academic Press; 1964: 50-161.
- Berruet N, Sentenac S, Auchere D, Gimenez F, Farinotti R, Fernandez C. Effect of efavirenz on intestinal p-glycoprotein and hepatic p450 function in rats. J Pharm Pharm Sci. 2005;8(2):226-234.
pubmed - Kishimoto W, Takano J, Senda C, Ishiguro N, Sakai K, Igarashi T. Quantitative prediction of in vivo drug interactions between nevirapine and antifungal agents from in vitro data in rats. Biol Pharm Bull. 2000;23(9):1027-1032.
pubmed - Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22(2):158-161.
pubmed doi - Mastan SK, Kumar KE. Influence of atazanavir on the pharmacodynamics and pharmacokinetics of gliclazide in animal models. International Journal of Diabetes Mellitus.2010;2:56–60.
- Kumar KE, Ramesh A, Yadav RS, Satyanarayana S. Determination of gliclazide in rabbit serum by RP-HPLC. Acta Ciencia Indica Chem.2007;33:273–278.
- Product Monograph. Gliclazide 80 mg Tablets, BP. Available at http://webprod3.hc-sc.gc.ca/dpd-bdpp/item-iteme.do?pm-mp=00003822. Accessed on 15th July 2011.
- SUSTIVA® U.S. FDA Approval: 1998. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021360s018,020972s031lbl.pdf. Accessed on 15th July 2011.
- VIRAMUNE® U.S. FDA Approval: 1996. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020636s027,020933s017lbl.pdf. Accessed on 15th July 2011.
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, et al.Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57-63.
pubmed doi - Satyanarayana S, Kumar KE, Rajasekhar J, Thomas L, Rajanna S, Rajanna B. Influence of aqueous extract of fenugreek-seed powder on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. Therapy.2007;4:457-463.
- Fang HM, Xu JM, Mei Q, Diao L, Chen ML, Jin J, Xu XH. Involvement of cytochrome P450 3A4 and P-glycoprotein in first-pass intestinal extraction of omeprazole in rabbits. Acta Pharmacol Sin. 2009;30(11):1566-1572.
pubmed doi - Nakamura T, Okada K, Nagata K, Yamazoe Y. Intestinal cytochrome P450 and response to rifampicin in rabbits. Jpn J Pharmacol. 2000;82(3):232-239.
pubmed doi - Kumar KE, Ramesh A, Satyanarayana S. Pharmacodynamic and pharmacokinetic drug interaction of gliclazide and lacidipine in animal models. Ind J Pharm Educ Res.2008;42:277-282.
- Balani SK, Kauffman LR, deLuna FA, Lin JH. Nonlinear pharmacokinetics of efavirenz (DMP-266), a potent HIV-1 reverse transcriptase inhibitor, in rats and monkeys. Drug Metab Dispos. 1999;27(1):41-45.
pubmed - Salami HA, Butt G, Tucker I, Skrbic R, Kon SG, Mikov M. Probiotic treatment proceeded by a single dose of bile acid and gliclazide exert the most hypoglycemic effect in type 1 diabetic rats. Med Hypotheses Res.2008;4:93-101.
- Obaid R, Ali O, Saify ZS, Kamil N, Khan MH, Ahmed T, Ahmed SW. Pharmacokinetic differences of some generic tablet gliclazide 80 mg on Pakistani population. Pak J Pharm Sci. 2004;17(1):55-64.
pubmed - Zhu M, Kaul S, Nandy P, Grasela DM, Pfister M. Model-based approach to characterize efavirenz autoinduction and concurrent enzyme induction with carbamazepine. Antimicrob Agents Chemother. 2009;53(6):2346-2353.
pubmed doi - Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72(1):1-9.
pubmed doi - Falloon J, Piscitelli S, Vogel S, Sadler B, Mitsuya H, Kavlick MF, Yoshimura K, et al. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: pharmacokinetic drug interactions and antiviral activity. Clin Infect Dis. 2000;30(2):313-318.
pubmed doi - Aarnoutse RE, Grintjes KJ, Telgt DS, Stek M, Jr., Hugen PW, Reiss P, Koopmans PP, et al. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin Pharmacol Ther. 2002;71(1):57-67.
pubmed doi - Liu P, Foster G, LaBadie RR, Gutierrez MJ, Sharma A. Pharmacokinetic interaction between voriconazole and efavirenz at steady state in healthy male subjects. J Clin Pharmacol. 2008;48(1):73-84.
pubmed doi - Robertson SM, Penzak SR, Lane J, Pau AK, Mican JM. A potentially significant interaction between efavirenz and phenytoin: a case report and review of the literature. Clin Infect Dis. 2005;41(2):e15-18.
pubmed doi - Smith PF, DiCenzo R, Morse GD. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin Pharmacokinet. 2001;40(12):893-905.
pubmed doi - Scientific Discussion on Sustiva. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000249/WC500058308.pdf. Accessed on July 15th 2011.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
