| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 3, Number 1-2, April 2013, pages 41-46
Central Diabetes Insipidus Diagnosed After Gynecologic Surgery: A Case Report
Nobuhiro Akuzawaa, c, Naoyuki Haradaa, Noriko Hasegawaa, Hidenori Sekia, Yuko Okua, Masayuki Totsukaa, Takashi Hatoria, Atsushi Murakamia, Kunihiko Imaia, Yonosuke Kitaharaa, Masahiko Tashiroa, Masahiko Kurabayashib
aDepartment of Internal Medicine, Social Insurance Gunma Chuo General Hospital, 1-7-13 Koun-cho, Maebashi, Gunma 371-0025, Japan
bDepartment of Medicine and Biological Science, Gunma University Graduate School of Medicine, 3-39-22 Showa-machi, Maebashi, Gunma 371-8511, Japan
cCorresponding author: Nobuhiro Akuzawa, Social Insurance Gunma Chuo General Hospital, 1-7-13 Koun-cho, Maebashi, Gunma 371-0025, Japan
Manuscript accepted for publication January 22, 2013
Short title: Central Diabetes Insipidus Diagnosed
doi: https://doi.org/10.4021/jem147e
| Abstract | ▴Top |
A 41-year-old woman with non-alcoholic fatty liver disease and complex endometrial hyperplasia with atypia was admitted to our hospital to undergo hysterectomy. Her weight was 107 kg, having doubled after delivery of her second child. After a routine hysterectomy, unexpected polyuria was observed. Investigation revealed a relatively low plasma vasopressin concentration, and absence of hyperintense signals in the region of the posterior lobe of the pituitary gland on T1-weighted magnetic resonance imaging, suggesting partial central diabetes insipidus. It was concluded that excessive intake of sweetened carbonated beverages due to thirst caused by diabetes insipidus had contributed to her obesity.
Keywords: Central diabetes insipidus; Magnetic resonance imaging; Obesity; Polyuria; Vasopressin
| Introduction | ▴Top |
Central diabetes insipidus (CDI) is caused by a decreased arginine vasopressin level, and is characterized by polyuria and polydipsia. This condition occurs equally in both sexes and affects all age groups, with the most frequent age of onset being between 10 and 20 years [1]. Patients are classified as having partial CDI or complete CDI based on the findings of a water deprivation test [2]. Complete CDI is less common than partial CDI [1]. In partial CDI, moderately excessive diuresis may be the only symptom, and plasma osmolarity usually only slightly exceeds 290 mOsm/kg (normal range: 280 - 295 mOsm/kg) [3].
CDI may result from any condition that impairs the synthesis, transport or release of arginine vasopressin. Known causes of secondary CDI include tumors, infections, trauma, and other conditions such as histiocytosis and vascular lesions [1, 2]. Idiopathic CDI is caused by selective hypofunction of the hypothalamic-neurohypophysial system, and accounts for 20-50% of all cases of CDI [4]. Patients often experience weight loss induced by excessive loss of free water [5].
Here, we describe a rare case of weight gain associated with partial CDI that was initially misdiagnosed as simple obesity. Observation of polyuria and hypernatremia after gynecologic surgery provided an opportunity to make the correct diagnosis. A detailed medical history revealed that excessive intake of sweetened carbonated beverages due to thirst caused by CDI may have contributed to her marked weight gain.
| Case Report | ▴Top |
A 41-year-old woman was admitted to the Department of Obstetrics and Gynecology at our hospital to undergo hysterectomy for complex endometrial hyperplasia with atypia. She had been diagnosed with simple endometrial hyperplasia 2 years previously, and surgical treatment was recommended because of progression of cellular atypia and endometrial thickening. Prior to surgery, she underwent medical review to evaluate the risks of general anesthesia in the presence of her impaired liver function.
When she first visited our department, she reported no thirst, polyuria or polydipsia. She had no other significant medical history or family history, was not taking any medications and denied alcohol intake. Her height was 153 cm and weight was 107 kg. Her body mass index was 45.7 kg/m2, indicating severe obesity. Her weight had been 58 kg at the time of the birth of her second child at age 35, and had increased by about 10 kg per year since then. Previous testing at another general hospital had shown normal plasma concentrations of thyroid and adrenocortical hormones. Her menstrual cycle had been irregular over the previous 2 years.
Physical examination revealed blood pressure 120/70 mmHg, pulse rate 85 beats/min, temperature 36.4 °C and oxygen saturation on room air 99%. She was alert and oriented. Her skin turgor was almost normal and she did not have a dry mouth. Her abdomen was obese and soft, with normal bowel sounds, no tenderness and no striae. Electrocardiography and chest X-ray showed no significant abnormalities. Laboratory testing 1 week before admission showed liver dysfunction and dyslipidemia, but no other abnormalities (Table 1). Her abnormal laboratory data were as follows: serum aspartate aminotransferase 49 IU/L (normal range: 10 - 38 IU/L), alanine aminotransferase 57 IU/L (normal range: 0 - 35 IU/L), γ-glutamyl transpeptidase 73 IU/L (normal range: 0 - 55 IU/L), alkaline phosphatase 321 IU/L (normal range: 105 - 320 IU/L) and low density lipoprotein-cholesterol 168 mg/dL (normal range: 70 - 139 mg/dL). Blood gas analysis on room air was normal. Urine analysis was normal, with a specific gravity of 1.012. Abdominal ultrasonography showed a normal-sized liver with poorly defined margins and a diffuse increase in echogenicity, consistent with fatty liver. No ascites was observed. It was concluded that her abnormal liver function test results were due to non-alcoholic fatty liver disease, and that general anesthesia was not contraindicated.
 Click to view | Table 1. Laboratory Test Results at 1 Week Before Admission |
During the first 24 h after hospitalization, her oral water intake was 3,200 mL and her urine output was 2,520 mL. She did not receive intravenous fluids during that time. On the day after admission, she underwent simple hysterectomy under general anesthesia with no complications. On the day of surgery, she received 3,200 mL of intravenous fluid and lost 520 mL of blood, and her urine output was 2,570 mL. No corticosteroids were administered during the perioperative period. No neoplasia was detected in the surgically resected specimen.
The next day after surgery, she received 3,200 mL of intravenous fluid and her urine output increased dramatically to 7,800 mL. Although oral food or water intake was permitted in the morning on that day, she complained of severe thirst and a compelling urge to drink water. Her plasma sodium concentration was high at 157 mEq/L. Although her urine osmolarity was < 180 mOsm/kg, her plasma osmolarity was high at 322 mOsm/kg. In spite of her hypernatremia, her urinary sodium excretion was only 22 mEq/day. Urine analysis showed a low specific gravity of 1.005 and no sediment. Her serum creatinine concentration was 1.17 mg/dL and her creatinine clearance rate was 51 mL/min. Her arterial blood pH remained normal, her serum potassium concentration was 3.9 mEq/L, and serum calcium concentration was 9.8 mg/dL, indicating that she was unlikely to be in the polyuric phase of acute tubular necrosis or to be developing secondary nephrogenic diabetes insipidus due to hypokalemia or hypercalcemia. Her basal concentrations of anterior pituitary hormones were largely within normal limits (Table 2). Her elevated plasma concentrations of aldosterone and renin were thought to be a response to volume depletion. However, her plasma arginine vasopressin concentration measured by radioimmunoassay was relatively low (0.9 pg/mL) considering her high plasma osmolarity. Testing for antipituitary, antithyroid and antinuclear antibodies was negative. T1-weighted magnetic resonance imaging of the pituitary gland showed absence of hyperintense signals and no enlargement of the neurohypophysis or thickening of the pituitary stalk (Fig. 1). She underwent a vasopressin test, and her urinary osmolarity increased to 512 mOsm/kg at 120 min after administration of 5 units of arginine vasopressin (Table 3). She was diagnosed with CDI, and was treated with 10 µg of intranasal 1-desamino-8-arginine vasopressin (DDAVP) daily. Over the following week, her serum sodium concentration gradually decreased to 140 mEq/L and her urine output decreased to < 2,500 mL/day. According to the Japanese guidelines for definitive diagnosis of CDI, administration of DDAVP was temporarily suspended, she was allowed free oral water intake, and a 5% hypertonic saline infusion test (0.05 mL/kg/min for 120 minutes) was performed. Her plasma arginine vasopressin concentration did not increase after the hypertonic saline infusion (Fig. 2), whereas her plasma osmolarity gradually increased. Both the results of the vasopressin test and the hypertonic saline infusion test met the criteria for CDI, but her 24-h urine output on the day of admission (< 3,000 mL) and her urine specific gravity (> 1.010) did not meet the criteria. We therefore made a diagnosis of partial CDI.
 Click to view | Table 2. Plasma Hormone Concentrations on the First Postoperative Day |
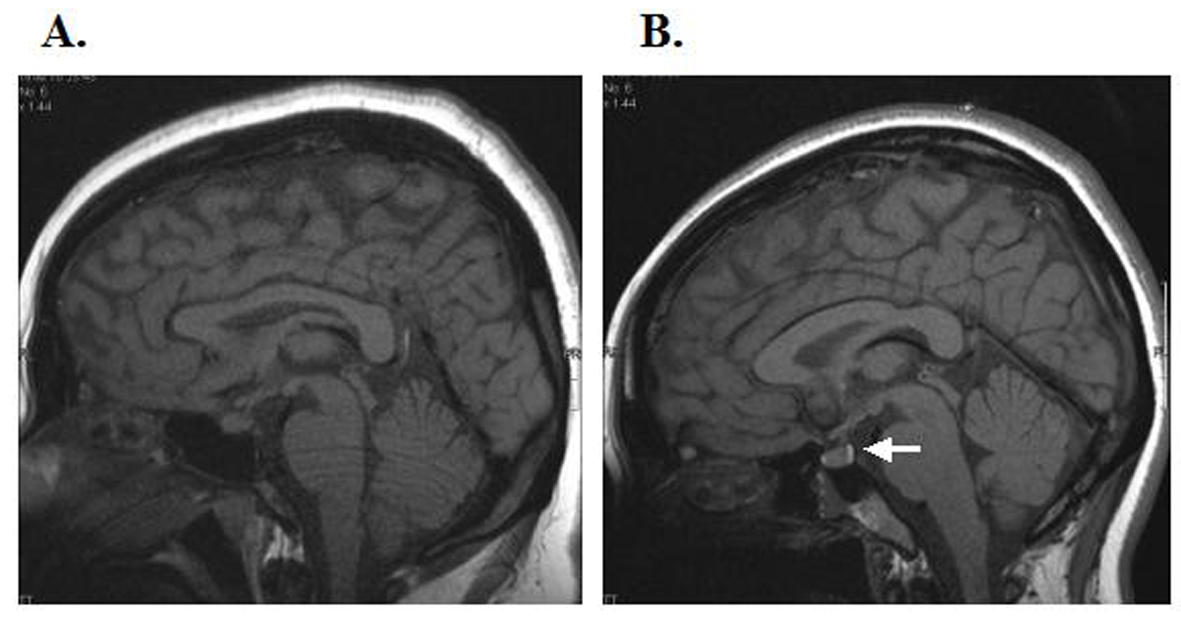 Click for large image | Figure 1. Pituitary T1-weighted magnetic resonance images of our patient (A) and a healthy young woman (B). In the young healthy woman, there is a hyperintense signal in the region of the posterior lobe of the pituitary gland (white arrow); this was absent in our patient. There was no thickening of the pituitary stalk or abnormality of the anterior lobe of the pituitary gland. |
 Click to view | Table 3. Results of Vasopressin Test |
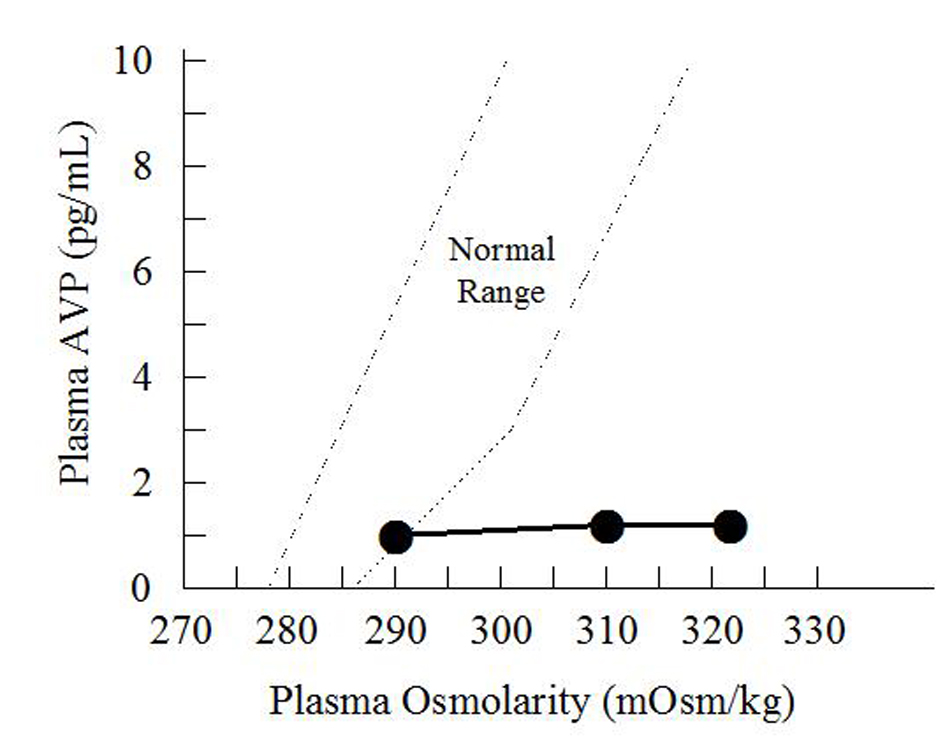 Click for large image | Figure 2. Hypertonic saline infusion test. The patient was given an intravenous infusion of 5% saline solution at 0.05 mL/kg/min for 120 min. Her arginine vasopressin (AVP) level remained low (•). The dotted lines indicate the normal range of arginine vasopressin concentration according to plasma osmolarity. |
Further questioning revealed that the patient had first noticed polyuria and extreme thirst during the last trimester of her second pregnancy. Her water intake had gradually increased from 2,500 mL/day to 3,500 mL/day over a few months. During this time she had started to drink large volumes of sweetened carbonated beverages and juice. Over the following 5 years she had gained 50 kg. It was therefore possible that the excessive consumption of these beverages due to the thirst caused by diabetes insipidus contributed to her obesity. Dietary therapy with caloric restriction initially resulted in a 5 kg weight loss over 2 months. Her DDAVP was reduced to 5 µg/day after 6 months. A year after discharge, she weighed 95 kg.
| Discussion | ▴Top |
CDI is a rare condition with an estimated prevalence of 3 per 100,000 persons [6]. We presume that our patient developed diabetes insipidus 6 years before diagnosis, during her second pregnancy. Soul et al [7] classified patients with transient diabetes insipidus during pregnancy into four major subgroups: (1) those with hepatic dysfunction such as acute fatty liver, which suppresses degradation of vasopressinase, resulting in a marked decrease in plasma arginine vasopressin concentration; (2) those with panhypopituitarism as a complication of obstetric shock, including Sheehan’s syndrome; (3) those with subclinical neurogenic diabetes insipidus and various degrees of pre-existing vasopressin deficiency; and (4) those with nephrogenic diabetes insipidus with concomitant resistance to arginine vasopressin. Our patient had not experienced severe liver dysfunction or obstetric shock associated with her second pregnancy, and her polyuria and polydipsia persisted after delivery. She also did not have a family history of diabetes insipidus, or significant abnormalities on magnetic resonance imaging. We therefore made a diagnosis of idiopathic CDI.
Undiagnosed CDI often causes a medical emergency due to severe hyperosomolarity and dehydration when fluid intake does not match obligate losses [8]. In the present case, we first noticed polyuria and hypernatremia postoperatively. Limitation of free oral water intake was probably the most significant cause of the intensification of her symptoms. Increased secretion of adrenocorticotropic hormone and corticosteroids induced by surgical stress might also have intensified her symptoms, because adrenocorticotropic hormone suppressesarginine vasopressin secretion [9] and an increased cortisol level causes sodium retention. Laboratory testing revealed a mild increase in plasma corticosteroid concentration, which may have contributed to her increased urine output. The possibility of potent adrenocortical insufficiency should also be considered because of her relatively low serum sodium concentration (139 mEq/L) and urine output (2,500 mL/day) during the preoperative period. Although no pituitary gland pathology was detected in this case, past studies have reported that lymphocytic infiltration of the neurohypophysis is associated with CDI, and lymphocytic infiltration of the adenohypophysis is associated with hypopituitarism [10, 11]. Lymphocytic adenohypophysitis occurs exclusively in young women during pregnancy [11], and causes preferential impairment of cells secreting adrenocorticotropic hormone and thyroid stimulating hormone [11]. It was previously considered that lymphocytic adenohypophysitis spared the neurohypophysis [12], but a review article reported that some patients with lymphocytic adenohypophysitis develop CDI [13]. Idiopathic adrenalitis may also occur in patients with lymphocytic adenohypophysitis [13]. We therefore suggested corticotropin-releasing hormone and adrenocorticotropic hormone tests, but the patient refused consent. Although she has shown no obvious signs of adrenocortical insufficiency since her discharge, careful follow-up is considered important.
In management of the present case, the most significant problem was that we did not diagnose CDI at the time of first contact. The main reason for this was the lack of a detailed medical history. Patients with CDI may not notice the onset of diabetes insipidus until urine output exceeds 4,000 mL/day [8]. Patients with an adequate thirst mechanism and free access to water may not develop dehydration or hypernatremia [5, 14]. However, the polydipsia and polyuria associated with CDI may be so disruptive to daily activities and sleep that patients seek medical attention [5]. Our patient had experienced interruption of sleep several times per night due to her polyuria for 6 years. The frequency of nocturnal awakening helps to determine the optimal DDAVP dose [5]. It is therefore important to obtain a careful history of sleep disturbances when evaluating patients with polyuria of unknown origin. It is also important to discriminate between solute diuresis and water diuresis [15]. For a rapid diagnosis of CDI, both elevated serum osmolarity due to an increased serum sodium concentration and inappropriately low urine osmolarity due to water diuresis are necessary [8]. Measurement of plasma arginine vasopressin concentration confirms the diagnosis, but is not available on an emergency basis [5].
In conclusion, we present a patient with weight gain associated with CDI that had been misdiagnosed as simple obesity. Observation of polyuria after gynecologic surgery provided an opportunity to diagnose CDI, and subsequently treat both her diabetes insipidus and obesity. Clinicians should be alert to the possibility of diabetes insipidus being masked by seemingly unrelated complications.
Acknowledgments
This work was done at Social Insurance Gunma Chuo General Hospital, Maebashi, Gunma, Japan.
Conflict of Interest
No conflict of interest.
Grant Support
No Funding.
| References | ▴Top |
- Makaryus AN, McFarlane SI. Diabetes insipidus: diagnosis and treatment of a complex disease. Cleve Clin J Med. 2006;73(1):65-71.
doi - Robertson GL. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24(3):549-572.
pubmed - Cagno JM. Diabetes insipidus. Crit Care Nurse. 1989;9(6):86-93.
pubmed - Ghirardello S, Garre ML, Rossi A, Maghnie M. The diagnosis of children with central diabetes insipidus. J Pediatr Endocrinol Metab. 2007;20(3):359-375.
doi pubmed - Singer I, Oster JR, Fishman LM. The management of diabetes insipidus in adults. Arch Intern Med. 1997;157(12):1293-1301.
doi pubmed - Saborio P, Tipton GA, Chan JC. Diabetes insipidus. Pediatr Rev. 2000;21(4):122-129; quiz 129.
doi pubmed - Soule SG, Monson JP, Jacobs HS. Transient diabetes insipidus in pregnancy—a consequence of enhanced placental clearance of arginine vasopressin. Hum Reprod. 1995;10(12):3322-3324.
pubmed - Buonocore CM, Robinson AG. The diagnosis and management of diabetes insipidus during medical emergencies. Endocrinol Metab Clin North Am. 1993;22(2):411-423.
pubmed - Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
pubmed - Imura H, Nakao K, Shimatsu A, Ogawa Y, Sando T, Fujisawa I, Yamabe H. Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med. 1993;329(10):683-689.
doi pubmed - Powrie JK, Powell M, Ayers AB, Lowy C, Sonksen PH. Lymphocytic adenohypophysitis: magnetic resonance imaging features of two new cases and a review of the literature. Clin Endocrinol (Oxf). 1995;42(3):315-322.
doi - Pestell RG, Best JD, Alford FP. Lymphocytic hypophysitis. The clinical spectrum of the disorder and evidence for an autoimmune pathogenesis. Clin Endocrinol (Oxf). 1990;33(4):457-466.
- Hashimoto K, Takao T, Makino S. Lymphocytic adenohypophysitis and lymphocytic infundibuloneurohypophysitis. Endocr J. 1997;44(1):1-10.
doi pubmed - Korzets A, Sachs D, Gremitsky A, Gershkovitz R, Farrage G, Chlibowsky A, Erlich N. Unexplained polyuria and non-obstructive hydronephrosis in a urological department. Nephrol Dial Transplant. 2004;19(9):2410-2412.
doi pubmed - Robertson GL. Differential diagnosis of polyuria. Annu Rev Med. 1988;39:425-442.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
