| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 3, Number 3, June 2013, pages 62-66
Thyroid Hormone Dysfunction and CRP Levels in Neonates With Sepsis
Shikha Sharmaa, Pradeep Kumar Dablaa, c, Santosh Kumara, Swati Dublisb
aDepartment of Biochemistry, Chacha Nehru Bal Chikitsalya, Geeta Colony, New Delhi -110031, India
bPediatric Medicine, Chacha Nehru Bal Chikitsalya, Geeta Colony, New Delhi -110031, India
cCorresponding author: Pradeep Kumar Dabla, Department of Biochemistry, Chacha Nehru Bal Chikitsalya, Geeta Colony, New Delhi -110031, India
Manuscript accepted for publication May 31, 2013
Short title: Thyroid Hormone Dysfunction and CRP Levels
doi: https://doi.org/10.4021/jem167w
| Abstract | ▴Top |
Background: Thyroid hormone abnormalities are frequently encountered in patients with critical illness. Sepsis is an important cause of death of neonates in developing countries. The aim of our study was to evaluate the prognostic role of thyroid hormones (FT3, FT4, TSH) in neonates specifically with sepsis and septic shock and to correlate their levels of C-reactive protein (CRP).
Methods: Forty neonates with sepsis were included in the study as cases. Neonates with gestational age less than 37 weeks, body weight less than 2,500 grams or with congenital abnormalities were excluded from the study. Septic neonates were further divided into sepsis survivors (n = 19), shock-survivors (n = 9) and non-survivors. Forty full term neonates without sepsis served as controls. Thyroid hormones and CRP were estimated by chemiluminescent immunometric assay and immunoturbidimetric assay respectively.
Results: The FT3 and FT4 hormones levels were significantly decreased (P < 0.001) in neonates with sepsis as compared to controls. No significant difference was observed in TSH levels. Non survivors had lower FT3 and FT4 levels (P < 0.05) compared to sepsis-survivor group. There was also a significant negative correlation between CRP and FT3 level in non-survivor group (r = -0.60; P = 0.02) and septic shock survivor group (r = -0.78; P =0.006).
Conclusions: Low levels of FT3 and elevation in CRP correlate closely with decreased survival in septic neonates.
Keywords: C-reactive protein (CRP); Thyroid hormones; Sepsis; Shock; Neonate; Outcome
| Introduction | ▴Top |
The incidence of neonatal sepsis in the developing world is 10-30% and mortality is between 30-45% [1]. Neonatal sepsis is a clinical syndrome characterized by signs and symptoms of infection with or without accompanying bacterimia in the first month of life. Many neuroendocrine changes take place during critical illness however reports of these changes in neonatal sepsis are limited. The hormonal changes can be attributed to various lymphokines and monokines which are able to influence the hypothalamic-pituitary-thyroid axis modulating either the thyroid hormone levels or the hormone cytokine production by thyrocytes [2]. IL-1 and IL-6 are inflammatory cytokines implicated in the pathogenesis of non-thyroidal illness [3]. C-reactive protein (CRP) is an acute phase reactant produced in the liver, induced by cytokine IL-6 and its levels are commonly assayed for diagnosis of neonatal sepsis.
Epidemiological studies have shown alterations in thyroid hormones levels in hospitalized patients. These alterations are more commonly observed in those with increased age or critical illness [4]. Low triiodothyronine (T3) and elevated reverse triiodothyronine (rT3) levels are commonly observed due to inhibition of 5’-deiodinase enzyme. With increasing severity of illness the levels of total thyroxin (TT4), free thyroxin (FT4) and thyroid stimulating hormone (TSH) may also decrease [5]. Few studies in critically ill children showed an association of decreased levels of TT3 and TT4 with mortality [6, 7]. In another study by Anand et al, no significant changes were observed in thyroid indices with CRP [8]. Very few studies have been reported in neonates. To date it is also unclear whether newborns respond in the same way as adults during critical illness. Therefore in this study we aimed at assessing the thyroid hormone and CRP levels in neonates with sepsis and correlating these levels with disease severity in sepsis survivor, sepsis shock survivor and sepsis non-survivor groups.
| Materials and Methods | ▴Top |
Study population and selection criteria
This study was conducted in Chacha Nehru Bal Chikitsalya Hospital, New Delhi over the period from January 1st till August 30th, 2012. The study population comprised of 40 newborns with sepsis characterized by diminished spontaneous activity, bradycardia, temperature instability, respiratory distress, seizures, jaundice and less vigorous sucking who were admitted to the NICU (Neonatal Intensive Care Unit) during this period. In addition, neonates were considered to have septic shock if they had hypotension and decreased end-organ perfusion. The neonates with sepsis were further divided into sepsis survivor, shock survivor and non-survivors groups which includes 19, 9 and 12 patients respectively.
Blood cultures showing positive growth were seen in 18 neonates and 4 neonates had meningitis as confirmed by CSF examination. Neonates with gestational age less than 37 weeks, birth weight less than 2,500 grams and those with multiple congenital anomalies were excluded from the study. Forty full term neonates without sepsis served as controls. This study has been cleared by Institution Ethics Review Board for human studies and patients have signed an informed consent.
Sample collection and processing
Under aseptic conditions venous blood samples were collected by the NICU staff into sterile blood collection tubes. The samples were used to monitor the levels of FT3, FT4, TSH and CRP with other investigations. Thyroid hormones were estimated after the third day of life using a chemiluminescent assay (Access-2, Beckman Coulter analyzer) [9] and CRP levels were determined by immunoturbidimetric assay (Olympus AU-400 analyzer) [10]. Thyroid function tests were evaluated among septic neonates and control group and further the relationship between thyroid hormones and CRP levels was determined among sepsis survivor, shock survivor and non-survivors on NICU admission.
Statistical analysis
Statistical analysis was carried out using SPSS for windows 12.0 software (SPSS Inc., Chicago, IL, USA). Data was expressed in mean ± standard deviation values. The data between the two groups was analyzed by using Students unpaired ‘t’ test and Pearson correlation test was used to define correlation between thyroid hormones and CRP levels. A P-value of less than 0.05 was accepted as significant.
| Results | ▴Top |
The mean serum CRP, FT3, FT4 and TSH levels in septic neonates and control group are presented in Table 1. There was a significant decrease in FT3 (P < 0.0001) and FT4 (P < 0.001) levels in neonates with sepsis as compared to controls. No significant difference was observed in TSH levels in neonates with sepsis and control group.
 Click to view | Table 1. Mean Serum CRP, FT3, FT4, and TSH Levels in Neonates With Sepsis and Controls |
The septic neonates were further divided according to the presence of shock and survival into the following sub-groups of sepsis survivors (n = 19), shock-survivors (n = 9) and non-survivors (n = 12). All septic neonates were treated with antibiotics and parenteral fluids as per protocol. Inotropics except dopamine or glucocorticoids were given to 15 neonates with septic shock and 6 neonates were mechanically ventilated.
The mean CRP level was significantly higher in the sepsis non-survivor group compared to the sepsis survivor and septic shock survivor groups (P < 0.05). The non-survivors also had statistically significantly lower FT3 and FT4 levels compared to the sepsis survivor group (P < 0.05). No significant difference was observed in FT3 and FT4 between septic shock survivors and nonsurvivors. No significant difference was also seen in TSH levels in sepsis survivors and septic shock survivors compared to non-survivors (Table 2).
 Click to view | Table 2. Mean Serum CRP, FT3, FT4 and TSH Levels in Sepsis Survivors, Septic Shock Survivors V/S Non-Survivors |
There was a significant negative correlation between CRP and FT3 levels in the septic shock survivors and non-survivor groups (Fig. 1, 2).
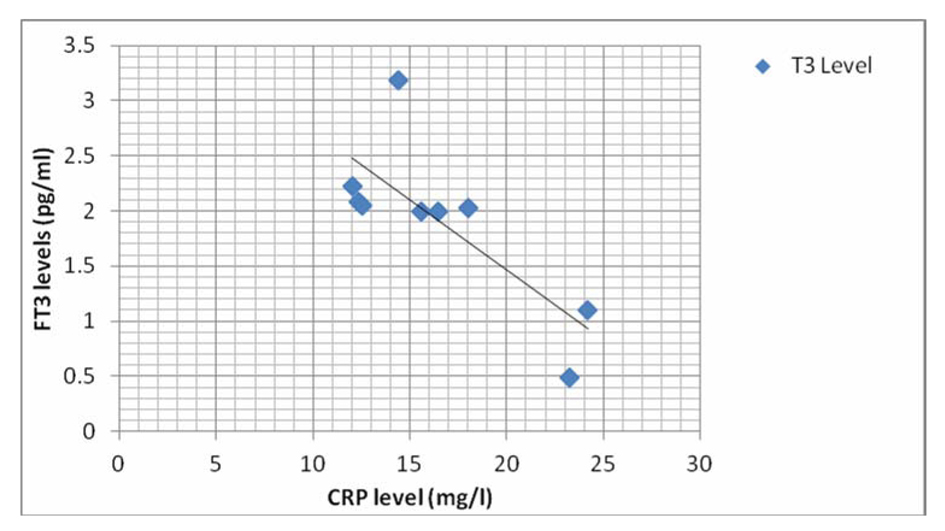 Click for large image | Figure 1. Graph showing correlation of CRP with FT3 level in septic shock survivor group. |
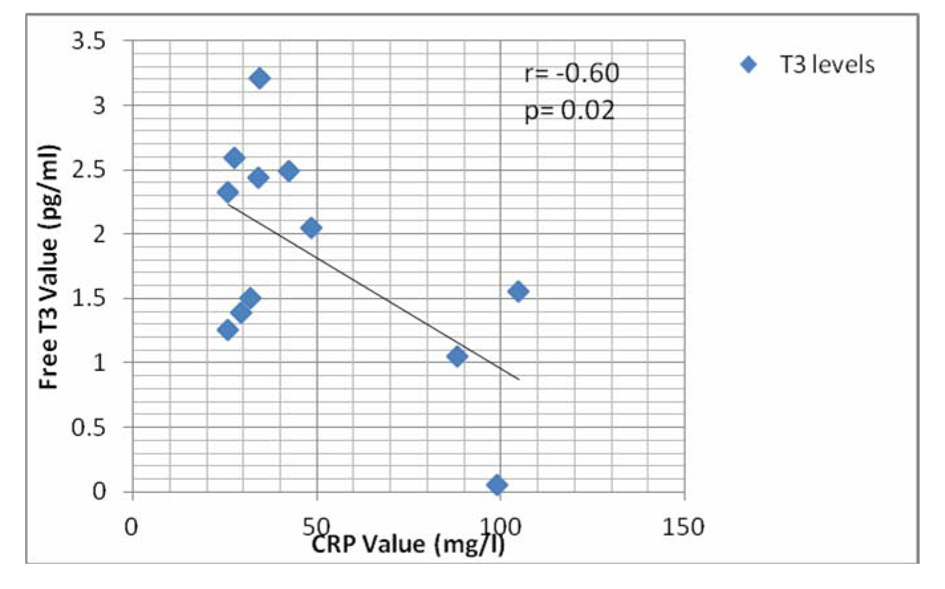 Click for large image | Figure 2. Graph showing correlation of CRP with FT3 level in non-survivor group. |
| Discussion | ▴Top |
The present study showed significantly lower serum levels of FT3 and FT4 in neonates with sepsis compared to age-matched non-septic controls. No difference was observed in levels of serum TSH. The levels of FT3 were also found to be significantly lower in non-survivor septic neonates compared to the survivor group. No statistical difference was observed in levels of FT4 and TSH. There was a significant negative correlation between high CRP levels in neonates with sepsis FT3 levels.
Thyroid hormones are important for the metabolic adaptations of the body in times of stress and critical illness [11]. Alterations in the hypothalamic-pituitary thyroid axis suggest a prognostic role of thyroid hormones in neonates with sepsis and septic shock. In a study by Kurt A et al [12], serum TT3 and TT4 levels of septic newborns were significantly decreased at the onset while serum TT4 level increased after the antibiotic treatment. In another prospective cohort study by B. K Das and his co-workers, low TT3, TT4 and elevated cortisol levels predicted adverse outcome in septic neonates [13]. Borkowski et al have shown that decreased levels of FT3 and TSH were associated with poor prognosis in patients with septic shock [14]. In another similar study, Lodha and his co-workers suggested that thyroid function derangement in children was not an important factor contributing to severity of septic shock [11]. They found lower levels of TT3, TT4, FT3, FT4 and TSH in children with septic shock compared to sepsis group.
It has been hypothesized that the immune system cells can affect the systemic thyroid hormone activity. This activity may be attributed to the complex pathophysiological interplay between TSH and the immune system. Expression of TSH receptors has been shown to be affected by the function of various types of hematopoietic and immune system cells that express the TSH receptor [15, 16]. It mainly includes subsets of hematopoietic cells in the bone marrow, as well as peripheral monocytes, dendritic cells, and T-lymphocytes.
Furthermore, some of the above cell types can produce biologically active TSH that can have autocrine and paracrine actions. It can influence the early stages of the immune response to an antigen [15, 16] which may includes the regulation of the synthesis and release of mediators of inflammation, such as IL6 and tumor necrosis factor-α (TNF-α) and T3 expression [17]. Various studies have demonstrated a significant negative correlation between cytokines and circulating thyroid hormone concentration in patients with non-thyroidal illness. Hashimoto et al have demonstrated that the levels of serum TT3 in patients with increased IL-6 were significantly reduced during acute infection in children [18]. In another study by Dilli D and his co-workers, CRP levels significantly correlated with T3 levels at the 4th week of life [19]. CRP is an acute phase protein produced in the liver under the influence of cytokines like IL-1, IL-6, TNF-α etc. The 5’-deiodinase which mediates the conversion of T4 to T3 is also found in rich quantities in the liver. It is possible that cytokines especially IL-6 play an important role in stimulating the production of CRP and simultaneously inhibit the activity of 5’-deiodinase [4].
Conclusion
To conclude, the present study on neonates with sepsis suggests that low levels of FT3 at baseline correlates closely with poor outcome in neonates with sepsis or septic shock and favour the existence of an association between lower FT3 and FT4 with worse outcome. We also showed an inverse relationship of thyroid hormones with CRP levels as limited studies are available. Our study supports the interplay between thyroid hormones and the immune system. The findings of our review regarding the association between thyroid hormone abnormalities and the outcome of patients with sepsis or septic shock indicate that these abnormalities could be of prognostic value, perhaps even independently of other such markers. Further studies are required on a larger sample size to substantiate the findings and should aim to more clearly establish the strength of the above-mentioned association in neonates with sepsis.
| References | ▴Top |
- Bhutta ZA. Neonatal infections. Curr Opin Pediatr. 1997;9(2):133-140.
doi pubmed - Fabris N, Mocchegiani E, Provinciali M. Pituitary-thyroid axis and immune system: a reciprocal neuroendocrine-immune interaction. Horm Res. 1995;43(1-3):29-38.
doi pubmed - Rapaport R, Rose SR, Freemark M. Hypothyroxinemia in the preterm infant: the benefits and risks of thyroxine treatment. J Pediatr. 2001;139(2):182-188.
doi pubmed - Simons RJ, Simon JM, Demers LM, Santen RJ. Thyroid dysfunction in elderly hospitalized patients. Effect of age and severity of illness. Arch Intern Med. 1990;150(6):1249-1253.
doi pubmed - Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88(7):3202-3211.
doi pubmed - Uzel N, Neyzi O. Thyroid function in critically ill infants with infections. Pediatr Infect Dis. 1986;5(5):516-519.
doi - Yildizdas D, Onenli-Mungan N, Yapicioglu H, Topaloglu AK, Sertdemir Y, Yuksel B. Thyroid hormone levels and their relationship to survival in children with bacterial sepsis and septic shock. J Pediatr Endocrinol Metab. 2004;17(10):1435-1442.
doi pubmed - Anand NK, Chandra V, Sinha RS, Chellani H. Evaluation of thyroid functions in critically ill infants. Indian Pediatr. 1994;31(10):1233-1237.
pubmed - Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45(12):2136-2141.
pubmed - Beckman Access Immunoassay System Operator's Guide and Reference Manual TSH kit inserts, Beckman Coulter, Inc. 2005 Beckman Coulter.
- Lodha R, Vivekanandhan S, Sarthi M, Arun S, Kabra SK. Thyroid function in children with sepsis and septic shock. Acta Paediatr. 2007;96(3):406-409.
doi pubmed - Kurt A, Aygun AD, Sengul I, Sen Y, Citak Kurt AN, Ustundag B Serum thyroid hormones levels are significantly decreased in septic neonates with poor outcome. J Endocrinol Invest. 2011; 34:92-96.
- Das BK, Agarwal P, Agarwal JK, Mishra OP. Serum cortisol and thyroid hormone levels in neonates with sepsis. Indian J Pediatr. 2002;69(8):663-665.
doi pubmed - Borkowski J, Siemiatkowski A, Wolczynski S, Czaban SL, Jedynak M. [Assessment of the release of thyroid hormones in septic shock—prognostic significance]. Pol Merkur Lekarski. 2005;18(103):45-48.
- Wang HC, Klein JR. Immune function of thyroid stimulating hormone and receptor. Crit Rev Immunol. 2001;21(4):323-337.
doi pubmed - Klein JR. Physiological relevance of thyroid stimulating hormone and thyroid stimulating hormone receptor in tissues other than the thyroid. Autoimmunity. 2003;36(6-7):417-421.
doi pubmed - Csaba G, Pallinger E. Thyrotropic hormone (TSH) regulation of triiodothyronine (T(3)) concentration in immune cells. Inflamm Res. 2009;58(3):151-154.
doi pubmed - Hashimoto H, Igarashi N, Yachie A, Miyawaki T, Sato T. The relationship between serum levels of interleukin-6 and thyroid hormone in children with acute respiratory infection. J Clin Endocrinol Metab. 1994;78(2):288-291.
doi pubmed - Dilli D, Dilmen U. The role of interleukin-6 and C-reactive protein in non-thyroidal illness in premature infants followed in neonatal intensive care unit. J Clin Res Pediatr Endocrinol. 2012;4(2):66-71.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
