| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 14, Number 4, August 2024, pages 184-193
Evaluation Effect of Low-Intensity Pulsed Ultrasound on Blood Insulin Secretion due to Pancreatic Stimulation in Type 2 Diabetic Male Rats
Niloufar Khalafpoura , Malakeh Malekzadeha, Razieh Haji Soltanib, Mitra Farbinb
, Mitra Nourbakhshc, Mohammad Bagher Shirana, d
aMedical Physics Department, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
bPhysiology Department, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
cFinetech in Medicine Research Center and Biochemistry Department, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
dCorresponding Author: Mohammad Bagher Shiran, Finetech in Medicine Research Center and Medical Physics Department, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Manuscript submitted June 8, 2024, accepted July 15, 2024, published online August 10, 2024
Short title: Low-Intensity Pulsed Ultrasound in T2D Rats
doi: https://doi.org/10.14740/jem1002
| Abstract | ▴Top |
Background: Ultrasound is a noninvasive, nonionizing radiation that can be focused to transfer acoustic energy into the body, inducing mechanical stimulation in cells. In this study, we evaluated the effect of low-intensity pulsed ultrasound (LIPUS) on insulin release in type 2 diabetes (T2D) male rats.
Methods: Twenty-eight white male Wistar rats were divided into four groups: non-diabetic control, diabetic control and diabetic treated with ultrasound at intensities of 1 W/cm2 and 1.5 W/cm2 (frequency = 1 MHz; pulsed = 1:2; exposure time = 15 min) in vivo. Diabetes was induced by a high-fat diet (HFD) and a low dose (35 mg/kg) of streptozotocin (STZ). After 14 days of LIPUS treatment, blood and tissue samples were analyzed.
Results: LIPUS treatment significantly increased insulin levels by 79.55% (P < 0.001) - 94.80% (P < 0.001) and decreased glucose levels by 44.55% (P < 0.001) - 45.64% (P < 0.01). Additionally, glucagon levels increased by 31.39% (P < 0.05) - 45.69% (P < 0.01), while somatostatin levels decreased by 12.12-21.50% (P > 0.05). The pancreas and surrounding tissues, such as the liver and spleen, were not affected by the LIPUS treatment.
Conclusions: Our findings indicate that LIPUS treatment improved glycemic control, insulin secretion and beta-cell function in T2D caused by HFD and low dose STZ (35 mg/kg) without tissue damage.
Keywords: Low-intensity pulsed ultrasound; Insulin secretion; Pancreas; Type 2 diabetes
| Introduction | ▴Top |
The 21st century has the most diabetogenic environment in human history [1]. type 2 diabetes (T2D) is a complex and chronic metabolic disease that results from the interplay of systemic insulin resistance in peripheral tissues and insufficient compensatory insulin secretion from pancreatic beta cells [2]. The pancreas is a complex gland that is active in digestion and metabolism through the secretion of digestive enzymes from the exocrine portion and hormones from the endocrine portion; each pancreatic islet is composed of alpha (α), beta (β), delta (δ), epsilon (ε) and PP (F) cells; beta cells are the most common cell type, making up 50-70% of the islet mass [3], and are significantly reduced at the time of diabetes diagnosis. Insulin secretion dysregulation characterized by hyperinsulinemia in the early stages and insufficient insulin secretion in later stages, plays a critical role in the onset and progression of T2D [4]. Any intervention aimed at improving blood glucose levels can improve pancreatic beta-cell function to a certain extent [5]. Controlling diabetes without side effects is still a challenge for the medical system. This leads to an effort to search for more effective, safer and cost-efficient treatments.
Low-intensity ultrasound (LIUS) has demonstrated therapeutic potential as a noninvasive, nonpharmaceutical and targeted treatment for internal organs, such as pancreas and kidney [6]. Studies have shown that ultrasound exposure stimulates the release of some biological macromolecules from cell membranes, such as cancer biomarkers [7], neurotransmitters [8], and insulin [2].
The primary objective of this study was to evaluate the efficacy of low-intensity pulsed ultrasound (LIPUS) as a treatment for diabetic rats in the early stages of T2D by evaluating its effects on blood glucose, insulin secretion, and pancreatic hormones.
We hypothesized that LIPUS would effectively stimulate insulin secretion, affecting blood glucose and pancreatic hormone levels, including glucagon and somatostatin, while maintaining pancreatic function without inducing tissue damage.
| Materials and Methods | ▴Top |
Experimental animals and study design
Animals and housing
Male Wistar rats (aged 4 weeks, average weight 90 ± 10 g) were purchased from Iran University of Medical Sciences, Tehran, Iran, and housed at room temperature (22 - 23 °C) with a 12-h light-dark cycle, free access to food and water. All the animal procedures were in accordance with the requirements of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (USA, National Institutes of Health (NIH) Publication No. 80-23, revised 1996), and experimental protocols were approved by the Iran University of Medical Sciences Ethics Committee (No.IR.IUMS.FMD.REC.1400.030).
Sample size determination
Sample size was estimated using a “Resource Equation” method [9], considering desired statistical power (80%), significance level (0.05), animal welfare and response detectability. The calculated sample size was six rats per control group, and eight rats per treatment group, totaling 28 animals in the study.
Experimental groups and T2D induction
Experimental groups and design
This study is a randomized controlled trial (RCT) with a parallel design. Rats were divided into four experimental groups in random order, consisting of n = 6 - 8/group, as follows: 1) non-diabetic control (NDC; n = 6); 2) diabetic control (DC; n = 6); 3) diabetic treated with ultrasound at intensity of 1 W/cm2 (DT + US1; n = 8); 4) diabetic treated with ultrasound at intensity of 1.5 W/cm2 (DT + US1.5; n = 8).
Induction of T2D
T2D was induced by combination a 52.82 kcal% high-fat diet (HFD) [10] and streptozotocin (STZ) (35 mg/kg; Sigma Aldrich S0130, Hamburg, Germany) injection [11]. Overnight-fasted rats received a single intraperitoneal (IP) injection of STZ dissolved in 1 mL of cold 0.9% NaCl. After 1 week, rats with fasting blood glucose (FBG) level ≥ 250 mg/dL were classified as diabetic [12]. NDC rats were fed a normal pelleted diet (NPD) without STZ injection.
Study timeline
Blood samples were analyzed at four time points: before T2D induction (baseline), after T2D induction (day 0) and after LIPUS treatment (day 7 and day 14). The profiles of the various treatment groups were plotted during the 14-day study period. Figure 1 shows the experimental timeline of this study
 Click for large image | Figure 1. Experimental timeline. *At 35 mg/kg body weight. NDC: non-diabetic control; DC: diabetic control; DT + US: diabetic treated with ultrasound at intensity of 1 W/cm2 (DT + US1), diabetic treated with ultrasound at intensity of 1.5 W/cm2 (DT + US1.5); STZ: streptozotocin. |
Primary and secondary endpoints
As the main focus of this study is to evaluate the efficacy of LIPUS in treating T2D, insulin and glucose levels in response to LIPUS treatment have been designed as primary endpoints. By assessing changes in these parameters, we can directly measure the effectiveness of LIPUS in improving glycemic control and insulin secretion, which are key factors in managing T2D.
To further explore the impact of LIPUS on pancreatic function and overall glucose homeostasis, we have identified several secondary endpoints. These include glucagon and somatostatin levels, histological alterations in the pancreatic islets of Langerhans, and homeostasis model assessment of beta-cell function (HOMA-B) levels in response to LIPUS. Investigating these additional outcomes will provide valuable insights into the broader effects of LIPUS on hormonal regulation, pancreatic health, and beta-cell function in the context of T2D.
Ultrasound experimental set-up and thermometry
Before ultrasound exposure, rats were anesthetized through IP injection of 90 mg/kg body weight (BW) ketamine and 10 mg/kg BW xylazine [13]. The abdominal area was prepared by shaving and covering with ultrasound coupling gel. The DT + US rats received 15-min ultrasound exposure every 48 h. Optimal intensity was determined by recording pancreatic temperature changes using a digital thermometer and an infrared camera (IR camera, resolution × 160, 120 pixels; Testo875-1i, Germany). The thermocouple probe of the thermometer (diameter: 125 µm; sensitivity: ± 0.1 °C) was inserted into the pancreas. A plane circular transducer (area: 1 cm2) operating at 1 MHz was placed on the abdomen surface and operated in duty cycle pulsating mode (1:2 and 1:8), with intensities of 0.5, 1 and 1.5 W/cm2, 100 Hz pulse repetition frequency (ultrasound generator: Phyaction190i, Germany). The entire pancreas region was stimulated, with no specific target. NDC and DC groups followed the same protocol (anesthesia, blood collection and necropsy) without ultrasound exposure. Figure 2 shows a schematic of the experimental setup [14]. Supplementary Material 1 (www.jofem.org) displays a real-time image of the sonication procedure, offering further insights into the practical application.
 Click for large image | Figure 2. Schematic of the sonication procedure for a diabetic rat under anesthesia. The probe ultrasound transducer was positioned transcutaneously over the abdomen, which was then shaved and covered with ultrasound coupling gel [14] (created with BioRender.com, by permission of use).. |
Biochemical analysis
Blood sample collection and assessment
Blood samples (whole blood = 2 mL) were collected following an overnight (12 h) fast and 30 min after ultrasound exposure using the retro-orbital bleeding (ROB) technique; blood serum was prepared by centrifugation (3,200 rpm, 15 min, 4 °C) and stored at -80 °C [15]. Serum levels of insulin, glucagon and somatostatin were assessed by enzyme-linked immunosorbent assay (ELISA) kits (ZellBio GmbH, Germany), with optical density read at 450 nm using an automatic multiple ELISA reader (BIO-RAD microplate reader, model680). Serum glucose levels were determined using colorimetric diagnostic kits based on the glucose oxidase-peroxidase method (Glucose (GOD-POX), Pars Azmoon Company, Tehran, Iran), and analyzed with a spectrophotometer at 546 nm (500 - 546 nm). The manufacturer’s instructions were followed.
Homeostasis model assessment of insulin resistance (HOMA-IR), HOMA-B, and quantitative insulin sensitivity check index (QUICKI) calculation
To assess insulin resistance, beta-cell function, and insulin sensitivity, the HOMA-IR, HOMA-B, and QUICKI were calculated by the following equations [16]:
Conversion factor: insulin (1 µIU/mL = 6 pmol/L) [17] and blood glucose (1 mmol/L = 18 mg/dL).
Histopathological analysis
At the end of the experiment, rats were sacrificed under ketamine-xylazine anesthesia, and pancreas, liver and spleen were removed and histopathologically prepared as follows: fixed in 10% neutral buffered formalin (NBF), dehydrated in a series of increasing alcohol concentrations (70-100%), immersed in xylene and embedded in paraffin. The paraffin blocks were 3 - 5 µm thick using a microtome, and then the sections were deparaffinized in xylene, rehydrated through a series of decreasing ethanol concentrations (100-70%) and stained with hematoxylin and eosin stain (H&E) solution [18]. Images of the slices were obtained at × 40, × 20 and × 4 magnifications using a light microscope (OPTIKA, Assembled in Italy).
For a concise visual representation of the experimental procedure, please refer to the diagram (Supplementary Material 2, www.jofem.org) [14].
Statistical analysis
Data analysis was performed using GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA). Following confirming normal data distribution, statistical analysis was conducted using two-way and one-way analysis of variance (ANOVA) followed by Tukey’s test. Statistical significance was set at P < 0.05, and results are presented as the mean ± standard error of the mean (SEM).
| Results | ▴Top |
Thermometry
The mean pancreas temperature increased by < 2 °C during ultrasound exposure (1 MHz; pulse (1:2 and 1:8); 15 min; intensities of 0.5, 1 and 1.5 W/cm2), staying below the hyperthermia effect range (ΔT ≥ 4 °C - 5 °C). A linear relationship was observed between temperature changes recorded by the IR camera (ΔT1) and thermometer (ΔT2). Pearson correlation between ΔT1 and ΔT2 in (1:2) and (1:8) pulses was positive and significant (P < 0.0003 - 0.0001; 0.94 ≤ r ≤ 0.99); these results are tabulated in Table 1.
 Click to view | Table 1. Changes in the Mean Temperature (ΔTmean) of the Pancreas After Ultrasound Exposure |
Effect of LIPUS treatment on serum levels of insulin, glucose, glucagon and somatostatin
Figure 3 demonstrates that on day 0, following T2D induction, significantly, insulin levels decreased by 32.80-38.86% (P < 0.05), glucose levels increased by 71.80-75.33% (P < 0.001), glucagon levels increased by 42.11% (P > 0.05) - 70.58% (P < 0.05) in DC, DT + US1 and DT + US1.5, compared to the NDC rats; and somatostatin levels increased by 3.67-5.03% (P > 0.05) without statistical significance. On day 14 of LIPUS treatment, significantly, insulin levels increased by 79.55% (P < 0.001) - 94.80% (P < 0.001) (Fig. 3a), glucose levels decreased by 44.55% (P < 0.001) - 45.64% (P < 0.01) (Fig. 3b), glucagon levels increased by 31.39% (P < 0.05) - 45.69% (P < 0.01) (Fig. 3c), respectively in DT + US1 and DT + US1.5, compared to DC rats; and somatostatin levels decreased by 12.12-21.50% (P > 0.05) (Fig. 3d) without statistical significance. The results are tabulated in Table 2.
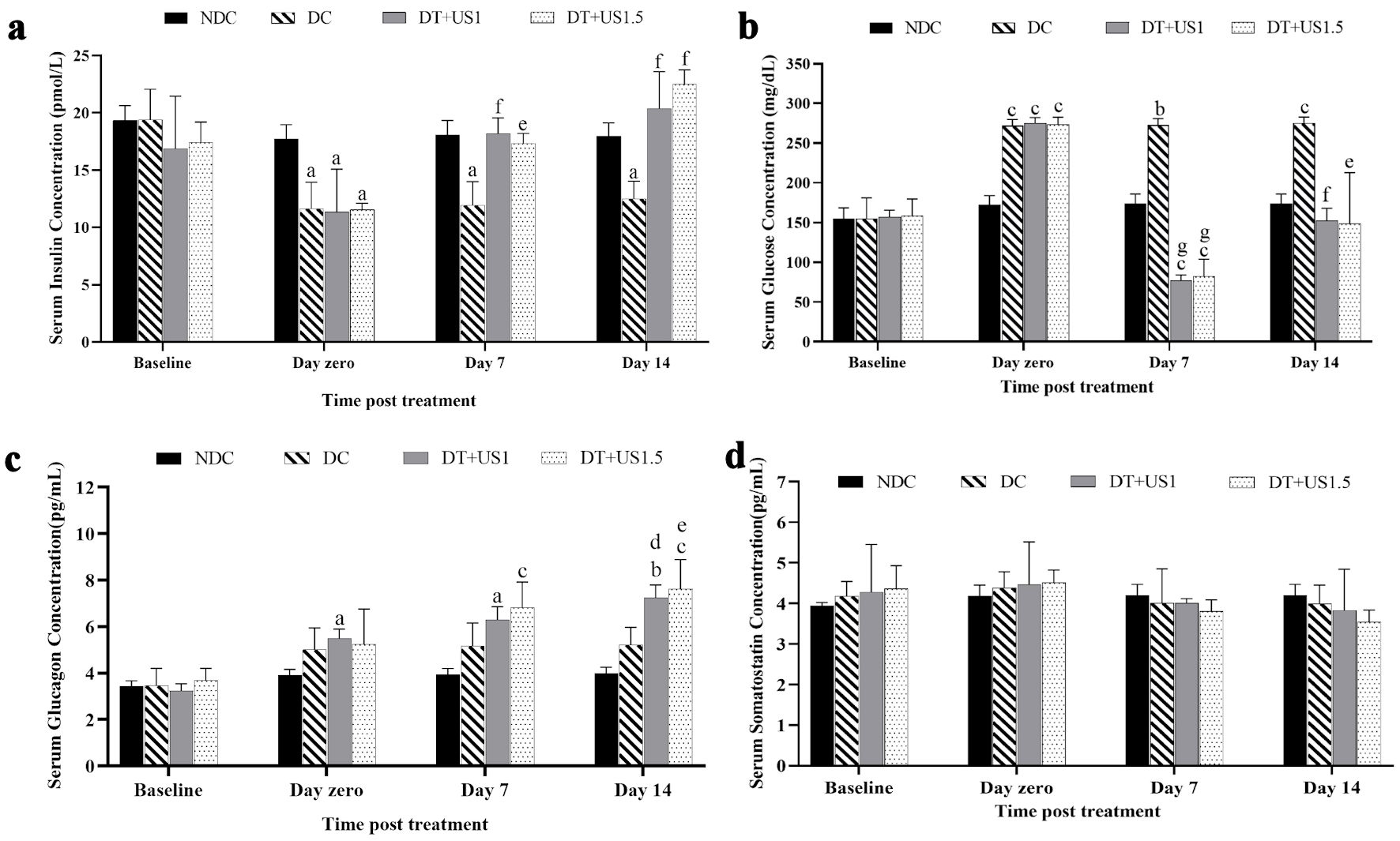 Click for large image | Figure 3. The graph of the biochemical analysis of the effects of ultrasound treatment on the serum levels of (a) insulin, (b) glucose, (c) glucagon, and (d) somatostatin. aP < 0.05. bP < 0.01. cP < 0.001 versus NDC. dP < 0.05. eP < 0.01. fP < 0.001. gP < 0.0001 versus DC. NDC: non-diabetic control; DC: diabetic control; DT + US: diabetic treated with ultrasound at intensity of 1 W/cm2 (DT + US1), diabetic treated with ultrasound at intensity of 1.5 W/cm2 (DT + US1.5). |
 Click to view | Table 2. Effect of LIPUS Treatment on Levels of Insulin, Glucose, Glucagon, Somatostatin, HOMA-IR, HOMA-B, and QUICKI |
Figure 4 compares insulin and glucose levels between DT + US1 vs. DT + US1.5 groups, showing no statistically significant differences (P > 0.05) when ultrasound intensity changed from 1 W/cm2 to 1.5 W/cm2 in terms of improved insulin secretion or reduced glucose levels.
 Click for large image | Figure 4. Comparison of (a) insulin and (b) glucose levels between treated groups (DT + US1 vs. DT + US1.5). There were no significant differences (P > 0.05) with increasing ultrasound intensity from 1 W/cm2 to 1.5 W/cm2 in terms of enhanced insulin secretion or reduced glucose levels. DT + US: diabetic treated with ultrasound at intensity of 1 W/cm2 (DT + US1), diabetic treated with ultrasound at intensity of 1.5 W/cm2 (DT + US1.5). |
Effects of LIPUS treatment on HOMA-IR, HOMA-B, and QUICKI scores
Table 2 presents calculated HOMA-IR, HOMA-B and QUICKI scores, after LIPUS treatment. The results demonstrated a significant decrease (two to three-fold (P < 0.01)) in pancreatic beta-cell function (HOMA-beta score) in DC compared to NDC, and significant increase in DT + US1 and DT + US1.5, compared to DC (three to four-fold increase, (P < 0.01) and (P < 0.05), respectively). However, no statistically significant differences were found in HOMA-IR and QUICKI scores among the groups (P > 0.05).
Histopathological evaluation
As illustrated in Figure 5, the NDC group showed a normal structure and appearance of the pancreas, liver and spleen without architectural or pathological changes such as necrosis (Fig. 5a, e, i), whereas the DC group showed degenerative and necrotic changes in the pancreas, including islet shrinkage and reduced or lost cells, increased vacuolation in liver hepatocytes due to glycogen infiltration, and diffuse white pulp in the spleen with reduced numbers of mature lymphocytes and dilated blood vessels (Fig. 5b, f, j). The DT + US groups showed minimal histopathological changes and improved morphology without gross visible damage, including bleeding, burns or discoloration (Fig. 5c, g, k, d, h, l).
 Click for large image | Figure 5. Photomicrographs of H&E sections of rat pancreas, liver and spleen from NDC, DC, and DT+US (diabetic treated with ultrasound at intensity of 1 W/cm2 (DT + US1); diabetic treated with ultrasound at intensity of 1.5 W/cm2 (DT + US1.5). (a) NDC rat pancreas showing normal islets of Langerhans (arrow). (b) DC rat pancreas showing shrinkage of islets of Langerhans with degeneration and necrosis of components cells (arrow). (c) DT + US1 and (d) DT + US1.5 rat pancreas showing the islets of Langerhans maintain their circular shape, no cell lysis or damage is visible throughout the samples and tissue integrity is retained (arrows). (e) NDC rat liver, showing normal hepatocytes (arrow). (f) DC rat liver showing increased vacuolation in the cytoplasm of hepatocytes appeared as indistinct clear vacuoles (arrows). (g) DT + US1 and (h) DT + US1.5 rat liver, showing no gross visible damage, including bleeding, burns or discoloration (arrows) (i) NDC rat spleen showing the normal architecture of the spleen (arrows). (j) DC rat spleen showing splenic lesions, such as histological changes, atrophy, and splenocyte apoptosis (arrow). (k) DT + US1 and (l) DT + US1.5 rat spleen, showing an absence of overt tissue damage, such as burns or bleeding (arrows). Magnification: (a-d): × 40, scale bar: 20 µm; (e-h): × 20, scale bar: 50 µm; (i-l): × 4, scale bar: 100 µm. H&E: hematoxylin and eosin stain; NDC: non-diabetic control; DC: diabetic control; DT + US: diabetic treated with ultrasound. |
| Discussion | ▴Top |
Our results showed lower serum insulin and higher serum glucose levels in diabetic rats compared to NDC rats, likely due to STZ-induced reductions in insulin secretion and alterations in pancreatic beta cells and islets of Langerhans [19, 20]. Additionally, insulin resistance caused by HFD leads to increased fat levels in tissues and muscles, which in turn decreases insulin secretion and utilization, resulting in elevated glucose concentrations [19]. Diabetic rats also displayed higher serum glucagon compared to NDC rats. Studies have shown that in T2D, chronic hyperglycemia inactivates factors inhibiting glucagon secretion, causing elevated glucagon levels [4, 21].
Ultrasound interacts with biological materials such as cells, tissues and organs, through thermal and mechanical (non-thermal) mechanisms [22]. Understanding these mechanisms is crucial for optimizing the therapeutic effects of ultrasound while minimizing potential adverse effects.
Thermal effects of ultrasound
Hyperthermia (temperature up to 39 - 45 °C), represents thermally induced cellular injury, membrane disruption, or cell death, through the induction of apoptosis [23, 24], and irreversible adverse effects (uncontrolled cellular stress response, induction of heat shock and tissue damages) [22]. To minimize these thermal effects and hence allow the mechanical effects of ultrasound waves, pulsed ultrasound can be applied, which reduces energy deposition and allows for cooling between pulses [25]. This approach keeps temperature below the hyperthermia threshold and minimizes the risk of thermal injury to pancreatic cells and surrounding tissues.
We investigated the thermal effects of LIPUS and based on the results, hypothesized that using ultrasound with parameters (frequency (f) = 1 MHz, intensity (I) = 1 and 1.5 W/cm2; pulse (1:2); 15 min exposure) may regulate pancreatic hormonal secretion and enhance non-thermal effects. Thus, these parameters were chosen for treating DT + US groups. Furthermore, the significant positive correlation between temperature recorded by thermocouple and IR camera, suggests that the IR camera can be effectively used for noninvasive thermal monitoring, ensuring optimal and safe application of LIPUS.
Mechanical effects of ultrasound
LIUS primarily induces changes in cellular function through non-thermal mechanisms, which are mediated by cavitation activity and non-cavitational factors, including acoustic radiation forces, acoustic microstreaming and shear stresses.
Our study hypothesized that ultrasound interacts with pancreatic tissue through mechanical (non-thermal) mechanisms, significantly enhancing insulin secretion from pancreatic beta cells, leading to reducing blood glucose levels. The increased glucagon levels beyond normal levels following LIPUS treatment, may be attributed to changes in concentration or bioactivity, potentially due to the excitability of glucagon-secreting alpha-cells being affected by LIPUS, while somatostatin-secreting delta-cells are less affected due to their low density.
The mechanical effects of ultrasound have been shown to modify cell membrane permeability, leading to altered ion and molecule transport rates across the membrane [26]. Two main hypotheses explain this phenomenon: 1) ultrasound-induced activation of voltage-gated Ca2+ channels; 2) ultrasound-enhanced cell membrane permeability.
Insulin secretion from pancreatic beta cells is naturally calcium-dependent [2]. Ultrasound-induced effects cause intracellular calcium transients in various cell types, such as pancreatic beta cells [27], potentially activating mechanosensitive membrane proteins. This activation may lead to membrane depolarization and increased insulin release. Enhanced insulin secretion is attributed to the stimulation of stretch-activated cation channels (SACs), and volume-regulated anion channels (VRAC). Ultrasound-induced transient changes in cell morphology and cytoskeletal disruptions may stimulate VRAC and SAC channels, leading to membrane depolarization and subsequent opening of voltage-gated Ca2+ channels [26]. These possible mechanisms could enhance membrane permeability and subsequent insulin secretion [2]. However, the exact underlying mechanisms remain unclear.
Studies have demonstrated interdependent behavior between insulin, glucagon, and somatostatin concentrations. The complex regulation of glucagon-secreting alpha cells involves various mechanisms, including direct/indirect paracrine signaling from beta and delta cells in the pancreatic islets of Langerhans. Studies propose that a decrease or increase in insulin secretion from beta cells serves as a reciprocal signal to alter the intra-islet insulin inhibition of alpha cells, leading to either increased or decreased glucagon secretion [28]. Other regulatory mechanisms within the islets, such as somatostatin secretion from delta cells, inhibit both insulin and glucagon secretion in a paracrine manner, ultimately leading to changes in glucose levels [29].
No statistically significant differences were observed between the DT + US1 and DT + US1.5 groups. We hypothesize that ultrasound at intensity of 1 W/cm2 may have fewer tissue effects, making it a potential candidate for future research and therapeutic application in managing T2D.
Effects of ultrasound on HOMA-IR, HOMA-B and QUICKI
Treatment strategies for beta-cell failure can be categorized into two groups: increasing cell number or enhancing insulin secretion [30]. Observations in humans and rodents suggest the possibility of resorting beta-cell function [30]. Factors like oxidative and endoplasmic reticulum (ER) stress and mitochondrial dysfunction contribute to beta-cell failure and altered beta-cell identity [30]. Our findings indicate that LIPUS treatment may improve pancreatic beta-cell function in, by increasing insulin secretion in T2D rats, as evidenced by the significant increase in HOMA-B score following treatment, opening a new approach for treating T2D by restoring beta-cell function. Although, we calculated this by using HOMA-B calculation [16], further research is needed to understand the precise effects of LIPUS on beta-cell stimulation and restoration.
The lack of significant changes in HOMA-IR and QUICKI scores suggests that LIPUS may not have substantial impact on overall insulin resistance and insulin sensitivity in this study. Further investigation is warranted to explore the potential of LIPUS in improving insulin resistance and sensitivity, as well as its long-term effects on beta-cell function.
Comparison with other studies
This study is the first to demonstrate that ultrasound therapy can effectively enhance insulin release, reducing hyperglycemia in a rat model of T2D induced by HFD and low-dose STZ (35 mg/kg, IP), without histopathological changes in pancreas, liver and spleen.
In previous studies, Suarez Castellanos et al [2] applied continuous ultrasound (I = 1 W/cm2; time (t) = 5 min; f = 800 kHz) to rat INS832/13 beta cells in vitro, observing an increase in insulin concentration through Ca2+ channel stimulation and enhanced cell membrane permeability in pancreatic beta cells. Singh et al [27] treated diabetic transgenic mice with ultrasound (f = 1 MHz; I = 1 W/cm2) and reported a 20% increase in insulin secretion from pancreatic beta cells with no pathological changes in the pancreatic tissue or islets of Langerhans cells; however, despite the increased in insulin levels, glucose levels did not significantly decrease. Suarez‐Castellanos et al [31] studied the effect of ultrasound (f = 800 kHz; I = 0.5 - 1 W/cm2, t = 5 min) on insulin, glucagon and alpha-amylase release from an ex vivo rabbit pancreatic model and cultured human pancreatic islets. Their findings showed an increase in insulin release from samples treated with ultrasound, but no statistically significant difference in extracellular glucagon and alpha-amylase concentrations. Chang et al [32] applied an ultrasound therapy (f = 1 MHz; t = 30 min) on diabetic Sprague-Dawley rats induced by HFD/STZ. They observed significantly increased serum concentrations of beta-endorphin, and decreased blood glucose levels, but found no statistically significant change in insulin release (P > 0.05).
Limitations
Our study had several limitations that should be considered when interpreting the results. One major limitation was the extended time needed for the HFD/STZ to induce T2D in rats. Additionally, other limitations include the short-term duration of treatment, and the use of a rat model necessitating validation in larger animals and human subjects. Monitoring other parameters, such as plasma lipid profiles (total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)), would be valuable for future studies.
Conclusions
In conclusion, this study suggests that LIPUS may be a potential therapeutic intervention for T2D. Following ultrasonic stimulation, insulin concentration and pancreatic beta-cell function improved, leading to reduced glucose levels without causing abnormal pathological changes. The mechanism underlying enhanced insulin release can be attributed to the mechanical force of ultrasonic waves, which increases cell membrane permeability and aids in resorting pancreatic beta-cell function. Future research should explore ion channel stimulation’s effects on hormone secretion in large animals and human cells. Optimizing ultrasound parameters, such as frequency, intensity, pulse mode and exposure time, is critical for understanding the potential therapeutic application of ultrasound.
| Supplementary Material | ▴Top |
Suppl 1. Real-time visualization of sonication procedure, offering further insights into the practical application.
Suppl 2. Visual representation of the experimental procedure (created with BioRender.com, by permission of use).
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the Iran University of Medical Sciences (IUMS) under grant (no. 20402).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Our study involved animal subjects, and did not involve any human participant; thus, the concept of informed consent is not applicable in our study.
Author Contributions
Niloufar Khalafpour: study design, data collection, literature review, data analysis and interpretation, and writing up manuscript. Malakeh Malekzadeh: supervision, study conception, reviewing and drafting manuscript. Razieh Haji Soltani: data collection. Mitra Farbin: data collection, reviewing and drafting manuscript. Mitra Nourbakhsh: supervision, study conception, reviewing and drafting manuscript. Mohammad Bagher Shiran: study design, supervision, data interpretation, reviewing and drafting manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author Note
This article is derived from M.Sc. dissertation of “Niloufar Khalafpour”, entitled “Evaluation effect of ultrasound on Insulin secretion in Blood due to stimulation of Pancreatic Beta cells in rats with type 2 diabetes.
Abbreviations
LIPUS: low-intensity pulsed ultrasound; T2D: type 2 diabetes; HFD: high-fat diet; NPD: normal pelleted diet; STZ: streptozotocin; ROB: retro-orbital bleeding; FBG: fasting blood glucose; ELISA: enzyme-linked immunosorbent assay; H&E: hematoxylin and eosin stain; HOMA-IR: homeostasis model assessment of insulin resistance; HOMA-B: homeostasis model assessment of beta-cell function; QUICKI: quantitative insulin sensitivity check index
| References | ▴Top |
- Atkins RC, Zimmet P. Diabetic kidney disease: act now or pay later. Nephrol Dial Transplant. 2010;25(2):331-333.
doi pubmed - Suarez Castellanos I, Jeremic A, Cohen J, Zderic V. Ultrasound stimulation of insulin release from pancreatic beta cells as a potential novel treatment for type 2 diabetes. Ultrasound Med Biol. 2017;43(6):1210-1222.
doi pubmed pmc - Bonner-Weir S, Sullivan BA, Weir GC. Human islet morphology revisited: human and rodent islets are not so different after all. J Histochem Cytochem. 2015;63(8):604-612.
doi pubmed pmc - Henquin JC, Ibrahim MM, Rahier J. Insulin, glucagon and somatostatin stores in the pancreas of subjects with type-2 diabetes and their lean and obese non-diabetic controls. Sci Rep. 2017;7(1):11015.
doi pubmed pmc - Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med. 2021;384(23):2219-2228.
doi pubmed pmc - Xu T, Lu X, Peng D, Wang G, Chen C, Liu W, Wu W, et al. Ultrasonic stimulation of the brain to enhance the release of dopamine - A potential novel treatment for Parkinson's disease. Ultrason Sonochem. 2020;63:104955.
doi pubmed - Kwong GA, Ghosh S, Gamboa L, Patriotis C, Srivastava S, Bhatia SN. Synthetic biomarkers: a twenty-first century path to early cancer detection. Nat Rev Cancer. 2021;21(10):655-668.
doi pubmed pmc - Meng Y, Hynynen K, Lipsman N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol. 2021;17(1):7-22.
doi pubmed - Castellanos I, Balteanu B, Singh T, Zderic V. Therapeutic modulation of calcium dynamics using ultrasound and other energy-based techniques. IEEE Rev Biomed Eng. 2016;9:177-191.
doi pubmed - Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303-306.
doi pubmed pmc - Binayi F, Moslemi M, Khodagholi F, Hedayati M, Zardooz H. Long-term high-fat diet disrupts lipid metabolism and causes inflammation in adult male rats: possible intervention of endoplasmic reticulum stress. Arch Physiol Biochem. 2023;129(1):204-212.
doi pubmed - Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14(3):140-162.
doi pubmed - Walle M, Whittier DE, Frost M, Muller R, Collins CJ. Meta-analysis of diabetes mellitus-associated differences in bone structure assessed by high-resolution peripheral quantitative computed tomography. Curr Osteoporos Rep. 2022;20(6):398-409.
doi pubmed pmc - BioRender: Scientific Image and Illustration Software. https://www.biorender.com (accessed 2024-07-22; Agreement number: PY273B2VM6, KI273B6JO3).
- Kumar AH, Clover AJ. Intraperitoneal co-administration of low dose urethane with xylazine and ketamine for extended duration of surgical anesthesia in rats. Lab Anim Res. 2015;31(4):174-179.
doi pubmed pmc - Giezenaar C, Hutchison AT, Luscombe-Marsh ND, Chapman I, Horowitz M, Soenen S. Effect of age on blood glucose and plasma insulin, glucagon, ghrelin, CCK, GIP, and GLP-1 responses to whey protein ingestion. Nutrients. 2017;10(1):2.
doi pubmed pmc - Tharaheswari M, Jayachandra Reddy N, Kumar R, Varshney KC, Kannan M, Sudha Rani S. Trigonelline and diosgenin attenuate ER stress, oxidative stress-mediated damage in pancreas and enhance adipose tissue PPARgamma activity in type 2 diabetic rats. Mol Cell Biochem. 2014;396(1-2):161-174.
doi pubmed - Knopp JL, Holder-Pearson L, Chase JG. Insulin units and conversion factors: a story of truth, boots, and faster half-truths. J Diabetes Sci Technol. 2019;13(3):597-600.
doi pubmed pmc - Alwahaibi NY, Alkhatri AS, Kumar JS. Hematoxylin and eosin stain shows a high sensitivity but sub-optimal specificity in demonstrating iron pigment in liver biopsies. Int J Appl Basic Med Res. 2015;5(3):169-171.
doi pubmed pmc - Magalhaes DA, Kume WT, Correia FS, Queiroz TS, Allebrandt Neto EW, Santos MPD, Kawashita NH, et al. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An Acad Bras Cienc. 2019;91(1):e20180314.
doi pubmed - Ren C, Zhang Y, Cui W, Lu G, Wang Y, Gao H, Huang L, et al. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int J Biol Macromol. 2015;72:951-959.
doi pubmed - Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine control of alpha-cell glucagon exocytosis is compromised in human type-2 diabetes. Nat Commun. 2020;11(1):1896.
doi pubmed pmc - Yu K, Niu X, Krook-Magnuson E, He B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat Commun. 2021;12(1):2519.
doi pubmed pmc - Maurici CE, Colenbier R, Wylleman B, Brancato L, van Zwol E, Van den Bossche J, Timmermans JP, et al. Hyperthermia enhances efficacy of chemotherapeutic agents in pancreatic cancer cell lines. Biomolecules. 2022;12(5):651.
doi pubmed pmc - Brero F, Calzolari P, Albino M, Antoccia A, Arosio P, Berardinelli F, Bettega D, et al. Proton therapy, magnetic nanoparticles and hyperthermia as combined treatment for pancreatic BxPC3 tumor cells. Nanomaterials (Basel). 2023;13(5):791.
doi pubmed pmc - Razavi M, Zheng F, Telichko A, Ullah M, Dahl J, Thakor AS. Effect of pulsed focused ultrasound on the native pancreas. Ultrasound Med Biol. 2020;46(3):630-638.
doi pubmed pmc - Singh T, Suarez Castellanos I, Bhowmick DC, Cohen J, Jeremic A, Zderic V. Therapeutic ultrasound-induced insulin release in vivo. Ultrasound Med Biol. 2020;46(3):639-648.
doi pubmed - Bisgaard Bengtsen M, Moller N. Mini-review: Glucagon responses in type 1 diabetes - a matter of complexity. Physiol Rep. 2021;9(16):e15009.
doi pubmed pmc - Yue JT, Burdett E, Coy DH, Giacca A, Efendic S, Vranic M. Somatostatin receptor type 2 antagonism improves glucagon and corticosterone counterregulatory responses to hypoglycemia in streptozotocin-induced diabetic rats. Diabetes. 2012;61(1):197-207.
doi pubmed pmc - Son J, Accili D. Reversing pancreatic beta-cell dedifferentiation in the treatment of type 2 diabetes. Exp Mol Med. 2023;55(8):1652-1658.
doi pubmed pmc - Suarez-Castellanos I, Singh T, Chatterjee Bhowmick D, Cohen J, Jeremic A, Zderic V. Effect of therapeutic ultrasound on the release of insulin, glucagon, and alpha-amylase from ex vivo pancreatic models. J Ultrasound Med. 2021;40(12):2709-2719.
doi pubmed - Chang CH, Fan KC, Cheng YP, Chen JC, Chen GS. Ultrasound stimulation potentiates management of diabetic hyperglycemia. Ultrasound Med Biol. 2023;49(5):1259-1267.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
