| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 14, Number 2, April 2024, pages 78-84
Multiple Metastatic Lesions on a Post-Ablative Whole-Body Scan Despite a Negative Serum Thyroglobulin Level in a Patient With Papillary Thyroid Cancer
Dohee Kim
Division of Endocrinology, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, 330-714, Korea
Manuscript submitted February 13, 2024, accepted April 6, 2024, published online April 16, 2024
Short title: False-Negative Tg Despite Positive WBS
doi: https://doi.org/10.14740/jem940
| Abstract | ▴Top |
Serum thyroglobulin (Tg) and radioactive iodine (RAI) whole-body scan (WBS) have been used to detect recurrent and metastatic differentiated thyroid cancer after total thyroidectomy and RAI remnant ablation. Discordant results of serum Tg and WBS are occasionally encountered, and a negative serum Tg and positive WBS are less common than a positive serum Tg and negative WBS. The author reports a rare case of a 41-year-old man with papillary thyroid cancer who showed unsuspected multiple metastatic lesions in both cervical lymph nodes and both lungs on post-ablative WBS despite a negative stimulated serum Tg level after total thyroidectomy and that an initial negative stimulated serum Tg level was converted to positive after RAI remnant ablation. In conclusion, RAI WBS can contribute to the detection of a relatively small number of unsuspected local and distant metastases despite a negative stimulated serum Tg.
Keywords: Differentiated thyroid carcinoma; Thyroglobulin; Whole-body scan; Metastasis
| Introduction | ▴Top |
In recent decades, the incidence of differentiated thyroid cancer (DTC) has been increasing rapidly worldwide, predominantly due to the increased detection of small papillary thyroid cancer (PTC), but thyroid cancer-related mortality rates have remained fairly stable [1, 2]. The long-term monitoring of patients with DTC is essential throughout the patients’ life after total thyroidectomy followed by 131I remnant ablation and thyroid hormone suppression of thyroid-stimulating hormone (TSH). Traditionally, radioactive iodine (RAI) whole-body scan (WBS) and the measurement of serum thyroglobulin (Tg) (stimulated Tg) after endogenous or exogenous TSH stimulation had been thought of as the main tools for the postoperative follow-up of DTC. However, radioiodine remnant ablation is not routinely recommended after thyroidectomy for low-risk patients with DTC [1-3]. Also, serum Tg and neck ultrasonography (US) have been recommended over RAI WBS, especially for the monitoring of low-risk patients with DTC because of low sensitivity of RAI WBS and the lack of additional information it provides as compared with serum Tg measurements [1-3].
TSH-stimulated serum Tg levels are the most sensitive marker for the detection of recurrence or metastasis and usually correlate with the results of RAI WBS. Occasionally, discordant results of serum Tg measurement and RAI WBS are encountered, and most of them are a positive serum Tg and a negative RAI WBS finding [4-6]. A few reports suggest that lesions detected only by RAI WBS with negative serum Tg levels could be recurrent or metastatic disease, routinely recommending RAI WBS to detect functioning recurrence and metastases regardless of serum Tg results [5, 6].
In the present study, the author reports a rare case of conversion from a negative (undetectable) stimulated serum Tg level and positive WBS to a positive (detectable) stimulated serum Tg level and positive WBS after RAI remnant ablation in a PTC patient with unsuspected multiple local and distant metastases on post-ablative WBS despite a negative stimulated serum Tg level.
| Case Report | ▴Top |
Investigations
A 41-year-old man was referred for the evaluation of thyroid nodules. He had no thyrotoxic symptoms or pain. He also had no other medical diseases or surgery in his history and had no specific diseases in his family history. Thyroid function tests were as follows: free thyroxine (fT4) level 1.08 ng/dL (reference, 0.78 - 1.94 ng/dL), TSH level 5.55 mIU/L (reference, 0.25 - 4.0 mIU/L). The thyroid US showed diffuse thyroid disease, a 2.4 × 2.1 × 3.35-cm-sized predominantly solid nodule with an isoechoic, parallel, ill-defined margin, punctate echogenic foci in the right mid-thyroid (2021 Korean Thyroid Imaging Reporting and Data System) [7] (K-TIRADS 4, intermediate suspicion, a fine-needle aspiration (FNA) was done), a 1.08 × 0.66 × 0.97-cm-sized pure cyst in the left upper thyroid (K-TIRADS 2, benign), a 1.89 × 1.07 × 1.87-cm-sized predominantly solid nodule with an isoechoic, parallel, ill-defined margin, no calcification in the left lower thyroid (K-TIRADS 3, low suspicion, an FNA was done), and multiple suspicious bilateral neck lymph nodes with cystic change, echogenic foci in a solid portion, and a loss of echogenic hilum. These lymph nodes were suspicious of malignancy (1.71 cm, 1.92 cm, 1.56 cm, 1.71 cm, 1.97 cm, 1.25 cm in the right and 1.4 cm in the left); however, the initial report suggested that multiple reactive lymph nodes were more likely. The FNA cytology findings were atypia of undetermined significance in the right nodule and were non-diagnostic in the left nodule (the 2023 Bethesda System for Reporting Thyroid Cytopathology) [8]. In addition, a repeated FNA was recommended for the right thyroid nodule, since PTC could not be excluded. There were multiple cold nodules in both thyroid lobes in the thyroid scintigraphy.
Diagnosis
After sharing decision-making with the patient, the patient first underwent a right thyroidectomy instead of re-aspiration. The frozen biopsy findings were consistent with a classic type of PTC with a perithyroidal extension to the fat. Finally, the patient underwent a total thyroidectomy with bilateral central neck dissection. The permanent pathologic findings were a 2.8 × 1.7-cm solid right PTC with a perithyroidal extension to the fat, a focally lymphovascular invasion, a focally suspicious involvement of the lateral resection margin, and a 3.5 × 3-cm ill-defined cystic-to-solid left PTC with multicentricity versus multifocal intrathyroidal micrometastases, minimal extension to the perithyroidal soft tissue, and a very close resection margin (a < 0.1 mm of safety margin). In addition, metastases were identified in 11 of the 12 cervical lymph nodes (nine of nine in the right). During postoperative care, multiple hard, fixed masses were palpable in the right neck. In addition, a right modified radical neck dissection type 1 which removed level II-V lymph nodes, the sternocleidomastoid (SCM) muscle and the jugular vein was performed 2 weeks after a total thyroidectomy. Metastatic PTCs in the conglomerated lymph nodes were noted, and the SCM muscle and the jugular vein were free of tumor. The patient’s postoperative tumor staging was American Joint Committee on Cancer (AJCC) eighth edition/TNM staging stage I/T2N1b, and the American Thyroid Association (ATA) risk stratification was intermediate.
Five weeks after a total thyroidectomy, 5.55 GBq (150 mCi) of 131I was administered without thyroid hormone replacement to ablate thyroid remnant. The stimulated serum Tg level was 0.3 ng/mL (reference, 0 - 40 ng/mL) with a fT4 level 0.42 ng/dL and a TSH level 58.72 mIU/L with negative anti-thyroglobulin antibody (Tg-Ab, 6.2 IU/mL, reference, 0 - 70 IU/mL) immediately before the RAI administration. A post-ablative WBS revealed multiple RAI uptakes at thyroid remnants and lymph nodes in both anterolateral neck and functioning metastases to both lungs (Fig. 1). Multiple 2 - 5 mm nodules in both lungs and a few small but enhancing lymph nodes in the left supraclavicular area suggesting metastases were observed in the chest computed tomography (CT) 2 months after RAI ablation. Two heterogeneous hypoechoic nodular lesions (1.5 and 1 cm) in the left supraclavicular fossa suggesting metastasis were also noted on neck US. Finally, the patient’s tumor staging was AJCC eighth edition/TNM staging stage II/T2N1bM1, and the ATA risk stratification was high.
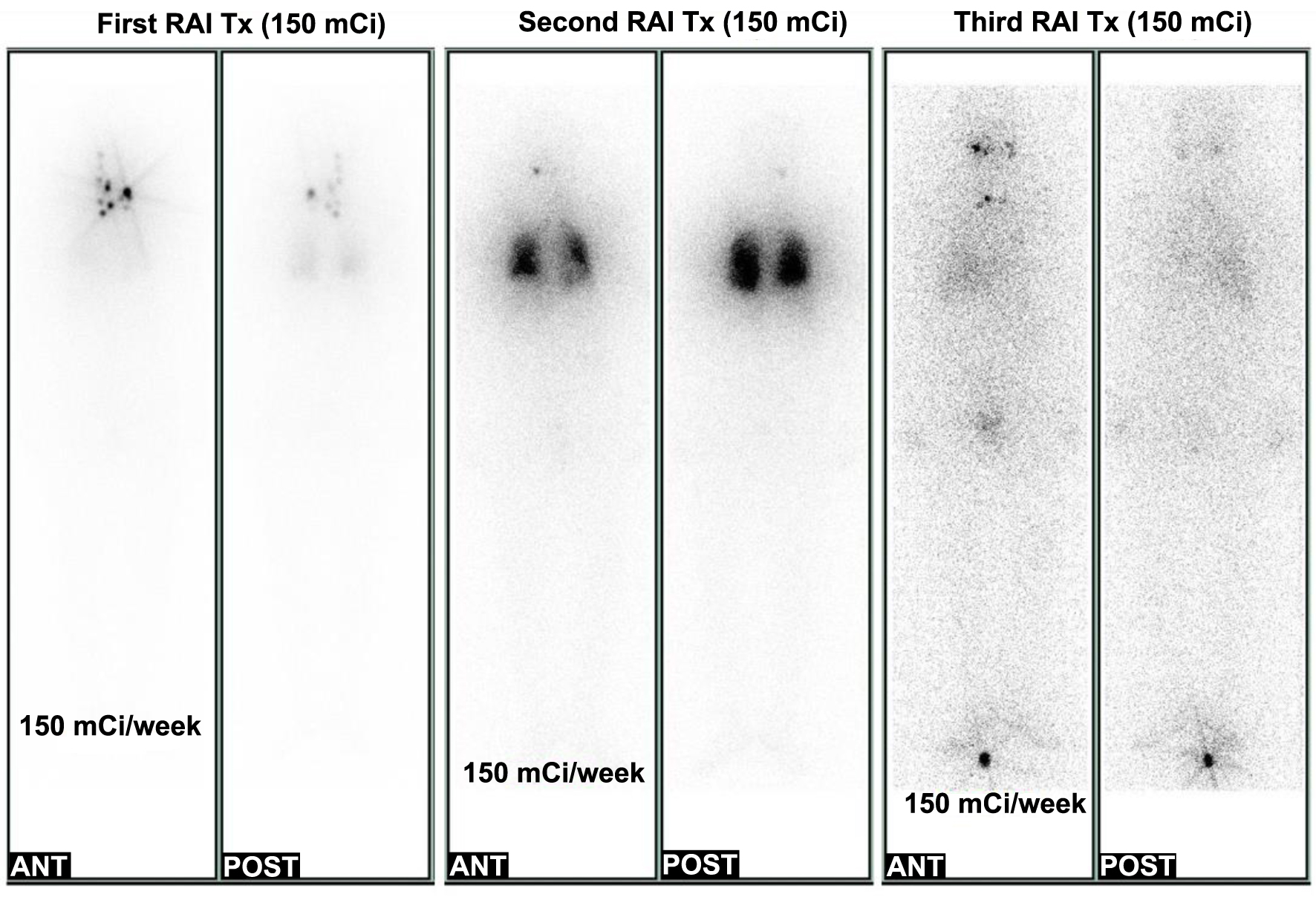 Click for large image | Figure 1. Follow-up of post-therapeutic whole-body scan (RxWBS) during the clinical course. The first RxWBS revealed multiple radioactive iodine (RAI) uptakes at thyroid remnants and multiple lymph nodes in the anterolateral neck and functioning metastases to both lungs (left). The second RxWBS showed focal hot uptake in the head (right retro-pharyngeal node) and diffuse RAI uptake in both lungs (center). The third RxWBS showed no RAI uptake in lung lesions and focal hot uptake in the right maxilla, left parotid gland, and isthmus of the thyroid (right). RAI Tx: RAI therapy; ANT: anterior; POST: posterior. |
Treatment, follow-up and outcomes
Immediately, left neck lymph node dissection in levels 3, 4, and 5 was performed, and metastatic papillary carcinomas in five out of 10 lymph nodes in levels 3 and 4 were identified. A suppressed serum Tg level was 8.3 ng/mL with a fT4 level of 1.52 ng/dL, TSH of 0.85 mIU/L and negative Tg-Ab (Fig. 2). The second round of RAI therapy (150 mCi of 131I) was administered 6 months after RAI ablation. A post-therapeutic WBS (RxWBS) showed diffuse RAI uptakes in both lungs, and the stimulated serum Tg level was 93 ng/mL with a TSH level of 65.33 mIU/L and negative Tg-Ab level. Nine months later, a third round of RAI therapy (150 mCi of 131I) was performed, and RxWBS showed no RAI uptake in the lesions of either lung. The stimulated serum Tg level was 59.2 ng/mL, with a TSH level of 65.46 mIU/L and negative Tg-Ab level. During the clinical course, all serum Tg levels were measured using the Tg-S immunoradiometric assay (IRMA) CT kit (RADIM, Pomezia, Italy).
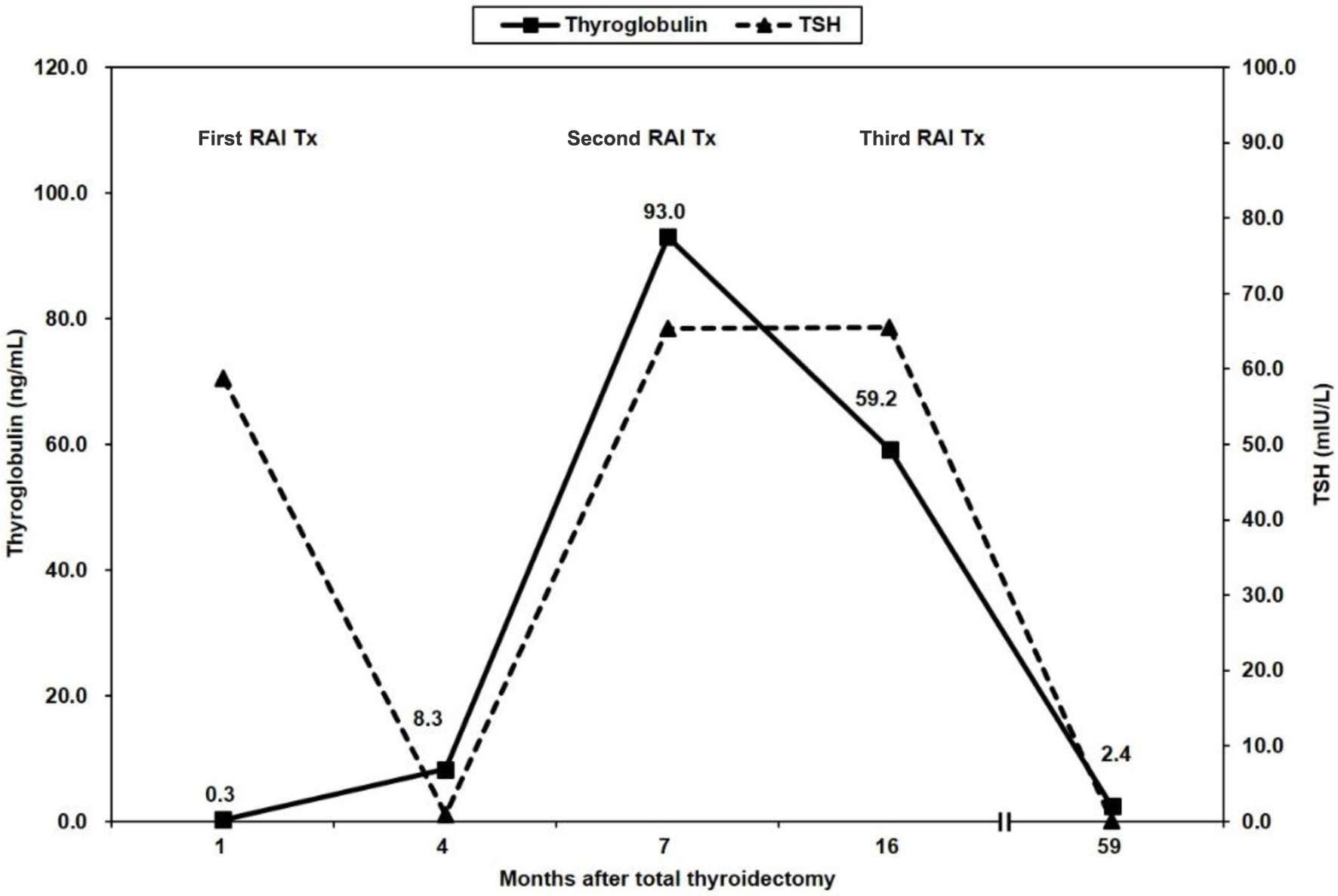 Click for large image | Figure 2. Follow-up of serum thyroglobulin levels during the clinical course. Negative serum thyroglobulin levels were increased after radioactive iodine (RAI) remnant ablation (first RAI Tx). Thereafter, serum thyroglobulin levels continuously decreased throughout the course. RAI Tx: RAI therapy; TSH: thyroid-stimulating hormone. |
Thereafter, any new recurrent or remnant lesions were not noted in the imaging study, including neck US, chest CT, and fluorine-18 fluorodeoxyglucose positron emission tomography (PET)-CT, although suppressed serum Tg levels were 1.7 - 3.1 ng/mL with a negative Tg-Ab level. The last suppressed serum Tg levels were 2.4 ng/mL at 58 months follow-up after initial ablative therapy.
| Discussion | ▴Top |
The most common postoperative tools used to define staging assessments and recurrence include serum Tg measurement, neck US, and RAI WBS. The importance of measuring serum Tg levels in the follow-up of DTC is well established. However, serum Tg measurements are performed using a number of assays that produce variable results, are influenced by the thyroid state (i.e., TSH levels), and are affected negatively by Tg-Ab. Neck US has high sensitivity for detection of cervical lymph node metastases, but is operator-dependent and has suboptimal specificity. Although RAI WBS is performed by different iodine isotopes and acquisition modalities with varying sensitivity and specificity, it has been shown to contribute to staging and risk stratification, especially by detection of unsuspected lymph nodes and distant metastases [9].
Cervical lymph nodes with any of the following sonographic features are regarded as suspicious: cystic changes, echogenic foci (calcifications), cortical hyperechogenicity (focal/diffuse), or abnormal vascularity (peripheral/diffuse) [7]. The patient’s initial preoperative thyroid US report was misdiagnosed as multiple reactive cervical lymph nodes, although they actually had suspicious imaging features. US-guided FNA and measurement of the tissue-washout Tg were recommended to assess for malignancy and determine the extent of initial surgery. Retrospectively, the inadequate preoperative assessment of the cervical lymph nodes leaded to persistent disease in the neck and reoperations in this case. The knowledge of US features is important for the diagnosis and optimal management plan of thyroid nodules.
This case showed the size discrepancy between the preoperative sonographic and the pathologic measurements for PTC. Deveci et al [10] reported that the concordance in the size of benign or malignant nodules measured by US and surgical pathology examination is ≤ 50%, except in the ≤ 1.0-cm size range (78.5%). In Hahn et al’s study [11], the agreement rate between US and gross pathological size measurements of PTC was 64.1%, and the tumor size between US and pathology was significantly discordant in tumors < 1.0 cm and cystic change. Size measurements by US may be of limited accuracy in nodules with a vague margin, irregular shape, or small size, in conglomerated masses of small nodules, and in patients with a short neck, a large goiter, or a thyroid nodule located in the lower portion of the neck [11].
In low-risk patients with DTC, neck US and serum Tg measurement are equivalent or superior in detecting and localizing residual disease compared to RAI WBS [1]. The majority of low-risk (AJCC stage I and II) patients with DTC did not demonstrate improvement in mortality or disease-specific survival from postoperative RAI remnant ablation, whereas a significant improvement in overall and disease-specific mortality, as well as disease-free survival, was confirmed for high-risk (AJCC stages III and IV) patients [2, 12]. RAI therapeutic efficacy in patients < 45 years of age with nodal metastases is unclear, because such patients are categorized as stage I with no benefit of RAI therapy [2]. The systematic reviews did not show a significant benefit of RAI remnant ablation on disease-related mortality in low-risk patients with DTC and demonstrated conflicting results in reducing disease recurrence [1, 12]. In these low-risk patients, the issue may not be the efficacy of RAI in treating residual disease, but rather the detection of the relatively small number of low-risk patients who truly have residual disease [9]. In 2015 ATA guidelines, RAI therapy was not routinely recommended in low-risk patients, should be considered in intermediate-risk patients, and was routinely recommended in high-risk patients [2]. Although there are insufficient and conflicting data for ATA intermediate-risk patients, postoperative RAI treatment in intermediate-risk PTC patients is associated with an improved overall survival in aggressive PTC histology, node-positive adult patients with PTC or pT3 node-negative PTC, in which the primary tumor is > 4 cm, where there is evidence of a microscopic extrathyroidal extension, or in patients with advanced age [2, 13]. First, this improved survival of postsurgical RAI treatment was associated in a subgroup analysis among patients < 45 years, as well as in all patients with intermediate risk [13]. For intermediate-risk patients, the evidence of the impact of RAI therapy on survival or disease recurrence is conflicting. The greatest potential benefit of RAI therapy can be expected primarily in patients with a higher risk of recurrent or persistent disease such as adverse thyroid cancer histologies, an increasing volume of nodal disease, lymph node disease outside of the central neck, or advancing patient age [2].
A negative serum Tg and positive WBS has been reported in a small, but significant number of cases [4-6, 14-16]. False-negative serum Tg occurred in patients with small PTC with cervical or mediastinal lymph node metastases, in follicular thyroid cancer (FTC) patients with bone metastases, and in 8.5% of DTC patients with metastases [4]. Caballero-Calabuig et al [5] reported that two (1.6%) of 128 patients with DTC had a negative serum Tg and positive post-ablative WBS revealing lymph node uptake. One year after RAI remnant ablation, six (5.2%) among 115 patients with initially a positive serum Tg and positive post-ablative WBS showed a negative serum Tg and positive diagnostic WBS due to lymph node uptake (one) and metastases (five). The authors proposed performing periodic diagnostic WBS to improve Tg specificity. Park et al [6] evaluated 824 patients with DTC who underwent post-ablation or therapeutic WBS. Fifty-two (6.3%) patients with functioning metastases had a negative serum Tg and positive WBS: 45 cases of cervical/mediastinal lymph node metastases and seven cases of distant metastasis to the lung or bone. The authors insisted that WBS should be undertaken routinely as a complimentary modality to detect functioning recurrence and metastasis regardless of serum Tg results. Cherk et al [14] documented that nine (4.7%) out of 193 patients with DTC had lateral neck uptake suspicious for loco-regional metastatic disease in post-ablative WBS without detectable Tg, recommending performing both serum Tg and WBS for the follow-up of higher risk patients. In a retrospective analysis of 52 post-ablative WBS positive patients, 10 patients had a negative serum Tg. After 1-year follow-up, four out of 42 patients with initially a positive serum Tg and positive WBS showed a negative Tg and positive WBS, suggesting that diagnostic WBS is a valuable tool in addition to the Tg level [15]. Shen et al [16] reported that 71 (2.1%) among a total of 3,367 DTC patients had a negative serum Tg and positive post-therapeutic WBS. Of these 71 patients, two (2.8%) patients had bone metastasis, 11 (15.5%) had lung metastasis, and 59 (83.1%) had lymph node metastasis. Fifty-six patients had cervical lymph node metastasis, and US was positive in 15 patients (26.8%) but negative in 41 (73.2%), showing a relatively low US positivity rate. The authors suggested an advantage of WBS in the detection of functioning metastasis despite low serum Tg levels in patients with metastatic DTC with limitations of US in negative serum Tg and positive WBS patients.
By contrast, Agate et al [17] analyzed post-ablative WBS in 545 low- and intermediate-risk DTC patients treated with 30 mCi of 131I after levothyroxine (LT4) withdrawal. In all, 16/545 (2.9%) cases showed metastatic lesions in WBS, including lateral cervical lymph nodes in 11/16 patients (10/11 were also detected by neck US), mediastinum in 1/16, lung in 3/16, and bone metastases in 1/16. In all, 6/545 (1.1%) metastases were detected by WBS alone, and 2/16 WBS positive patients had a negative serum Tg [17]. The same authors showed that only 1.3% of the low- and intermediate-risk DTC patients showed a positive post-ablative WBS when treated with 30 mCi of 131I after recombinant human TSH (rhTSH) stimulation [18]. The authors suggested that the cost-effectiveness of performing RAI remnant ablation and post-ablative WBS in all low- and intermediate-risk DTC patients to find 1-2% of the cases with distant metastases remains controversial [17].
The possible causes of false-negative Tg are the following: low functional sensitivity of Tg assay, interference from the presence of Tg-Ab, the hook effect, and immunologically inactive Tg [4]. The functional sensitivity of Tg methods is low enough to detect small amounts of thyroid tissue or heterogeneity in circulating Tg. Endogenous Tg-Ab is a known confounder of serum Tg measurements and similar to Tg, conformational epitopes of Tg-Ab can be highly variable between individuals. Hook effects can occur in the presence of very high Tg levels that exceed the binding capacity of antibody. The marginally differentiated metastatic tumors can still concentrate iodine, but are prone to association with low Tg levels via decreased normal Tg synthesis and/or release, by the synthesis of a Tg variant undetected by routine assays, or by a more rapid clearance of Tg from the plasma [4, 6, 14]. On the other hand, false-positive WBS occurs by external contamination through saliva and sweat and internal contamination through nasopharyngeal secretion, as wells as physiologic uptake in nonthyroidal tissue such as the choroid plexus, salivary glands, gastric mucosa, and urinary tract [4]. False-positive 131I uptakes may also occur secondary to non-thyroid conditions (bronchiectasis, lung infection, subcutaneous injection into gluteal fatty tissue, aortic calcification, benign bone cyst, vertebral hemangioma, recent non-thyroid surgical procedure site, rotator cuff injury, mature cystic teratoma, and ovarian follicle cyst) and its underlying mechanisms with inflammation, trapping, increased perfusion, etc. [19]. Furthermore, there was a recent case report for false-positive WBS due to trapping of the isotope in an intraosseous hemangioma with slow blood clearance [20].
Tg is known to increase with benign thyroid swelling and after trauma or surgery, as well as with FNA of the intact thyroid gland itself [21]. The half-life of circulating Tg is 65 h [21, 22]. A previous study reported that there is a transient increase in serum Tg levels 48 h to 7 days after RAI ablation and that serum Tg levels decreased below baseline by 6 months [22, 23]. In previous studies, the serum Tg level immediately after RAI therapy was frequently elevated and reflected destruction of follicular cells from cancer and normal thyroid tissue, which is significantly higher in patients with midline uptake on post-ablation WBS [24, 25]. This is presumed to reflect RAI-induced thyroid tissue destruction/inflammation with subsequent release of Tg from the thyroid remnant [23]. In addition, several reports revealed that a greater increase in Tg levels after RAI administration showed better treatment outcomes, including successful RAI ablation of remnant thyroid tissue, reflecting the destruction of cancer and thyroid cells [22, 23, 26].
The case presented herein showed unsuspected multiple metastatic lesions in both cervical lymph nodes and both lungs on WBS after RAI remnant ablation despite an initial negative stimulated serum Tg level, which was converted to positive after RAI remnant ablation. The patient’s serum Tg levels were not evaluated until the stimulated serum Tg levels were checked immediately before RAI remnant ablation. During the clinical course, all Tg levels were evaluated using the same assay method, thus mitigating the risk of false-negative Tg and false-positive WBS. There are two possible causes of false-negative Tg levels despite local and distant metastases in post-ablative WBS. One is that marginally differentiated metastatic tumors can still concentrate iodine but are not associated with normal Tg synthesis or release. False-negative Tg is also associated with small PTC. Because 1 g of cancer tissue elevates serum Tg levels by 0.5 - 1 ng/mL, smaller cancers secrete less Tg into the bloodstream. It has been previously reported that patients with bone and lung metastases have high Tg levels, whereas those with small PTC with cervical or mediastinal lymph node metastases have low Tg levels [6]. The patient’s negative stimulated serum Tg levels were increased after RAI remnant ablation despite normal TSH levels, which indicates RAI-induced destruction of thyroid remnant and cancer cells with subsequent release of Tg. Although suppressed serum Tg levels were detectable, the patient’s imaging studies, including WBS, eventually became negative after three cycles of radioiodine therapy.
Conclusion
In conclusion, it is important that clinicians are familiar with thyroid sonographic features for the optimal management of thyroid nodules. Despite a negative stimulated serum Tg, RAI WBS can contribute to the detection of a relatively small number of unsuspected local and distant metastases. Increased serum Tg levels after RAI remnant ablation may reflect RAI-induced destruction of thyroid remnant and cancer cells with better treatment outcomes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent has been obtained.
Data Availability
The data supporting the finding of this study are available from the corresponding author upon reasonable request.
Abbreviations
AJCC: American Joint Committee on Cancer; ATA: American Thyroid Association; CT: computed tomography; DTC: differentiated thyroid cancer; FNA: fine-needle aspiration; FTC: follicular thyroid cancer; fT4: free thyroxine; IRMA: immunoradiometric assay; K-TIRADS: Korean Thyroid Imaging Reporting and Data System; PET: positron emission tomography; PTC: papillary thyroid cancer; RAI: radioactive iodine; rhTSH: recombinant human TSH; RxWBS: post-therapeutic WBS; SCM: sternocleidomastoid; Tg: thyroglobulin; Tg-Ab: anti-thyroglobulin antibody; TSH: thyroid-stimulating hormone; US: ultrasonography; WBS: whole-body scan
| References | ▴Top |
- Lamartina L, Durante C, Filetti S, Cooper DS. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 2015;100(5):1748-1761.
doi pubmed - Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
doi pubmed pmc - Schlumberger M, Borget I, Nascimento C, Brassard M, Leboulleux S. Treatment and follow-up of low-risk patients with thyroid cancer. Nat Rev Endocrinol. 2011;7(10):625-628.
doi pubmed - Ma C, Kuang A, Xie J, Ma T. Possible explanations for patients with discordant findings of serum thyroglobulin and 131I whole-body scanning. J Nucl Med. 2005;46(9):1473-1480.
pubmed - Caballero-Calabuig E, Cano-Terol C, Sopena-Monforte R, Reyes-Ojeda D, Abreu-Sanchez P, Ferrer-Rebolleda J, Sopena-Novales P, et al. Influence of the thyroid remnant in the elevation of the serum thyroglobulin after thyroidectomy in differentiated thyroid carcinoma. Importance of the diagnostic iodine total-body scanning. Eur J Nucl Med Mol Imaging. 2008;35(8):1449-1456.
doi pubmed - Park EK, Chung JK, Lim IH, Park DJ, Lee DS, Lee MC, Cho BY. Recurrent/metastatic thyroid carcinomas false negative for serum thyroglobulin but positive by posttherapy I-131 whole body scans. Eur J Nucl Med Mol Imaging. 2009;36(2):172-179.
doi pubmed - Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, Park JS, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2021;22(12):2094-2123.
doi pubmed pmc - Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid. 2023;33(9):1039-1044.
doi pubmed - Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, Dillehay G, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019;29(4):461-470.
doi pubmed - Deveci MS, Deveci G, LiVolsi VA, Gupta PK, Baloch ZW. Concordance between thyroid nodule sizes measured by ultrasound and gross pathology examination: effect on patient management. Diagn Cytopathol. 2007;35(9):579-583.
doi pubmed - Hahn SY, Shin JH, Oh YL, Son YI. Discrepancies between the ultrasonographic and gross pathological size of papillary thyroid carcinomas. Ultrasonography. 2016;35(3):220-225.
doi pubmed pmc - Sacks W, Fung CH, Chang JT, Waxman A, Braunstein GD. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20(11):1235-1245.
doi pubmed - Ruel E, Thomas S, Dinan M, Perkins JM, Roman SA, Sosa JA. Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J Clin Endocrinol Metab. 2015;100(4):1529-1536.
doi pubmed pmc - Cherk MH, Francis P, Topliss DJ, Bailey M, Kalff V. Incidence and implications of negative serum thyroglobulin but positive I-131 whole-body scans in patients with well-differentiated thyroid cancer prepared with rhTSH or thyroid hormone withdrawal. Clin Endocrinol (Oxf). 2012;76(5):734-740.
doi pubmed - Kucukalic-Selimovic E, Alagic J, Valjevac A, Hadzovic-Dzuvo A, Begic A, Beslic N. The value of serum thyroglobulin levels and whole body (I-131) scintigraphy in the follow-up of the thyroid cancer patients after thyroidectomy. Coll Antropol. 2012;36:67-71.
- Shen CT, Wei WJ, Qiu ZL, Song HJ, Luo QY. Value of post-therapeutic (1)(3)(1)I scintigraphy in stimulated serum thyroglobulin-negative patients with metastatic differentiated thyroid carcinoma. Endocrine. 2016;51(2):283-290.
doi pubmed - Agate L, Bianchi F, Brozzi F, Santini P, Molinaro E, Bottici V, Viola D, et al. Less than 2% of the low- and intermediate-risk differentiated thyroid cancers show distant metastases at post-ablation whole-body scan. Eur Thyroid J. 2019;8(2):90-95.
doi pubmed pmc - Matrone A, Gambale C, Piaggi P, Viola D, Giani C, Agate L, Bottici V, et al. Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. J Clin Endocrinol Metab. 2017;102(3):893-902.
doi pubmed - Oral A, Yazici B, Eraslan C, Burak Z. Unexpected false-positive I-131 uptake in patients with differentiated thyroid carcinoma. Mol Imaging Radionucl Ther. 2018;27(3):99-106.
doi pubmed pmc - Kang H, Drake FT, McAneny D, Lee SL. False-positive imaging for papillary thyroid cancer caused by intraosseous hemangioma. JCEM Case Rep. 2023;1(5):luad102.
- Moosavi M, Kreisman S. A case report of dramatically increased thyroglobulin after lymph node biopsy in thyroid carcinoma after total thyroidectomy and radioiodine. Case Rep Endocrinol. 2016;2016:6471081.
doi pubmed pmc - Choi JH, Lim I, Lee I, Byun BH, Kim BI, Choi CW, Lim SM. An enhanced treatment effect can be expected from a higher serum thyroglobulin level after radioactive iodine therapy. Ann Nucl Med. 2019;33(2):128-134.
doi pubmed - Stevic I, Dembinski TC, Pathak KA, Leslie WD. Transient early increase in thyroglobulin levels post-radioiodine ablation in patients with differentiated thyroid cancer. Clin Biochem. 2015;48(10-11):658-661.
doi pubmed - Jeong GC, Song M, Park HJ, Min JJ, Bom HS, Cho SG, Park KS, et al. Iodine uptake patterns on post-ablation whole body scans are related to elevated serum thyroglobulin levels after radioactive iodine therapy in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2016;50(4):329-336.
doi pubmed pmc - Kim HK, Yoon JH, Cho JS, Kwon SY, Yoo SW, Kang HC. The clinical meaning of pre- and post-ablation thyroglobulin levels at first radioiodine therapy in patients with papillary thyroid cancer. Korean J Intern Med. 2020;35(5):1164-1172.
doi pubmed pmc - Song M, Jeon S, Kang SR, Jabin Z, Yoo SW, Min JJ, Bom HS, et al. Response prediction of altered thyroglobulin levels after radioactive iodine therapy aided by recombinant human thyrotropin in patients with differentiated thyroid cancer. Nucl Med Mol Imaging. 2018;52(4):287-292.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
