| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Letter to the Editor
Volume 13, Number 4, November 2023, pages 170-173
The Safety of Sodium Glucose Cotransporter 2 Inhibitors for the Elderly Diabetic Patients
Hidekatsu Yanaia, b, Hiroki Adachia, Mariko Hakoshimaa, Hisayuki Katsuyamaa
aDepartment of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, Chiba, Japan
bCorresponding Author: Hidekatsu Yanai, Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted August 4, 2023, accepted September 5, 2023, published online October 21, 2023
Short title: Safety of SGLT2i for the Elderly DM Patients
doi: https://doi.org/10.14740/jem892
| To the Editor | ▴Top |
Sodium glucose cotransporter 2 inhibitors (SGLT2i) have shown to prevent the hospitalization for heart failure and retard the progression of chronic kidney disease (CKD), and then, SGLT2i is increasingly being used in the elderly. One of SGLT2i, canagliflozin has shown to improve glycemic control, reduced body weight and systolic blood pressure, and was generally well tolerated in patients aged 55 - 80 years with type 2 diabetes over 104 weeks [1]. The meta-analysis was performed to compare the influence of SGLT2i on the efficacy and safety in elderly type 2 diabetic patients (≥ 65 years) with those in young type 2 diabetic patients (< 65 years) [2]. Although the improvement of hemoglobin A1c (HbA1c) in elderly patients receiving SGLT2i was not as good as that in young patients, relatively satisfying effectiveness was observed in the fasting plasma glucose, body weight, blood pressure in the elderly patients. Some adverse events (AEs), such as volume depletion (odds ratio (OR): 2.80; 95% confidence interval (CI): 1.82 - 4.32; P < 0.001), and urinary tract infection (OR: 1.37; 95% CI: 1.18 - 1.60; P < 0.001), and renal impairment (OR: 2.61; 95% CI: 1.78 - 3.81; P < 0.001) had a higher risk in old patients than young ones, but most of these events were generally mild, rarely led to treatment discontinuation, or recovered following appropriate treatments. To prevent the occurrence of such AEs, the instruction to drink an appropriate amount of water, and the elderly patients’ cognitive ability to be able to bathe and excrete independently and to understand instructions regarding drinking water are required.
Since the adherence to diabetic treatment including anti-diabetic medications and insulin injection, and endogenous insulin secretion decrease in the elderly, there is concern about the development of euglycemic diabetic ketoacidosis (DKA) by SGLT2i. The underlying mechanism for SGLT2i-induced euglycemic DKA was shown in Figure 1. Insulin deficiency or relative insulin deficiency and an increase in glucagon activate hormone sensitive lipase (HSL), which hydrolyses triglyceride (TG) into free fatty acids (FFA). Increased FFA are transported into hepatic mitochondria by carnitine palmitoyl-transferase I (CPT-I), which increases the rate of β-oxidation and results in the development of euglycemic DKA due to overproduction of ketone bodies. Insulin deficiency and an increase in glucagon reduce the activity of acetyl-CoA carboxylase (ACC) which produces malonyl-CoA, a potent inhibitor of CPT-I, further enhancing β-oxidation. Possible euglycemic DKA-inducing factors were shown in Table 1. Low carbohydrate and energy intake and excessive energy expenditure by exercise decrease plasma glucose, which decreases insulin secretion [3, 4]. Intrinsic insulin secretion is absent or reduced in patients with type 1 diabetes, latent autoimmune diabetes in adults, a long-standing type 2 diabetes, and pancreatic diseases [4]. In the meta-analyses, a higher risk of DKA (relative risk (RR): 5.042, 95% CI: 3.160 - 8.046, P < 0.001) was associated with SGLT2i compared with placebo in patients with type 1 diabetes [5]; however, SGLT2i were not significantly associated with an increased risk of DKA when compared with placebo (OR: 1.98; 95% CI: 0.56 - 6.94) in patients with type 2 diabetes [6]. Reduced intrinsic insulin secretion can induce euglycemic DKA under the SGLT2i use. Cessation of insulin and reduction of insulin dose and cessation of insulin secretagogues such sulfonyl urea can be the cause of the SGLT2i-associated euglycemic DKA [3, 4]. In situations where anti-insulin hormones such as catecholamines are increased due to stress such as surgery or acute illness and insulin demand is increased, SGLT2i induces euglycemic DKA [3, 4, 7, 8].
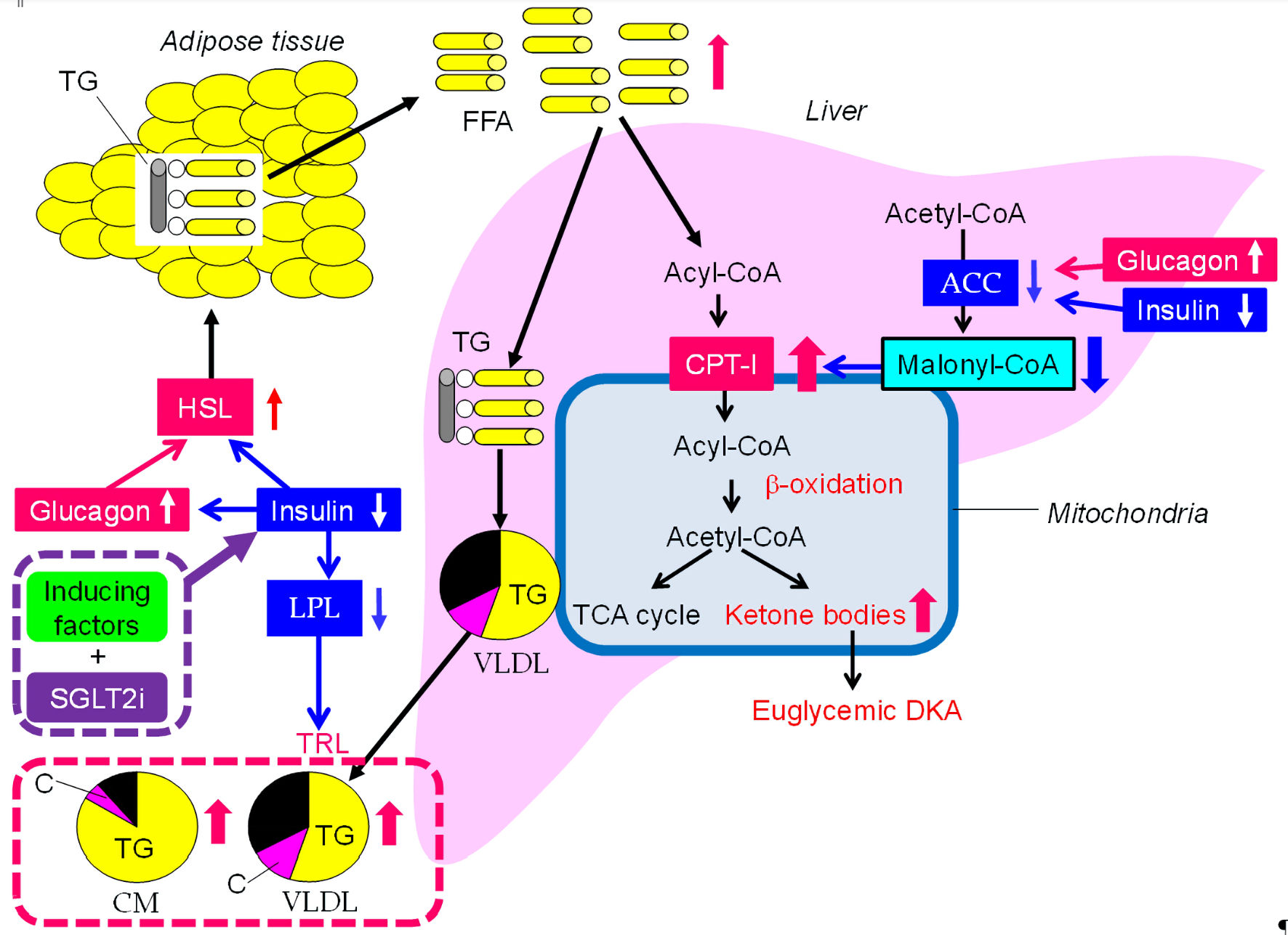 Click for large image | Figure 1. The underlying mechanism for SGLT2i-induced euglycemic DKA and elevation in triglyceride. Insulin with downward arrows include relative insulin deficiency due to increased insulin demand. SGLT2i: Sodium glucose cotransporter 2 inhibitors; DKA: diabetic ketoacidosis; ACC: acetyl-CoA carboxylase; C: cholesterol; CM: chylomicron; CPT-1: carnitine palmitoyl-transferase I; FFA: free fatty acids; HSL: hormone sensitive lipase; LPL: lipoprotein lipase; TCA: tricarboxylic acid; TG: triglyceride; TRL: TG-rich lipoproteins; VLDL: very-low-density lipoprotein. |
 Click to view | Table 1. Possible Inducing Factors for Euglycemic Diabetic Ketoacidosis |
To prevent the development of euglycemic DKA in the elderly, an adequate assessment of intrinsic insulin secretory capacity is required. To assess intrinsic insulin secretary capacity, what should we measure? SGLT2i improve metabolic parameters induced by insulin resistance such as reduced high-density lipoprotein-cholesterol (HDL-C) level and elevated levels of TG by reducing body weight. Therefore, SGLT2i should be used for elderly patients with insulin resistance and/or hyperinsulinemia. The homoeostasis model assessment of insulin resistance (HOMA-IR) is a useful index for insulin resistance, and it has actually been widely used to evaluate insulin resistance in subjects with prediabetes or early type 2 diabetes. However, the HOMA-IR using serum insulin concentration in patients treated with insulin or insulin secretagogues need to be interpreted with caution. C-peptide and insulin are secreted in equimolar amounts from pancreatic beta cells. Intrinsic insulin secretion can be assessed even in the presence of exogenous insulin by measuring C-peptide. We had previously reported that fasting serum C-peptide levels were significantly correlated with visceral fat area, measured by abdominal computed tomography (r = 0.634, P < 0.001) in Japanese patients with type 2 diabetes, who had relatively long durations of diabetes (9.1 ± 9.9 years) and were treated by hypoglycemic agents including insulin secretagogues or insulin [9]. Finally, we found that fasting serum C-peptide levels (> 1.6 ng/mL) can predict the presence of insulin resistance in Japanese patients with type 2 diabetes [10]. When using SGLT2i in the elderly, if the fasting serum C-peptide is 1.6 ng/mL or higher, it may be relatively safe to use SGLT2i. If an improvement in TG and non-HDL-C which reflects TG-rich lipoproteins is obtained at 1 month after SGLT2i administration, it would be considered that SGLT2i acted safely and effectively. If a deterioration in such parameters is observed, increases in FFA and TG-rich lipoproteins would have developed due to activation of HSL and reduction of lipoprotein lipase (LPL) activity by relative insulin deficiency. The reports on serum lipids in euglycemic DKA due to SGLT2i were very limited, and there were only two case reports. Serum TG was extremely elevated to greater than 8,300 mg/dL in type 2 diabetic patients with euglycemic DKA due to SGLT2i, however, unfortunately, only TG levels among serum lipids was described in this case report [11]. This case developed hypertriglyceridemia-induced acute pancreatitis, suggesting an elevation in chylomicron (CM). In another case, serum TG level peaked at 2,050 mg/dL, however, total cholesterol (TC) was relatively low (122 mg/dL) [12], suggesting a remarkable increase in TG-rich lipoproteins. Since hypertriglyceridemia is observed even in insulin resistance accompanied by hyperinsulinemia, an increase in TG-rich lipoproteins induced by relative insulin deficiency may precede euglycemic DKA.
The advantages and disadvantages of measurements of fasting C-peptide and TG for the evaluation of intrinsic insulin secretion were shown in Table 2.
 Click to view | Table 2. Advantages and Disadvantages of Measurements of Fasting C-Peptide and Triglyceride for the Evaluation of Intrinsic Insulin Secretion |
We have to mention the limitations of our article. Since euglycemic DKA is rare, and almost such case reports lack data on serum lipids and C-peptide, we cannot provide an estimate of euglycemic DKA risk for elderly patients such as low prior risk, which should be studied in the future.
In conclusion, euglycemic DKA is a life-threatening condition for the elderly type 2 patients. The establishment of predictive factors for the development of euglycemic DKA is desired.
Acknowledgments
We thank the staff of the Division of Research Support, National Center for Global Health and Medicine Kohnodai Hospital.
Financial Disclosure
Authors have no financial disclosure to report.
Conflict of Interest
The authors declare that they have no conflict of interest concerning this article.
Informed Consent
Not applicable.
Author Contributions
HY designed the research. MH, HK, and HA collected and analyzed data. HY wrote the paper, and all authors approved the final version of the paper.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Bode B, Stenlof K, Harris S, Sullivan D, Fung A, Usiskin K, Meininger G. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17(3):294-303.
doi pubmed - Wang Y, Shao X, Liu Z. Efficacy and safety of sodium-glucose co-transporter 2 inhibitors in the elderly versus non-elderly patients with type 2 diabetes mellitus: a meta-analysis. Endocr J. 2022;69(6):669-679.
doi pubmed - Goldenberg RM, Berard LD, Cheng AYY, Gilbert JD, Verma S, Woo VC, Yale JF. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38(12):2654-2664.e2651.
doi pubmed - Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
doi pubmed pmc - Zou H, Liu L, Guo J, Wang H, Liu S, Xing Y, Deng C, et al. Sodium-glucose cotransporter inhibitors as add-on therapy in addition to insulin for type 1 diabetes mellitus: A meta-analysis of randomized controlled trials. J Diabetes Investig. 2021;12(4):546-556.
doi pubmed pmc - Tang H, Li D, Wang T, Zhai S, Song Y. Effect of sodium-glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2016;39(8):e123-124.
doi pubmed - Branco A, Fatima R, Liblik K, Jackson R, Payne D, El-Diasty M. Euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitors after cardiac surgery: a review of current literature. J Cardiothorac Vasc Anesth. 2022;36(10):3877-3886.
doi pubmed - Hitsuwari T, Tsurutani Y, Yamane T, Sunouchi T, Horikoshi H, Hirose R, Hoshino Y, et al. Two cases of thyrotoxicosis and euglycemic diabetic ketoacidosis under sodium-glucose transport protein 2 inhibitor treatment. Intern Med. 2022;61(20):3069-3075.
doi pubmed pmc - Yanai H, Hirowatari Y. Different associations of body mass index and visceral fat area with metabolic parameters and adipokines in Japanese patients with type 2 diabetes. Diabetes Metab. 2015;41(3):261-262.
doi pubmed - Yanai H, Hirowatari Y. Fasting serum C-peptide levels (>1.6ng/mL) can predict the presence of insulin resistance in Japanese patients with type 2 diabetes. Diabetes Metab. 2017;43(1):97-98.
doi pubmed - Acevedo-Mendez BA, Ye Y, Hajizadeh N, Myers A. Hypertriglyceridemia-induced acute pancreatitis, euglycemic diabetic ketoacidosis and COVID-19 infection in a patient with type 2 diabetes taking a sodium-glucose cotransporter 2 inhibitor. Cureus. 2021;13(11):e19828.
doi pubmed pmc - Gajjar K, Luthra P. Euglycemic diabetic ketoacidosis in the setting of SGLT2 inhibitor use and hypertriglyceridemia: a case report and review of literature. Cureus. 2019;11(4):e4384.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
