| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 13, Number 4, November 2023, pages 164-169
A Challenging Case of Cushing’s Syndrome Due to Ectopic Adrenocorticotropic Hormone Secretion
Claudia Alexandrina Irimiea, b , Marius Irimiea, c
, Mihai Stelian Varciua, b
aFaculty of Medicine, Transilvania University of Brasov, Brasov, Romania
bDepartment of Endocrinology, Medlife-PDR Hyperclinic, Brasov, Romania
cCorresponding Author: Marius Irimie, Faculty of Medicine, Transilvania University of Brasov, Brasov 500019, Romania
Manuscript submitted July 7, 2023, accepted August 31, 2023, published online October 21, 2023
Short title: A Challenging Case of Cushing’s Syndrome
doi: https://doi.org/10.14740/jem889
| Abstract | ▴Top |
Adrenocorticotropic hormone (ACTH)-dependent Cushing’s syndrome secondary to an ectopic source is an uncommon condition. Hypercortisolism due to ectopic ACTH syndrome is usually rapidly progressive, and excess cortisol levels can lead to severe clinical manifestations, potentially life-threatening. We present the case of a 67-year-old woman who presented to the endocrinological evaluation for severe hypokalemia and massive edema of the upper and lower limbs. Hormonal investigations showed elevated plasma total cortisol, 24-h urinary free cortisol, and unsuppressed cortisol following 1 mg and 8 mg dexamethasone suppression test, high level of ACTH suggestive for ACTH-dependent Cushing’s syndrome. Calcitonin and chromogranin A levels were also elevated. Head magnetic resonance imaging (MRI) evaluation revealed a pituitary microadenoma. However, in the context of the onset of severe symptoms in the short term and the association of chronic hypokalemia with the failure of plasma cortisol suppression and the very high value of ACTH, a Cushing’s syndrome with ectopic secretion of ACTH was suspected. Neck and abdomen computed tomography (CT) scan showed a heterogeneous nodular mass of 14 × 13 mm in size with macrocalcifications in the right thyroid lobe and bilateral adrenal enlargement, confirming the diagnosis of ectopic ACTH syndrome, with thyroid nodule being the likely source of ectopic ACTH secretion. Due to hypokalemia refractory to treatment, severe hypercortisolism in the conditions of unavailability of steroidogenesis inhibitors, emergency bilateral adrenalectomy was performed. Diagnosis of ectopic ACTH syndrome is challenging and requires a multidisciplinary approach. Thyroid evaluation is important to identify a possible source of ectopic secretion of ACTH in the context of medullary thyroid carcinoma (MTC).
Keywords: Ectopic ACTH secretion; Cushing’s syndrome; Medullary thyroid carcinoma
| Introduction | ▴Top |
Adrenocorticotropic hormone (ACTH)-dependent Cushing’s syndrome (CS) secondary to an ectopic source is an uncommon condition, accounting for about 10% of all cases of CS [1]. Hypercortisolism due to ectopic ACTH syndrome (ECS) is usually rapidly progressive, and excess cortisol levels can lead to severe clinical manifestations, potentially life-threatening without timely diagnosis and intervention. Refractory hypokalemia is a common presenting feature in patients with ECS being observed in up to 90% of cases [2]. We present the case of a patient diagnosed with ECS with refractory hypokalemia and rapid clinical deterioration despite adrenal surgery.
| Case Report | ▴Top |
Investigations
A 67-year-old woman with medical history of obesity, dyslipidemia, hypertension, recently diagnosed type 2 diabetes mellitus (3 months ago), breast carcinoma (hormone-treated, previously refusing surgical therapy, 4 years ago), endometrial adenocarcinoma (with recommendation for surgery, 2 months ago), come to the endocrinological evaluation at the request of the cardiologist in the context of the electrolyte imbalance represented by severe hypokalemia and massive edema of the upper and lower limbs. The patient was being in preoperative evaluation for the surgical intervention of endometrial adenocarcinoma.
Diagnosis
At clinical examination, a body mass index of 31.2 kg/m2 with central adiposity, mild hirsutism (Ferriman-Gallwey scoring of 12), and massive persisting edema of the upper and lower limbs for about 3 months were noted. The patient also presented with polyuria-polydipsia syndrome (water intake about 4 L/day). The patient received recommendation for admission, and the initial investigations revealed: leukocytosis with neutrophilia, eosinopenia, hyperglycemia (167 mg/dL) with glycated hemoglobin of 10%, severe hypokalemia (1.9 mmol/L), and hypernatremia (150 mmol/L) (Table 1). Evaluation of the hypothalamic-pituitary-adrenal axis indicated hypercortisolism with significantly elevated value of serum cortisol at 8 am (2,039 nmol/L) and altered night plasma cortisol at 11 pm (1,923 nmol/L) (result obtained from 1:2 serum dilution), indicating an impaired diurnal cortisol rhythm. Determination of 24-h urine free cortisol also showed a very high level (15,555 nmol/24 h). The extremely high level of ACTH (346 pg/mL) and the lack of plasma cortisol suppression at 1 mg (2,754 nmol/L) and 8 mg dexamethasone (2,142 nmol/L), respectively, established the diagnosis of ACTH-dependent CS.
 Click to view | Table 1. Laboratory Values of the Patient |
Head magnetic resonance imaging (MRI) evaluation revealed a pituitary microadenoma of 5 × 3 × 4 mm, which did not exceed the pituitary gland and did not cause compressions on adjacent structures (Fig. 1). Abdominal computed tomography (CT) revealed bilaterally adrenal hyperplasia (Fig. 2). However, in the context of the onset of severe symptoms in the short term and the association of chronic hypokalemia with the failure of plasma cortisol suppression and the very high value of ACTH, a CS with ectopic secretion of ACTH was suspected.
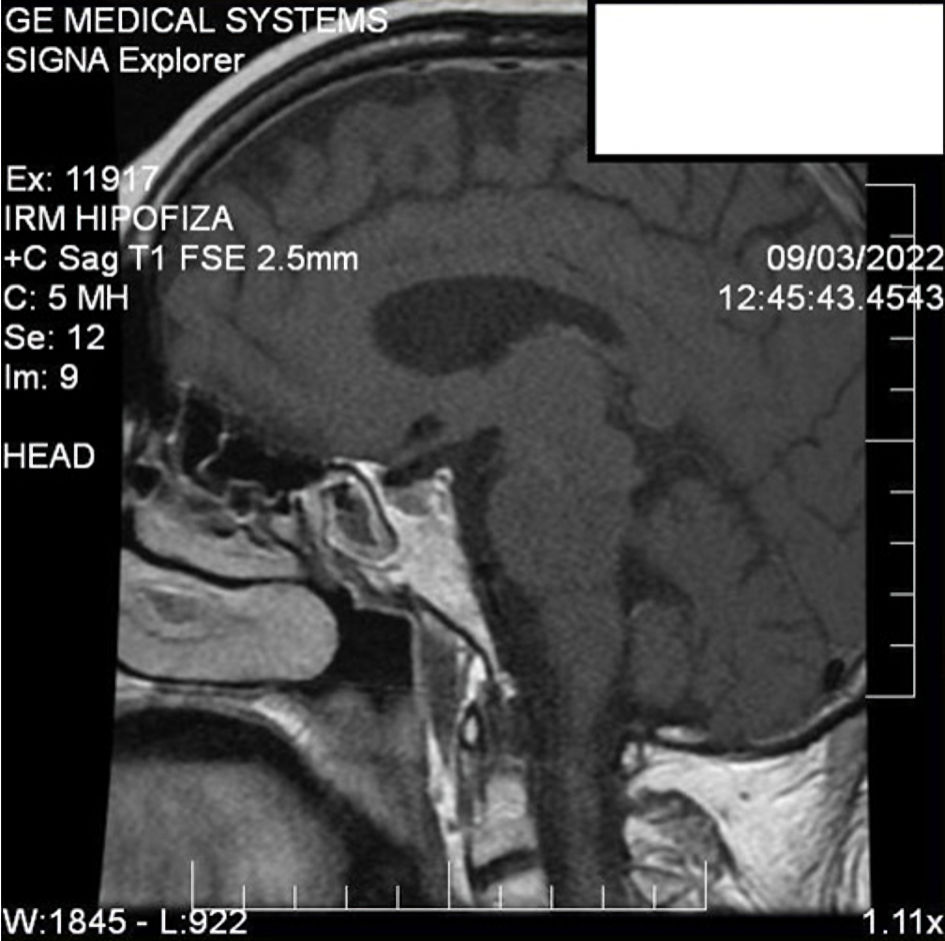 Click for large image | Figure 1. A sagittal view of the computed tomography of head with contrast showing pituitary microadenoma. |
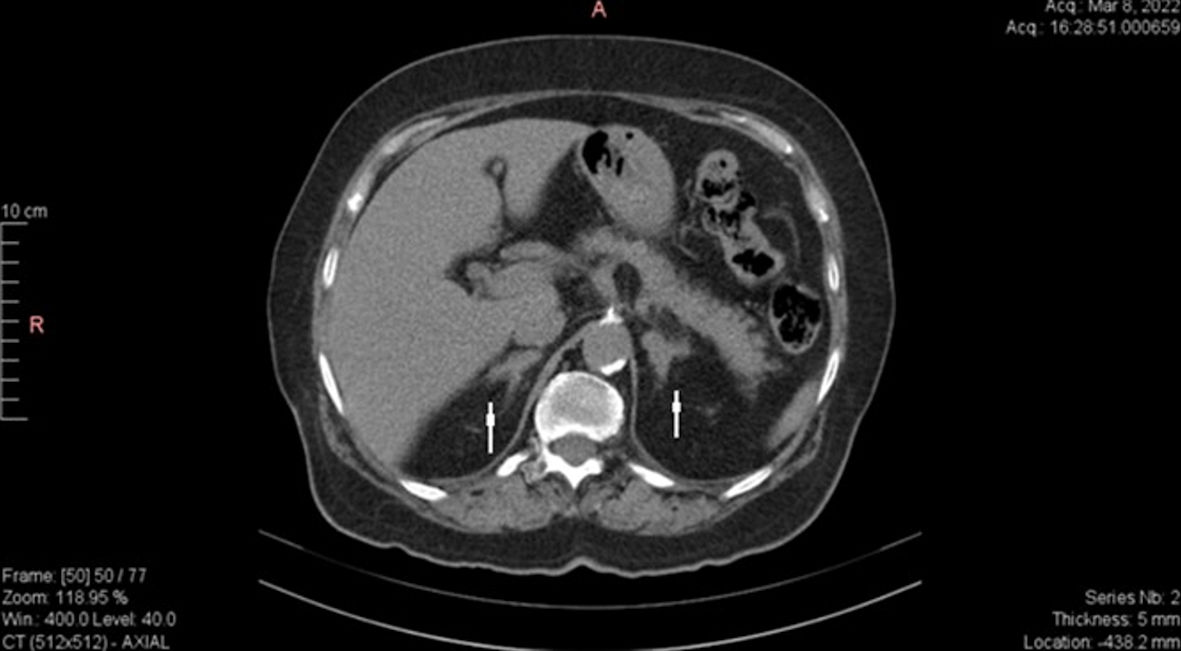 Click for large image | Figure 2. Abdomen CT scan showing bilaterally adrenal hyperplasia (arrows). CT: computed tomography. |
Further investigations were needed to identify ectopic ACTH secretion. Plasma metanephrines showed normal values. Calcitonin and chromogranin A levels were elevated (Table 1). Thyroid ultrasound revealed an irregular nodular mass of about 23 × 17 mm in size, but the evaluation was difficult in the context of the presence of the central venous catheter. The CT assessment of the neck area revealed a 20 × 15 × 18 mm heterogeneous nodular mass in the right thyroid lobe. Posterior to the former nodular mass, another nodular mass of 14 × 13 mm in size with macrocalcifications was highlighted (which was not identified on ultrasound). Small lymphadenopathies were noted in the upper latero-cervical area (Figs. 3, 4). Thyroid function tests indicated a slightly suppressed level of free thyroxine (FT4) with normal level of thyroid-stimulating hormone (TSH). Consequently, these results increased our suspicion for ectopic ACTH secretion within medullary thyroid carcinoma (MTC).
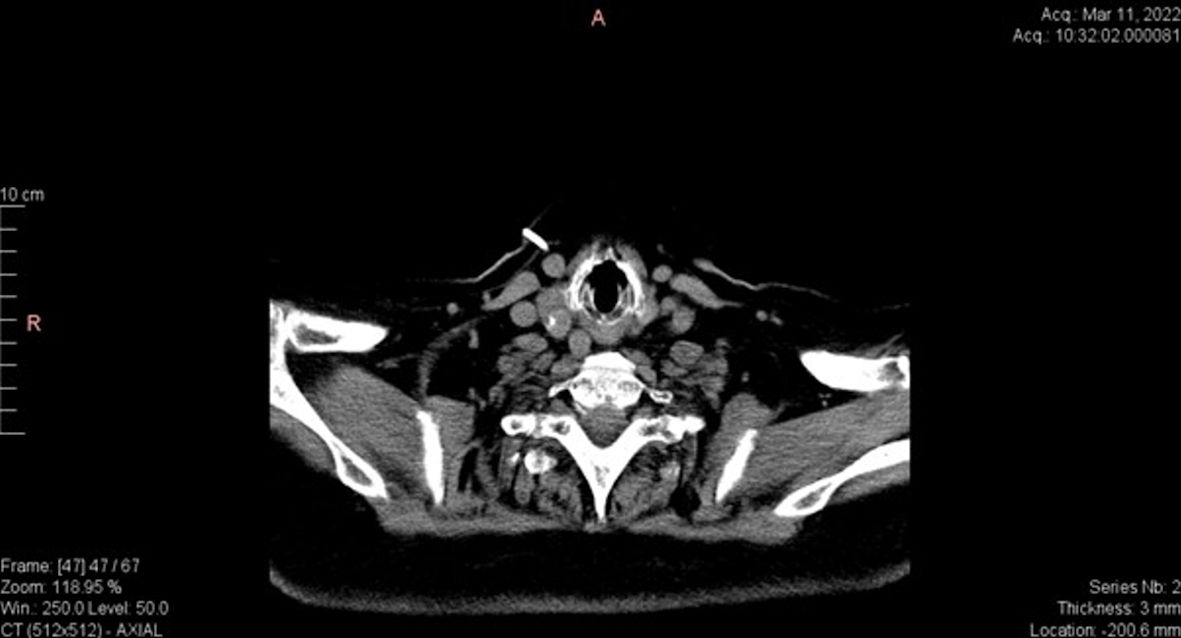 Click for large image | Figure 3. Axial CT scan of the neck showing heterogeneous nodular mass with macrocalcifications in the right thyroid lobe. CT: computed tomography. |
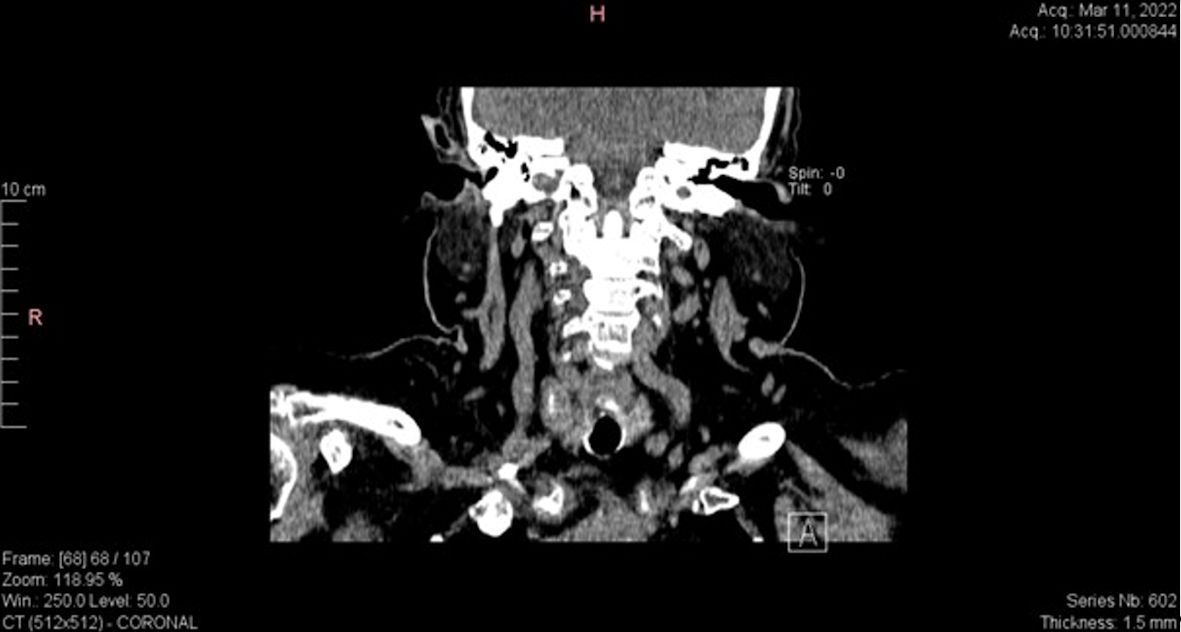 Click for large image | Figure 4. Coronal CT scan of the neck showing heterogeneous nodular mass with macrocalcifications in the right thyroid lobe. CT: computed tomography. |
Treatment
Despite therapy with spironolactone 200 mg twice a day (BID) and intravenous (IV) KCl 120 mEq/day, the normalization of the serum potassium value was not achieved. As steroidogenesis inhibitors were unavailable in our country, for a rapid control of severe hypercortisolism and severe hypokalemia, a specialized team consisting of endocrinologists, cardiologist, oncologist, general surgeon, intensive care specialist, established to perform emergency bilateral adrenalectomy approximately 10 days after admission. The histopathological examination of the adrenal glands did not show malignancy.
Follow-up and outcomes
Substitution treatment with glucocorticoids was administered after bilateral adrenalectomy. In the subsequent 4 - 5 days after surgery, the patient was slightly confused, depressive, hemodynamically and electrolytical balanced. On the seventh day post-surgery, the patient started to have fever, cough, with left basal lung crackles. Pulmonary X-ray revealed left basal pneumonia. Under antibiotic therapy with meropenem and vancomycin, the evolution was favorable. Despite our recommendations, the patient requested to leave the hospital. The recommendations received at discharge were antibiotic therapy for approximately 7 days at home and therapy with apixaban in accordance with the recommendations of the cardiologist and infectious disease doctor along with adrenal replacement therapy. The patient refused to take the substitute medication with glucocorticoids and mineralocorticoids at home, so that death occurred 9 days after discharge. Her family refused an autopsy. The probable cause of death was adrenal crisis.
| Discussion | ▴Top |
Ectopic secretion of ACTH (ECS) represented a rare cause of CS, which due to the severity of the clinical manifestations, must be evaluated and treated as an endocrinological emergency [3]. The severity of the clinical manifestations of the ECS is explained by how high and how fast cortisol levels rise. In the case of ECS, it is difficult to distinguish between pituitary and nonpituitary sources. Most frequently, ectopic ACTH syndrome is caused by a bronchial carcinoid tumor or by a small cell lung cancer (45%), thymic (11%), pancreas (8.5%), MTCs (6%), gastrointestinal neuroendocrine tumors (5%), and pheochromocytomas (5%) [4]. MTC represents 3-10% of all thyroid carcinoma and is a rare etiology of ECS (< 1%) [4]. Only up to 100 cases about ECS induced by MTC have been reported in literature [5, 6]. In 35% of the cases of MTC with ECS, CS is detected first, later MTC is diagnosed as the source of the ACTH excess [7]. Patients with MTC and ECS generally have a worse prognosis compared to patients with MTC without hypercortisolism [8]. The clinical and metabolic manifestations in Cushing disease (CD) appear progressively (over months to years), while in ECS due to the very high levels of cortisol, the clinical and metabolic disturbances are noted early (over 1 month) [9]. ECS is more frequent in males and appears at older ages than CD. The metabolic disturbances represented by hypokalemic metabolic alkalosis occur in a high percentage in patients with ECS compared to patients with CD (90% vs. 10-15%) [4]. It is reported that the clinical manifestations are different in ECS, thus in neuroendocrine tumors the manifestations of hypertension, diabetes, weakness, hypokalemia, neuropsychiatric, edema predominate [10]. In the present case, the patient suffered from hypertension and recent onset diabetes mellitus due to hypercortisolism and complained of fatigue and weakness.
Establishing the diagnosis of CS is based on the documentation of the excessive production of glucocorticoids in the blood and urine. Several screening tests are used: cortisol rhythm, midnight salivary cortisol, 24-h urinary free cortisol, and 1 mg overnight dexamethasone suppression. After confirming the diagnosis of CS, the next step is the etiological establishment through ACTH dosing. We identify ACTH-dependent forms when its value is increased. Although the value of ACTH in ECS is much higher compared to CD, this evaluation alone is not sufficient. Using the 8 mg dexamethasone suppression test and inferior petrosal sinus sampling, and according to the findings, pituitary MRI, or nuclear imaging (Octreoscan, 68Ga-DOTATATE), may be required.
Sometimes the diagnosis of forms of ACTH-dependent CS may be difficult to establish, especially in the context of the prevalence of nonfunctioning pituitary microadenomas. It is important to remember that 10% of the population may experience nonfunctional micronodular pituitary lesions that usually do not exceed the size of 4 - 5 mm [11]. In the presented case, the patient had a pituitary microadenoma below 6 mm in size.
Moreover, our patient showed a low value of FT4 with TSH in the normal ranges at the evaluation of thyroid function, consistent with central hypothyroidism, but this change is also suggestive for hypercortisolism that through inhibition of thyroid releasing hormone (TRH) and TSH secretion, as well as suppression of the 5’-deiodinase enzyme can suppress thyroid function [11].
The prognosis of ECS is generally poor and the main causes of death are either complications of CS or disease progression. The management of hypercortisolism is challenging in most cases, with nearly 50% ultimately requiring bilateral adrenalectomy [6]. For cases where the ectopic source of ACTH has been established, the optimal treatment is surgical resection of the causative tumor. When the causative tumor cannot be found or surgical intervention is unsuccessful or not possible, control of hypercortisolism must be achieved by medical therapy. Ketoconazole, mitotane, and metyrapone are oral medications that reduce cortisol synthesis by inhibiting adrenal enzymes. Etomidate is the only IV drug that can be used when rapid reduction of cortisol production is desired. Several kinase inhibitors (vandetanib, sorafenib, or sunitinib) are effective in treating ECS secondary to medullary thyroid cancer [12]. In our presented case, in the context of severe hypercortisolism and the lack of immediate availability of cortisol synthesis inhibitor medication, bilateral adrenalectomy was performed.
Conclusions
A high degree of clinical suspicion is needed for early diagnosis of ECS, to establish emergency investigations and treatment to reduce complications and increase life expectancy. Diagnosis of ECS is often challenging and requires a multidisciplinary approach. ECS is a rapidly progressive and life-threatening situation that can be fatal if diagnosis or timely intervention is delayed. Thyroid evaluation is important to identify a possible source of ectopic secretion of ACTH in the context of MTC. Through this report, we would like to present the case of a patient with ECS with severe and rapid course, with probable MTC origin, which is associated to other oncological pathologies.
Acknowledgments
None to declare.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
All appropriate consent forms were obtained from the patient’s daughter for the publication of the case report. Consent was also given for images and other clinical information to be reported in the journal.
Author Contributions
CAI did the literacy search. MSV contributed to the study design. MI wrote the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CS: Cushing’s syndrome; ECS: ectopic ACTH syndrome; MTC: medullary thyroid carcinoma; TSH: thyroid-stimulating hormone; FT4: free thyroxine; ACTH: adrenocorticotropic hormone; FBG: fasting blood glucose; HbA1c: glycated hemoglobin; PTC: plasma total cortisol; UFC: urine free cortisol; DST: dexamethasone suppression test
| References | ▴Top |
- Valassi E, Santos A, Yaneva M, Toth M, Strasburger CJ, Chanson P, Wass JA, et al. The European Registry on Cushing's syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165(3):383-392.
doi pubmed - Raj R, Taylor RK, Owen D. A rapidly progressive case of ectopic adrenocorticotropic hormone (ACTH) syndrome. Am J Case Rep. 2021;22:e934437.
doi pubmed pmc - Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, et al. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117-123.
doi pubmed - Cohen EG, Shaha AR, Rinaldo A, Devaney KO, Ferlito A. Medullary thyroid carcinoma. Acta Otolaryngol. 2004;124(5):544-557.
doi pubmed - Sand M, Uecker S, Bechara FG, Gelos M, Sand D, Wiese TH, Mann B. Simultaneous ectopic adrenocorticotropic hormone syndrome and adrenal metastasis of a medullary thyroid carcinoma causing paraneoplastic Cushing's syndrome. Int Semin Surg Oncol. 2007;4:15.
doi pubmed pmc - Corsello A, Ramunno V, Locantore P, Pacini G, Rossi ED, Torino F, Pontecorvi A, et al. Medullary thyroid cancer with ectopic Cushing's syndrome: a case report and systematic review of detailed cases from the literature. Thyroid. 2022;32(11):1281-1298.
doi pubmed - Matheny LN, Wilson JR, Baum HB. Ectopic ACTH Production Leading to Diagnosis of Underlying Medullary Thyroid Carcinoma. J Investig Med High Impact Case Rep. 2016;4(2):2324709616643989.
doi pubmed pmc - Koehler VF, Fuss CT, Berr CM, Frank-Raue K, Raue F, Hoster E, Hepprich M, et al. Medullary thyroid cancer with ectopic Cushing's syndrome: A multicentre case series. Clin Endocrinol (Oxf). 2022;96(6):847-856.
doi pubmed - Stewart P. The adrenal cortex. In: Williams Textbook of Endocrinology 13th ed. 2016. p. 507-511
- Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, Jenkins PJ, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006;91(2):371-377.
doi pubmed - Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602.
doi pubmed - Young J, Haissaguerre M, Viera-Pinto O, Chabre O, Baudin E, Tabarin A. MANAGEMENT OF ENDOCRINE DISEASE: Cushing's syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur J Endocrinol. 2020;182(4):R29-R58.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
