| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 13, Number 1, February 2023, pages 13-19
Metabolically Obese Normal-Weight Phenotype as a Risk Factor for High Blood Pressure: A Five-Year Cohort
Victor Juan Vera-Poncea, b, f , Jamee Valencia Guerrac
, Jenny Raquel Torres-Malcaa, b
, Fiorella E. Zuzunaga-Montoyad
, Joan A. Loayza-Castroa
, Norka Rocio Ponce Guillena
, Gianella Zulema Zenas-Trujilloa
, Mario J. Valladares-Garridoe
, Willy Cesar Munoz Ramosa
, Jhony A. De La Cruz-Vargasa
aInstituto de Investigaciones en Ciencias Biomedicas, Universidad Ricardo Palma, Santiago de Surco, Peru
bFacultad de Psicologia, Universidad Tecnologica del Peru, Lima, Peru
cFacultad de Ciencias de la Salud, Universidad Privada del Norte, Lima, Peru
dFacultad de Ciencias de la Salud, Universidad Cientifica del Sur, Lima, Peru
eSouth American Center for Education and Research in Public Health, Universidad Norbert Wiener, Lima, Peru
fCorresponding Author: Victor Juan Vera-Ponce, Instituto de Investigacion en Ciencias Biomedicas de la Universidad Ricardo Palma, Santiago de Surco, Peru
Manuscript submitted December 6, 2022, accepted December 30, 2022, published online February 25, 2023
Short title: MONW as a Risk Factor for HBP
doi: https://doi.org/10.14740/jem855
| Abstract | ▴Top |
Background: The metabolically obese normal-weight (MONW) phenotype has been considered a risk factor for different chronic diseases, but its role in high blood pressure (HBP) is still unclear. The aim of the study is to determine if the MONW phenotype constitutes a risk factor for hypertension in Peruvian adults belonging to a 5-year cohort.
Methods: This is a retrospective cohort study. A secondary analysis from the database of the PERU MIGRANT study was carried out from the MONW and non-MONW cohorts; after a 5-year follow-up, the appearance of HBP was evaluated in the subjects of both cohorts. To assess the strength and magnitude of the association, a Poisson regression model (crude and adjusted) with robust variance was used. The measure of association was the relative risk (RR).
Results: The incidence of HBP was 11.30%. In the multivariable analysis, subjects with the MONW phenotype had a 2.879-fold risk of presenting HBP in 5 years compared with those who were not MONW at the beginning of the study; this was adjusted for categorized age, sex, group, and state of smoker and alcohol drinker (RR: 2.055; 95% confidence interval (CI): 1.118 - 3.777; P = 0.020).
Conclusions: The presence of the MONW phenotype doubled the incidence of HBP, even after adjusting for other covariates. However, studies in this field should continue. If these findings are confirmed, it should be considered that presenting an adequate weight for height should not be interpreted as a condition free of metabolic alterations, so screening for hypertension should be carried out regardless of whether or not the body mass index obtained is considered normal.
Keywords: Obesity; Metabolism; Hypertension; Peru
| Introduction | ▴Top |
High blood pressure (HBP) is one of the main risk factors for the development of ischemic heart disease, cerebrovascular disease, and other cardiovascular diseases [1]. By the year 2019 the number of people affected by hypertension was more than 1 billion, and this figure has doubled since 1990 [2]. Globally, the prevalence of HBP oscillates around 30% [3]. In Peru, the prevalence of hypertension is estimated at 21.7% [4] and the associated financial resources represent around 6% of the country’s total health budget [5].
The association between obesity and hypertension was established prospectively from the Framingham study [6], and has been extensively documented by other studies, with even 65% to 78% of cases of primary hypertension [7, 8]. However, there are different obesity phenotypes, including those that present metabolic alterations despite maintaining an average body mass index (BMI) [9, 10], which is called metabolically obese normal-weight (MONW). As previously reported in different studies, this phenotype is more common than expected [11-13].
Different studies, mainly carried out in the Asian population, have reported an association between the MONW phenotype and the increased risk of developing HBP [12, 14] or cardiovascular risk [15], when compared to the healthy population. In these works, follow-up of cohorts was carried out between 4 to 8 years, and it was observed that the risk of developing hypertension showed a pattern of increase over 4 years of follow-up [1, 12, 14]. However, not all studies have found a relationship between this obesity phenotype and the risk of hypertension, as is the case in the work by Ding et al, in which the 6-year follow-up of a cohort of children and adolescents did not report a significant difference between the incidence of hypertension compared with healthy peers [16]; therefore, the age at which the association occurs could be a factor to consider [15]. In turn, elevations in blood pressure have previously been associated with different components of MONW criteria, such as waist circumference (WC) [17].
Since there are still controversies, and the lack of conclusive evidence of this in the Latin American population, the objective of the present study was to determine the risk of the MONW phenotype for HBP in Peruvians belonging to a 5-year cohort.
| Materials and Methods | ▴Top |
Design
This is a retrospective cohort study. The study constitutes a secondary analysis of the PERU MIGRANT study cohort database, designed to assess the magnitude of the differences between groups of rural, rural to urban, and urban migrants concerning cardiovascular risk factors [18].
Study population
It was evaluated in two periods: the first was between the period 2007 - 2008, then carried out a follow-up and subsequent evaluation of the population at 5 years, between the period 2012 - 2013. Said database is freely accessible to the public [19].
At the beginning of the study, the study groups were defined by a unique random sampling of participants aged 30 years or older from the rural site of Ayacucho, the urban area of Lima, and migrants from the countryside to the city of Ayacucho who now reside in Lima. Information on selection criteria, sample size, and participation rates have been previously published [18]. Initially, the total number of participants that were recruited in the primary study was 989 people. For the present manuscript, patients with a diagnosis of HBP and type 2 diabetes mellitus were excluded at the first evaluation. Those who did not present the main variables of interest (HBP and MONW) were also excluded.
Variables and measurement
The response variable was the development of hypertension, defined as the presence of systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg, according to international guidelines, or the current use of antihypertensive medication prescribed by a physician.
MONW phenotype is defined as a person who presents an average BMI but also metabolic alteration [9, 10]. Two cohorts were reconstructed based on the absence or presence of the MONW phenotype: the cohort exposed to the MONW phenotype and the non-exposed cohort. The MONW phenotype was defined using the cut-off points of the metabolic syndrome criteria proposed by the International Diabetes Federation (IDF) [20], as well as those for the homeostasis model assessment of insulin resistance (HOMA-IR) index and C-reactive protein (CRP) described by previous studies [21]. Finally, subjects were classified as having MONW if they presented at least two of the following criteria: WC ≥ 80 cm for women or ≥ 90 for men (the cut-off point for South Asia was used according to the IDF proposal), triglycerides ≥ 150 mg/dL, fasting glucose ≥ 100 mg/dL (or if receiving glucose-lowering treatment), SBP ≥ 130 mm Hg, or DBP ≥ 85 mm Hg (or if receiving treatment to lower blood pressure levels), high-density lipoprotein (HDL)-cholesterol < 50 mg/dL in women or < 40 mg/dL in men, insulin resistance (IR) through HOMA-IR ≥ 2.80 and CRP ≥ 3 mg/dL.
The covariates that were considered in the first data collection were age group 29 to 44, 45 to 59 and 60 years and over); sex (male vs. female); group (urban vs. rural vs. migrant); current smoking status (yes vs. no); alcohol consumption (low vs. high); level of physical activity (high vs. medium vs. low).
Data collection and procedure
Participants initially enrolled in the PERU MIGRANT study between 2007 and 2008 were contacted again between 2012 and 2013 for follow-up purposes. After oral consent, the participants were asked to respond to a detailed questionnaire.
Weight and WC were measured in triplicate using standardized techniques. Blood pressure was taken using an OMROM HEM-780 digital blood pressure monitor (OMRON, Tokyo, Japan). Blood samples were obtained in the fasting period. Glucose was measured in plasma using an enzymatic colorimetric method (GOD-PAP; Modular P-E/Roche-Cobas, Grenzach-Whylen, Germany). To know the level of physical activity of the participant, the International Physical Activity Questionnaire (IPAQ) was used, which was categorized according to the total number of days of physical activity and the metabolic equivalent in minutes/week in the three aforementioned categories.
Statistical analysis
All statistical analyzes were performed with Stata 17.0. The descriptive analysis was done with all the subjects of the first evaluation. The categorical variables were presented in frequencies and percentages. Then, comparisons were made with subjects who remained until the second evaluation. Since the response variable and the exposure variables were categorical, the Chi-square test was used.
To assess the strength and magnitude of the association, a Poisson regression model (crude and adjusted) was used with robust variance between the development of obesity and the MONW phenotype, considering the possible confusion variables in the adjusted model. The association measure was the relative risk (RR) with its respective confidence interval (CI) of 95%.
Ethical considerations
Approval was obtained from the Ethics Committee of the Universidad Peruana Cayetano Heredia. The purpose of the study was detailed to each participant, and informed consent was subsequently obtained. Participants who were originally part of the PERU MIGRANT 2007 - 2008 study were contacted in the years 2012 to 2013. Additionally, the present study was conducted in compliance with the ethical standards of the Helsinki Declaration. The present study is an analysis of secondary data, in which there was no human contact with the subjects who signed part of the study. Therefore, there was no risk to the participants.
| Results | ▴Top |
We only worked with those with a normal weight (n = 405). Subsequently, hypertensive patients (n = 24), those with DM2 (n = 3) and those with missing values for the formation of the MONW variable (n = 11) were excluded.
After applying the selection criteria, 367 people were available for analysis; 43.9% were female; 15.5% were 60 or older. Regarding harmful habits, 12.6% were currently smokers, while 10.9% consumed alcohol in high quantities (Table 1).
 Click to view | Table 1. Baseline Characteristics of the Study Sample |
From the sample, the MONW cohort was reconstructed, which consisted of 85 people; while, the people who did not present this phenotype, who constituted the unexposed cohort, were 221.
After 5 years of follow-up, 345 subjects completed the study, 22 subjects due to loss to follow-up. Arterial hypertension (AHT) was diagnosed in 20.6% of the MONW cohort and in 7.1% of the unexposed cohort (Chi-square test; P < 0.001); thus, the incidence in the MONW cohort was 3.8 cases per 100 person-years and 1.4 cases per 100 person-years (Fig. 1). The age group (P < 0.001) and the migration group (P = 0.042) also had a statistically significant association with the development of this disease (Table 2).
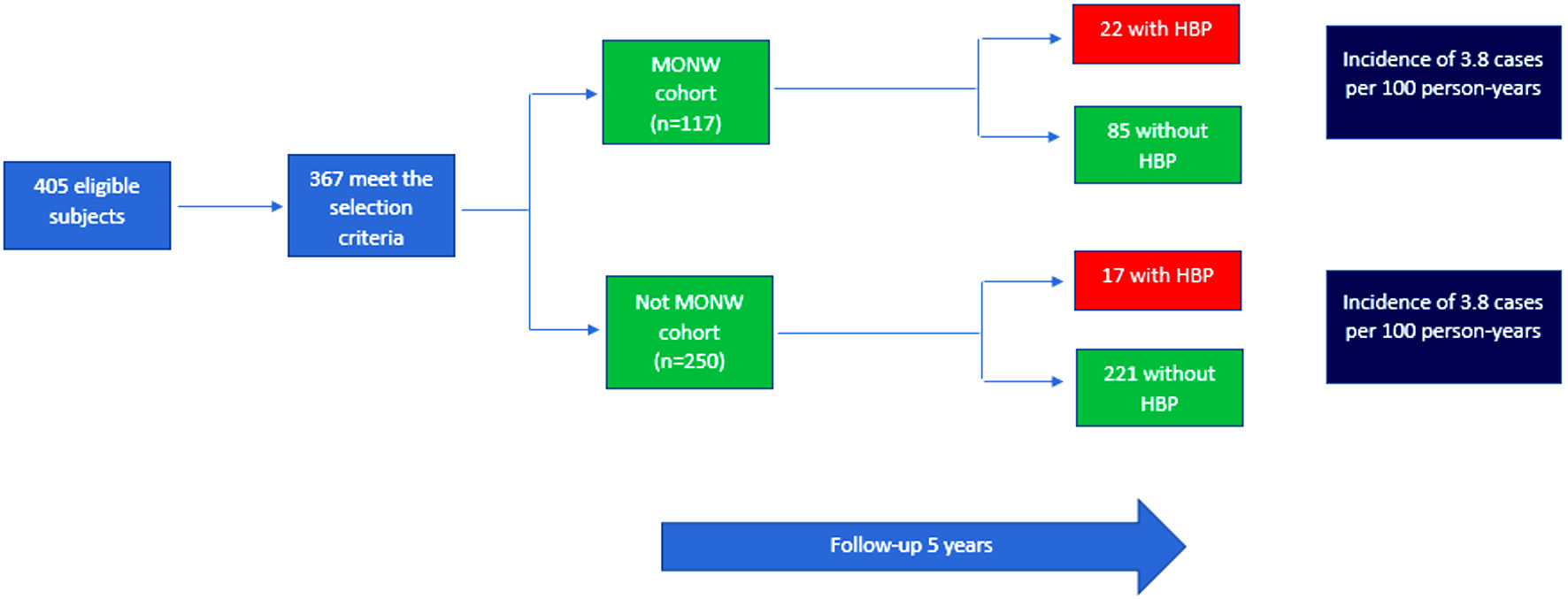 Click for large image | Figure 1. Reconstruction of cohorts from the PERU MIGRANT 2007 - 2008 study. HBP: high blood pressure; MONW: metabolically obese normal-weight. |
 Click to view | Table 2. Bivariate Analysis of the Characteristics Associated With the Presence of Hypertension |
In the multivariable analysis, the subjects who presented the MONW phenotype had a 2.879-fold risk of presenting HBP in 5 years, compared to those who were not MONW at the beginning of the study. This was adjusted by categorized age, sex, group, state of smoker and alcohol drinker (RR: 2.055; 95% CI: 1.118 - 3.777; P = 0.020) (Table 3).
 Click to view | Table 3. Relative Risks of the Association Between the Metabolically Obese Thin and the Development of HBP: Crude and Adjusted Models |
| Discussion | ▴Top |
Main findings
After the 5-year follow-up, the MONW phenotype was a risk factor for hypertension in the sample studied, observing that those exposed to the MONW phenotype doubled the risk of developing hypertension than those who did not present the MONW phenotype, considering the adjustment of the confusing variables.
Comparison with previous studies
The incidence of hypertension in the MONW group was lower than that reported by previous studies [14, 21, 22] in which it was between 15% and 20.4%. These differences can be explained when considering that how said studies defined MONW was ≥ 1 altered cardiometabolic factor, while our research thought the presence of at least two of these.
Although it has been reported that the increase in BMI is a factor that increases the risk of HBP [14, 23], the findings in this study would indicate that the idea that maintaining an average weight would be an exclusive condition for developing metabolic diseases should be discarded. The choice to consider metabolic factors has been reported in other studies; for example, the Kip KE study found that metabolic syndrome, but not BMI, predicted future cardiovascular risk in a sample of women [24].
Other previous works, mainly in the Asian population, both in adolescents and in adults, reported that being MONW presented a risk of between 23% and 77% for HBP [14, 16, 21, 22]. Although there are differences in the number of factors included to define MONW (≥ 1 vs. ≥ 2 altered factors) between the different studies, the association between the MONW phenotype and the development of HBP seems consistent. In turn, comparisons with other studies worldwide have the problem of using different definitions of the metabolically healthy state. Some authors considered that metabolically healthy individuals should not have more than one [21] or two [25] of the five metabolic risk factors, whereas in other studies, metabolically healthy participants were defined as those who had none or one of the six metabolic risk factors [26]. Furthermore, the cut-off point must be considered to define said alterations (Adult Treatment Panel III, IDF, Wildman, or others), the number of factors used, and regardless of the presence or not of a higher-than-normal BMI and/or WC.
However, as the study by Zhao et al [14], an altered metabolic state is related to greater cardiovascular risk than metabolically normal subjects. In addition, a reported feature of the MONW phenotype is the increased deposit of visceral adiposity [10], same as in our work it was considered to include the WC as a MONW criterion and that has been shown to be associated with the prevalence and incidence of HBP [14], regardless of BMI [27].
Results interpretation
This research shows the importance of detecting the MONW phenotype in individuals from the general population due to their risk of HBP intervening early in their lifestyle. The mechanisms to explain the association of MONW and hypertension go beyond the increase in fat linked to the increase in BMI, chronic diseases could be further related to the accumulation of intraorganic fat, which can induce the development of hypertension through various mechanisms that lead to chronic systemic inflammation and oxidative stress in obesity such as hyperactivation of the sympathetic nervous system, increased action of the renin angiotensin aldosterone system, changes in the structure and function of the left ventricle [21], chronic vascular inflammation [28], insulin resistance [29] and consequent endothelial dysfunction [10, 30].
Strengths and limitations
The present study had limitations as strengths that deserve to be considered. The first is that since our study was an analysis of secondary data, we could not control for some important confounding variables, such as family history of HBP [16] and intake of critical nutrients such as sodium and potassium that play a critical role in regulating blood pressure [23]. Furthermore, the results are limited to the Peruvian population, so they may not represent other races or ethnicities fully. Additionally, our study did not consider any socioeconomic variable. However, despite the limitations, the study had strengths such as the fact that, to the authors’ knowledge, no previous studies have evaluated the association in the Peruvian population of the MONW phenotype with the incidence of HBP. Secondly, the study considered the inclusion of criteria factors proposed by the Wildman model [26] and concordant with the IDF [20], as well as using previously defined HOMA-IR and CRP cut-off points for the Peruvian population [31], which would increase internal validity. Finally, the study design considered objective measures of anthropometry, biochemical and metabolic markers.
Conclusions
The MONW phenotype was a risk factor for hypertension in the sample studied. Those exposed to the MONW phenotype doubled the risk of developing HBP compared to those without the MONW phenotype.
If these findings are confirmed, it should be considered that presenting an adequate weight for height should not be interpreted as a condition free of metabolic alterations. Therefore, HBP screening should be carried out regardless of whether or not a normal BMI is obtained. Likewise, the importance of detecting the MONW phenotype in individuals from the general population is evidenced due to their risk of cardiometabolic diseases in order to intervene early in their lifestyle.
Acknowledgments
A special thanks to the members of INICIB who provided valuable comments during the preparation of this study.
Financial Disclosure
This study is self-financed.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The informed consent was obtained.
Author Contributions
Victor Juan Vera-Ponce, Jamee Valencia Guerra and Jenny Raquel Torres-Malca participated in the genesis of the idea and project design. Fiorella E. Zuzunaga-Montoya, Norka Rocio Ponce Guillen, Mario J. Valladares-Garrido, Willy Cesar Munoz Ramos and Jhony A. De La Cruz-Vargas were in charge of the data collection, interpretation and analysis of results. Joan A. Loayza-Castro and Zulema Zenas-Trujillo contributed to the preparation of the manuscript of this research paper.
Data Availability
The data supporting the findings of this study can be accessed by the original research paper in the follow link: https://doi.org/10.1186/1471-2261-9-23.
| References | ▴Top |
- Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785-802.
doi pubmed - N. C. D. Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957-980.
doi pubmed - Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441-450.
doi pubmed - INEI - Peru: Enfermedades No Transmisibles y Transmisibles, 2020 [Internet]. [citado el 30 de noviembre de 2021]. Disponible en: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1796/.
- Castillo N, Malo M, Villacres N, Chauca J, Cornetero V, de Flores KR, Tapia R, et al. [Methodology for estimating total direct costs of comprehensive care for non-communicable diseases]. Rev Peru Med Exp Salud Publica. 2017;34(1):119-125.
doi pubmed - Kannel WB, Brand N, Skinner JJ, Jr., Dawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med. 1967;67(1):48-59.
doi pubmed - Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the The Obesity Society and The American Society of Hypertension. Obesity (Silver Spring). 2013;21(1):8-24.
doi pubmed - Faulkner JL, Belin de Chantemele EJ. Sex differences in mechanisms of hypertension associated with obesity. Hypertension. 2018;71(1):15-21.
doi pubmed - Gomez-Zorita S, Queralt M, Vicente MA, Gonzalez M, Portillo MP. Metabolically healthy obesity and metabolically obese normal weight: a review. J Physiol Biochem. 2021;77(1):175-189.
doi pubmed - Pluta W, Dudzinska W, Lubkowska A. Metabolic obesity in people with normal body weight (MONW)-review of diagnostic criteria. Int J Environ Res Public Health. 2022;19(2):624.
doi pubmed - Benziger CP, Bernabe-Ortiz A, Gilman RH, Checkley W, Smeeth L, Malaga G, Miranda JJ, et al. Metabolic abnormalities are common among south American Hispanics subjects with normal weight or excess body weight: the CRONICAS cohort study. PLoS One. 2015;10(11):e0138968.
doi pubmed - Jia A, Xu S, Xing Y, Zhang W, Yu X, Zhao Y, Ming J, et al. Prevalence and cardiometabolic risks of normal weight obesity in Chinese population: A nationwide study. Nutr Metab Cardiovasc Dis. 2018;28(10):1045-1053.
doi pubmed - Goday A, Calvo E, Vazquez LA, Caveda E, Margallo T, Catalina-Romero C, Reviriego J. Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: results from the Icaria study. BMC Public Health. 2016;16:248.
doi pubmed - Zhao Y, Qin P, Sun H, Liu Y, Liu D, Zhou Q, Guo C, et al. Metabolically healthy general and abdominal obesity are associated with increased risk of hypertension. Br J Nutr. 2020;123(5):583-591.
doi pubmed - Choi KM, Cho HJ, Choi HY, Yang SJ, Yoo HJ, Seo JA, Kim SG, et al. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clin Endocrinol (Oxf). 2013;79(3):364-370.
doi pubmed - Ding WQ, Yan YK, Zhang MX, Cheng H, Zhao XY, Hou DQ, Mi J. Hypertension outcomes in metabolically unhealthy normal-weight and metabolically healthy obese children and adolescents. J Hum Hypertens. 2015;29(9):548-554.
doi pubmed - Sun JY, Hua Y, Zou HY, Qu Q, Yuan Y, Sun GZ, Sun W, et al. Association between waist circumference and the prevalence of (Pre) hypertension among 27,894 US adults. Front Cardiovasc Med. 2021;8:717257.
doi pubmed - Miranda JJ, Gilman RH, Garcia HH, Smeeth L. The effect on cardiovascular risk factors of migration from rural to urban areas in Peru: PERU MIGRANT Study. BMC Cardiovasc Disord. 2009;9:23.
doi pubmed - PERU MIGRANT Study | Baseline and 5yr follow-up dataset [Internet]. Figshare. 2017 [citado el 29 de julio de 2021]. https://figshare.com/articles/dataset/PERU_MIGRANT_Study_Baseline_and_5yr_follow-up_dataset/4832612/3.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645.
doi pubmed - Lee SK, Kim SH, Cho GY, Baik I, Lim HE, Park CG, Lee JB, et al. Obesity phenotype and incident hypertension: a prospective community-based cohort study. J Hypertens. 2013;31(1):145-151.
doi pubmed - Cao ZK, Huang Y, Yu HJ, Yuan S, Tang BW, Li QX, Li XT, et al. Association between obesity phenotypes and incident hypertension among Chinese adults: a prospective cohort study. Public Health. 2017;149:65-70.
doi pubmed - Jayedi A, Rashidy-Pour A, Khorshidi M, Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes Rev. 2018;19(5):654-667.
doi pubmed - Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109(6):706-713.
doi pubmed - Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97(7):2482-2488.
doi pubmed - Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168(15):1617-1624.
doi pubmed - Ostchega Y, Hughes JP, Terry A, Fakhouri TH, Miller I. Abdominal obesity, body mass index, and hypertension in US adults: NHANES 2007-2010. Am J Hypertens. 2012;25(12):1271-1278.
doi pubmed - Ferreira J, Cunha P, Carneiro A, Vila I, Cunha C, Silva C, Longatto-Filho A, et al. Is obesity a risk factor for carotid atherosclerotic disease? - Opportunistic review. J Cardiovasc Dev Dis. 2022;9(5):162.
doi pubmed - Ramesh R, Pandurangan V, Madhavan S, Srinivasan D, Bhaskar E, Marappa L, Nair AM, et al. Comparison of fasting insulin level, homeostatic model of insulin resistance, and lipid levels between patients with primary hypertension and normotensive subjects. Rambam Maimonides Med J. 2022;13(2):e0009.
doi pubmed - Shariq OA, McKenzie TJ. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020;9(1):80-93.
doi pubmed - Carrillo-Larco RM, Miranda JJ, Gilman RH, Checkley W, Smeeth L, Bernabe-Ortiz A, Cronicas Cohort Study G. The HOMA-IR performance to identify new diabetes cases by degree of urbanization and altitude in Peru: the CRONICAS cohort study. J Diabetes Res. 2018;2018:7434918.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
