| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 12, Number 6, December 2022, pages 198-201
Mediastinal Mass as First Clinical Manifestation of Medullary Thyroid Microcarcinoma in a Patient With Hashimoto’s Thyroiditis
Victor Raul Garcia-Ruiza, b , Jacsel Suarez-Rojasa, c, f
, Julio Cesar Alvarez-Gameroa, d
, Jose Rene Somocurcio-Peraltab, e
, Jose Luis Paz-Ibarraa, b
aEndocrinology Division, Hospital Edgardo Rebagliati Martins, Lima, Peru
bUniversidad Nacional Mayor de San Marcos, Lima, Peru
cUniversidad Cientifica del Sur, Lima, Peru
dUniversidad Peruana Cayetano Heredia, Lima, Peru
ePathology Division, Hospital Edgardo Rebagliati Martins, Lima, Peru
fCorresponding Author: Jacsel Suarez-Rojas, Universidad Cientifica del Sur, Lima, Peru
Manuscript submitted September 27, 2022, accepted November 3, 2022, published online December 1, 2022
Short title: Micro-MTC Presenting With a Mediastinal Mass
doi: https://doi.org/10.14740/jem841
| Abstract | ▴Top |
Medullary thyroid cancer is a rare neuroendocrine tumor with aggressive behavior and an uncertain prognosis. Calcitonin is the associated tumor marker; however, neuroendocrine tumors of the lung or intestine can also present high values. We report the case of a 53-year-old patient presenting with dry cough for 3 years. Computed tomography (CT) showed a 58-mm expansive lesion in the right anterior mediastinum and cervical ultrasound informed a 9-mm hypoechoic nodule with a lobulated edge and thick calcifications. Cytology reported Hashimoto’s thyroiditis. He underwent emergent surgery for respiratory failure. The pathological study informed G2 neuroendocrine carcinoma, immunohistochemistry showed: cytokeratin (+), vimentin (+), alpha-actin (-), synaptophysin (+), chromogranin (+), Ki-67: 10%. He progressed with bone metastasis visualized in scintigraphy and mediastinal tumor remnants, adenopathy, and pulmonary nodules on CT scan, prompting chemotherapy. Due to elevated and increasing calcitonin, an ultrasound study with needle washout for calcitonin resulted in 1,724 pg/mL, and cytology of the nodule reported medullary carcinoma. He was finally diagnosed with medullary thyroid microcarcinoma with metastasis to the mediastinum. Coexistence with Hashimoto’s thyroiditis could alter the initial fine-needle aspiration results. We conclude that in the case of calcitonin-secreting neuroendocrine tumors or medullary thyroid cancer, calcitonin fine needle aspirate washout may be helpful in elucidating a diagnosis. Due to its severity, medullary thyroid cancer should always be approached and managed aggressively, even microcarcinomas.
Keywords: Medullary carcinoma; Thyroid neoplasms; Hashimoto’s disease; Neoplasm metastasis; Calcitonin
| Introduction | ▴Top |
Medullary thyroid cancer (MTC) is a rare neuroendocrine tumor with aggressive behavior and an uncertain prognosis [1]. Medullary thyroid microcarcinoma (micro-MTC) is a malignancy of less than or equal to 1 cm, with poorly defined data regarding its natural history compared to MTC of more than 1 cm [2]. Calcitonin is a tumor marker for diagnosis and follow-up and its serum concentration is directly related to C cell mass. Neuroendocrine tumors of the lung or intestine can also present high calcitonin levels [3]. Therefore, coexistence of a thyroid nodule leads to a diagnostic question on the source of calcitonin excess production.
The association of Hashimoto’s thyroiditis with conditions such as thyroid adenoma, papillary thyroid carcinoma, and thyroid lymphomas has been well described [4]. However, Hashimoto’s thyroiditis along with MTC is considered rare [5]. Herein we report the case of a middle-aged man with micro-MTC presenting with a mediastinal mass, very high calcitonin levels, and Hashimoto’s thyroiditis.
| Case Report | ▴Top |
A previously healthy 53-year-old man presented without significant past medical history, without tobacco and alcohol use. He complained of nonproductive cough and dysphagia to solids for 5 months and was diagnosed with a mediastinal mass on chest X-ray by another provider at an outpatient clinic. Acid fast bacillary on sputum were negative for tuberculosis. Computed tomography (CT) scan of the chest showed an expansive lesion of 58 mm in diameter located in the right anterior mediastinum, displacing vascular structures and the trachea (Fig. 1). Technetium-99m scintigraphy was performed to rule out substernal goiter, which revealed a normal shape, size, and location of the thyroid (Fig. 2). Laboratory exams showed thyroid-stimulating hormone (TSH) 2.11 µIU/L (reference range 0.4 - 4), free T4 (FT4; thyroxine) 1.66 ng/dL (reference range 0.8 - 1.9), anti-thyroid peroxidase (anti-TPO) > 600 IU/mL (reference range < 40 IU/mL), anti-thyroglobulin (anti-Tg) 138 IU/mL (reference range < 40 IU/mL), and serum calcitonin 265 pg/mL (reference range < 10 pg/mL). Thyroid ultrasound showed a hypoechoic nodule in the right thyroid lobe of 9 × 7 × 9 mm, lobulated edges, and thick calcification. Rest of the thyroid parenchyma is heterogeneous and compatible with thyroiditis (Fig. 3). Fine needle aspiration (FNA) biopsy of thyroid micronodule concluded Hashimoto’s thyroiditis.
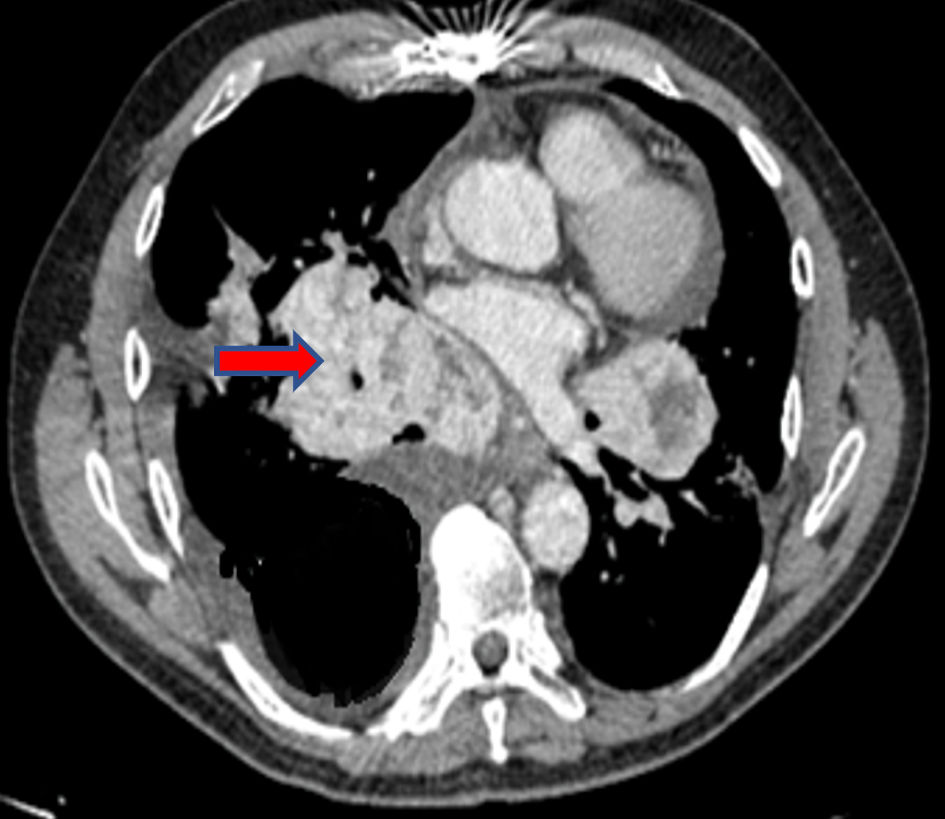 Click for large image | Figure 1. Chest CT scan: expansive lesion in the right anterior mediastinum (arrow). CT: computed tomography. |
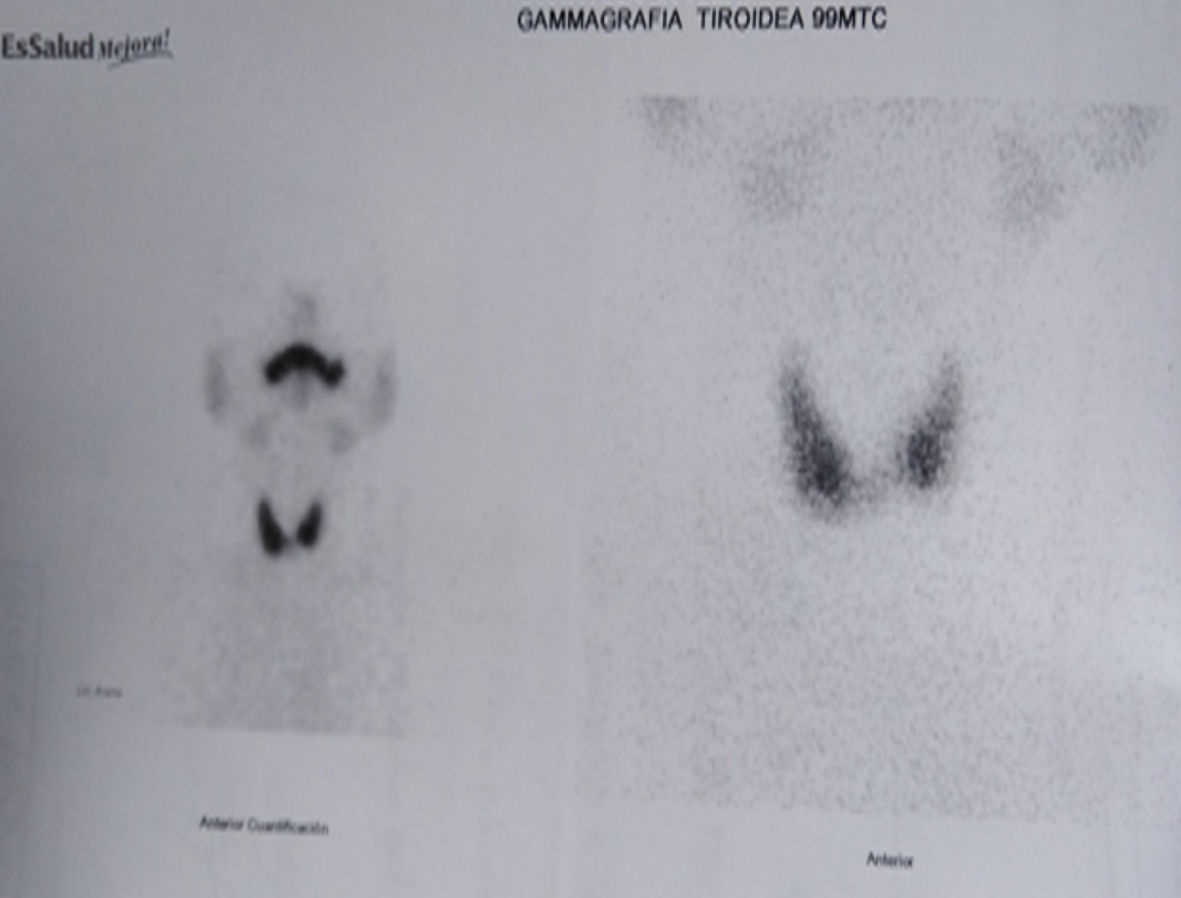 Click for large image | Figure 2. Tc-99m scan: thyroid in normal position. Tc: technetium. |
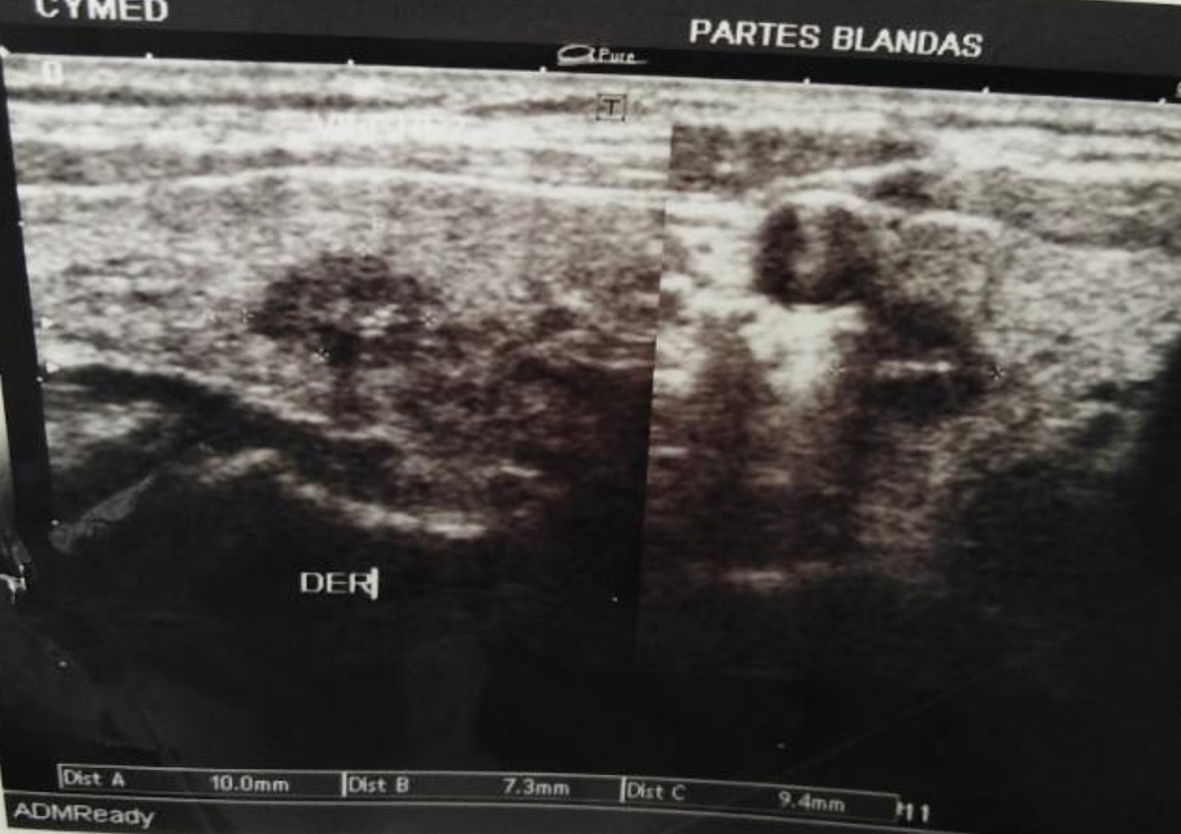 Click for large image | Figure 3. Cervical ultrasound shows hypoechoic nodule in the right thyroid lobe of 9 × 7 × 9 mm. |
During follow-up, 10 months after symptoms onset he developed severe hemoptysis and respiratory failure, which prompted emergent surgery. Surgical findings exhibited a mediastinal tumor with adhesions to supra-aortic vessels. Pathology results showed neuroendocrine carcinoma of nodular and trabecular histological type with fusiform areas of expansive growth measuring 10 × 7 × 6 cm. Immunohistochemistry exhibited cytokeratin (+), vimentin (+), alpha-actin (-), synaptophysin (+), chromogranin (+), and Ki67: 10%. Elevated levels of calcitonin were associated with production by this tumor. The patient was followed by medical oncology, with 5-hydroxy indole acetic acid levels 2.6 mg/24 h (reference range 2 - 6 mg/24 h). Six months after surgery, he presented lumbar pain and persistent dyspnea. A follow-up CT of the chest reported a right pleural effusion, and a conglomerate of mediastinal and hilar adenopathies of up to 4 cm. Calcitonin was 1,113 pg/mL on follow-up. Oncology proposed radiotherapy 45 Gy/28 to the mediastinum and 40 Gy/16 to the vertebral injury in T9, and later, five courses of chemotherapy with cisplatin plus etoposide. Follow-up at 6 months showed serum calcitonin of 1,411 pg/mL.
Due to persistent high calcitonin levels, he underwent a new ultrasound and FNA with calcitonin measurement in needle washout. Cytology reported a Bethesda VI nodule with MTC characteristics. Calcitonin concentration in the needle washout was 1,724 pg/mL. Carcinoembryonic antigen (CEA) was 520.34 ng/mL (reference range 5 ng/mL), and positron emission tomography (PET)-CT scan with fluorodeoxyglucose (18F-FDG) showed hypermetabolic lesions in the cervical, mediastinal, vertebral, and pelvic lymph node levels.
Finally, a review of the pathology of the mediastinal tumor was requested, which showed multifocal positivity for calcitonin and the presence of amyloid material (Fig. 4). We assume that the excess production of calcitonin was due to a micro-MTC with an unusual presentation of metastasis to the mediastinum. Thyroidectomy was proposed, but patient died due to pneumonia before surgery.
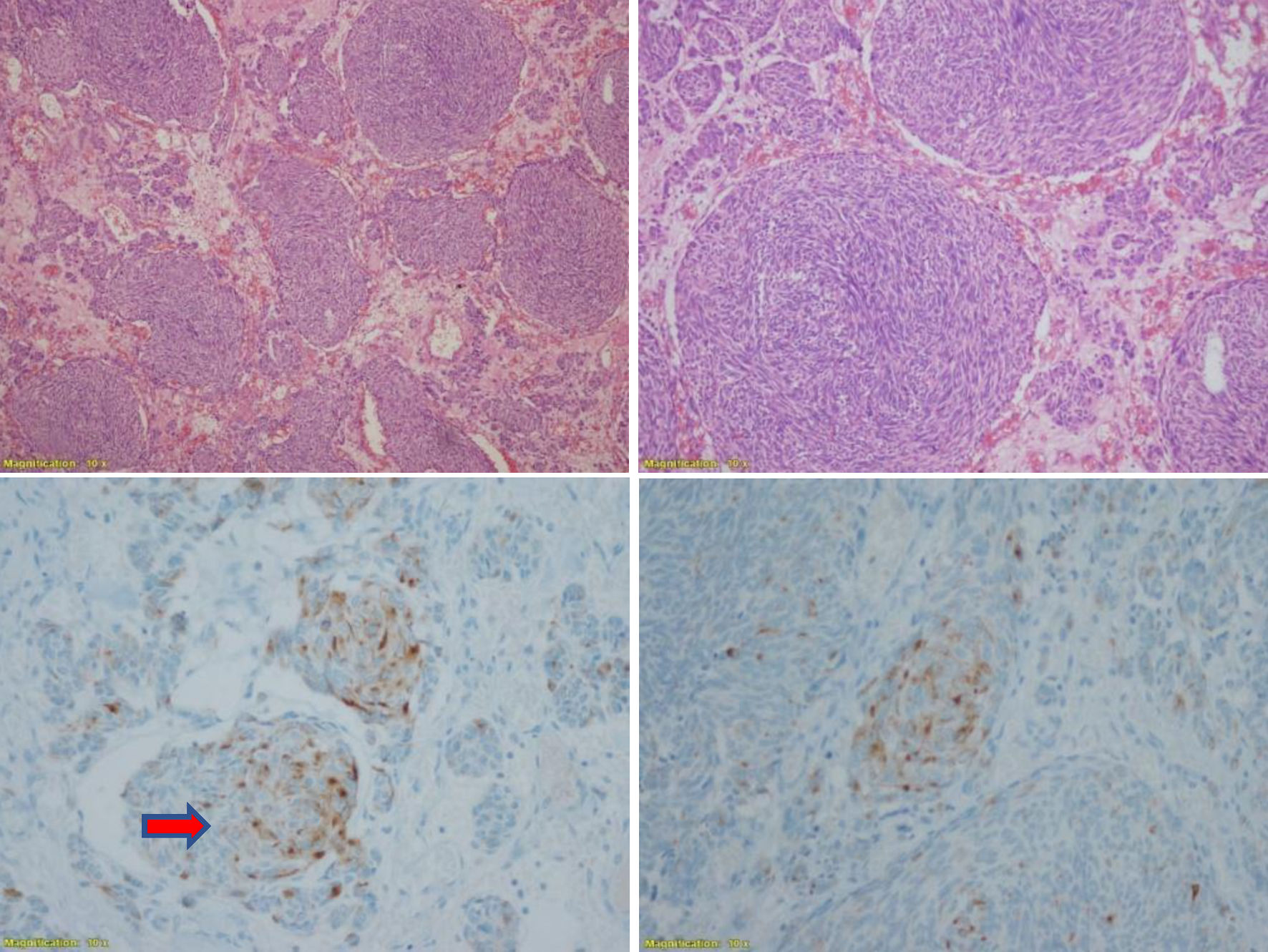 Click for large image | Figure 4. Pathological anatomy: neuroendocrine carcinoma. Nodular and trabecular histological type with fusiform areas of expanding growth delimited in the area of the thymus CK (+), CD5 (+), vimentin (+), synaptophysin (+), calcitonin (+) (arrow). CK: cytokeratin. Magnification: × 10. |
| Discussion | ▴Top |
The presence of a tumor with neuroendocrine characteristics and high levels of calcitonin in association with a thyroid nodule is an unusual finding. In this context, it is a diagnostic challenge to determine the source of excess production with a nodule smaller than 1 cm [1]. Micro-MTC is a tumor less than or equal to 1 cm, with controversies regarding its clinical relevance due to the lack of data on its natural history [2]. It can present in two different forms, hereditary in 25% to 30% of cases and sporadic in 70% [6].
In 2017, a meta-analysis on micro-MTC found rates of multifocality and distant metastases similar to those larger than 1 cm, which suggests that the treatment could have a similar approach [7]. A study of 310 subjects showed that patients with micro-MTC presented regional metastasis in almost 20% and distant metastasis in 5%, with higher probability of regional metastasis in those with tumor size greater than 5 mm [8]. Another study showed that patients with regional lymph node involvement at initial staging had concurrent bone metastases in 16% and, less frequently, visceral involvement including the liver, lungs, and very rarely the brain [9]. This evidence supports that MTC aggressive behavior can occur even in small tumors, as it is the case with our patient.
Unlike papillary carcinoma, the treatment of MTC is primarily surgical. Early surgical intervention is critical, as the MTC cells do not absorb radioactive iodine [1]. In the follow-up, calcitonin is the main marker of MTC, with values greater than 150 pg/mL suggestive of distant metastasis. Calcitonin along with CEA are indicative of disease progression [3]. Calcitonin levels of 1,000 pg/mL indicate at least 1 mL of tumor tissue [9]. Less frequently, calcitonin can also be produced by neuroendocrine tumors of the lungs, larynx, pancreas, bladder, and ovaries, which could be confused with MTC, particularly in the presence of a thyroid nodule [10, 11]. If ultrasound studies reveal a suspicious thyroid nodule, FNA is mandatory [11]. Cases in which calcitonin-producing neuroendocrine tumors metastasize to the thyroid are rare, complicating the diagnosis. In these cases, histopathology of the thyroid following thyroidectomy may allow to clarify the diagnosis [12]. Calcitonin levels in needle washout fluid of greater than 100 pg/mL is practically diagnostic of MTC. The calcitonin levels are undetectable in the cervical metastasis [3]. Even more exceptional are cases of MTC diagnosed with a clinically evident extrathyroidal mass, which often confuses the primary tumor origin [13, 14].
Immunohistochemically, calcitonin-producing neuroendocrine tumors and MTC are indistinguishable. Hence, the presence of amyloid material and calcitonin in the surgical specimen, although suggestive, are not decisive to specify the source of elevated peripheral calcitonin levels [15]. The association of Hashimoto’s thyroiditis with follicular and papillary cancer and thyroid lymphoma has been previously described [16]. Its presentation along with MTC is rare, making our case even more unique. The presence of Hashimoto’s thyroiditis could make it harder to study the cytology of the thyroid nodules, which may explain the result of the initial cytology in our patient [17].
Based on the clinical evolution, the very high and rising calcitonin and CEA levels, the expression of progressive and metastatic MTC, along with the thyroid cytology, we conclude that this is a rare case of micro-MTC presenting with a metastatic mass in the mediastinum.
Conclusions
Very high values of serum calcitonin and CEA are typical of advanced MTC. Measurement of calcitonin in FNA washout fluid may be helpful in order to discern MTC from other calcitonin-secreting neuroendocrine tumors. The MTC is typically aggressive and even micro-MTCs can present with distant metastasis.
Acknowledgments
We appreciate the support of the Pathological Anatomy Service and the Hormone Laboratory of the Edgardo Rebagliati National Hospital.
Financial Disclosure
No funding was obtained for this work.
Conflict of Interest
No potential conflict of interest.
Informed Consent
Informed consent for publication was obtained from the patient’s relatives.
Author Contributions
Concept: VRGR. Data collection and processing: VRGR, JSR, JCAG, JRSP. Writing: VRGR, JSR, JCAG, JLPI. Analysis and interpretations: VRGR, JSR, JRSP, JLPI, JCAG. Literature search: VRGR, JSR, JCAG, JLPI, JRSP.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Kim BH, Kim IJ. Recent updates on the management of medullary thyroid carcinoma. Endocrinol Metab (Seoul). 2016;31(3):392-399.
doi pubmed - Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998;83(12):2638-2648.
doi - Wells SA, Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610.
doi pubmed - Resende de Paiva C, Gronhoj C, Feldt-Rasmussen U, von Buchwald C. Association between Hashimoto's Thyroiditis and Thyroid Cancer in 64,628 Patients. Front Oncol. 2017;7:3.
doi pubmed - Zayed AA, Ali MK, Jaber OI, Suleiman MJ, Ashhab AA, Al Shweiat WM, Momani MS, et al. Is Hashimoto's thyroiditis a risk factor for medullary thyroid carcinoma? Our experience and a literature review. Endocrine. 2015;48(2):629-636.
doi pubmed - Machens A, Dralle H. Biological relevance of medullary thyroid microcarcinoma. J Clin Endocrinol Metab. 2012;97(5):1547-1553.
doi pubmed - Kim JH, Pyo JS, Cho WJ. Clinicopathological significance and prognosis of medullary thyroid microcarcinoma: a meta-analysis. World J Surg. 2017;41(10):2551-2558.
doi pubmed - Kazaure HS, Roman SA, Sosa JA. Medullary thyroid microcarcinoma: a population-level analysis of 310 patients. Cancer. 2012;118(3):620-627.
doi pubmed - Hassan A, Siddique M, Riaz S, Khan AI, Nawaz MK, Bashir H. Medullary thyroid carcinoma: prognostic variables and tumour markers affecting survival. J Ayub Med Coll Abbottabad. 2018;(Suppl 1):S627-S632.
- Schneider R, Waldmann J, Swaid Z, Ramaswamy A, Fendrich V, Bartsch DK, Schlosser K. Calcitonin-secreting pancreatic endocrine tumors: systematic analysis of a rare tumor entity. Pancreas. 2011;40(2):213-221.
doi pubmed - Giannetta E, Guarnotta V, Altieri B, Sciammarella C, Guadagno E, Malandrino P, Puliani G, et al. ENDOCRINE TUMOURS: Calcitonin in thyroid and extra-thyroid neuroendocrine neoplasms: the two-faced Janus. Eur J Endocrinol. 2020;183(6):R197-R215.
doi pubmed - LaBryer L, Sawh R, McLaurin C, Scofield RH. Calcitonin-Secreting Neuroendocrine Carcinoma of Larynx with Metastasis to Thyroid. Case Rep Endocrinol. 2015;2015:606389.
doi pubmed - White J, Mohyeldin A, Schwartz A, Bielamowicz S. Medullary thyroid carcinoma presenting as a supraglottic mass. Ear Nose Throat J. 2014;93(10-11):E40-42.
doi pubmed - Daraki V, Koukouraki S, Velegrakis G, Mamalaki E, Haniotis VT, Kalikakis G, Stathaki MI, et al. Rare presentation of occult medullary carcinoma of the thyroid as a mediastinal mass. Hormones (Athens). 2012;11(2):210-214.
doi pubmed - Vahidi S, Stewart J, Amin K, Racila E, Li F. Metastatic medullary thyroid carcinoma or calcitonin-secreting carcinoid tumor of lung? A diagnostic dilemma in a patient with lung mass and thyroid nodule. Diagn Cytopathol. 2018;46(4):345-348.
doi pubmed - Choi MR, Yoo SB, Kim JH. Sporadic medullary microcarcinoma in a male patient with concurrent Hashimoto's hypothyroidism and Kikuchi disease. Korean J Intern Med. 2016;31(6):1184-1186.
doi pubmed - Malpani S, Tandon A, Panwar H, Khurana U, Kapoor N, Behera G, Gupta V. Medullary thyroid carcinoma co-existent with Hashimoto's thyroiditis diagnosed by a comprehensive cytological approach. Diagn Cytopathol. 2020;48(4):386-389.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
