| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 2, Number 3, June 2012, pages 145-150
Two Cases of Rapidly Growing Papillary Thyroid Carcinoma After Radioactive Iodine Therapy for Graves’ Disease
Seigo Tachibanaa, d, Tadao Yokoib, Shinya Satob, Nobuhiro Nakatakec, Shuji Fukatac, Junichi Tajiric, Hiroyuki Yamashitab
aDepartment of Endocrinology, Yamashita Thyroid and Parathyroid Clinic, Fukuoka-city, Hakata-ku, Shimogofukumachi 1-8, 812-0034, Japan
bDepartment of Surgery, Yamashita Thyroid and Parathyroid Clinic, Fukuoka-city, Hakata-ku, Shimogofukumachi 1-8, 812-0034, Japan
cTajiri Thyroid Clinic, Kumamoto-city, Suizenji 2-6-3, 862-0950, Japan
dCorresponding author: Seigo Tachibana
Manuscript accepted for publication April 6, 2012
Short title: Thyroid Carcinoma
doi: https://doi.org/10.4021/jem84w
| Abstract | ▴Top |
We herein report two cases of papillary thyroid carcinoma (PTC) after radioactive iodine therapy (RIT) for Graves’ disease (GD). Case 1: A 25-year-old man visited to our clinic. He had undergone RIT for GD at 23 years old. His thyroid function was severe hypothyroidism because of poor compliance of taking levothyroxine (LT4), and thyroid nodule was detected by neck ultrasonography (US). After his first visit, his compliance of taking LT4 maintained poor, then his thyroid nodule grew up from 7 mm to 12 mm for 9 months. Fine needle aspiration biopsy (FNAB) was performed and the result of FNAB suggested PTC. Therefore, total thyroidectomy was done. Case 2: A 40-year-old woman was referred to our clinic for the treatment of PTC. She had also undergone RIT for GD at 34 years old. When she was 39 years old, a thyroid nodule was detected by neck US. Her compliance of taking LT4 was also poor, and the nodule grew up from 11 mm to 19 mm for 12 months. The result of FNAB was suggestive of PTC. Therefore, total thyroidectomy was done. In both two cases, thyroid nodular lesion could not be detected by neck US before RIT. In conclusion, we considered that this rapid growth of PTC in the irradiated thyroid gland may be induced by TSH stimulation, and that routine neck US should be continued after RIT for GD, even if there is no tumor in the thyroid before RIT.
Keywords: Radioactive iodine therapy; Papillary thyroid carcinoma; Graves’ disease; Hypothyroidism
| Introduction | ▴Top |
It is well known that radioactive iodine therapy (RIT) for Graves’ disease (GD) is a safe and useful treatment. It is also well known that the risk of RIT-induced thyroid cancer is not increased significantly [1]. In this paper, we report two cases of papillary thyroid carcinoma (PTC) which shows rapid growth after RIT for GD.
| Case Report | ▴Top |
Case 1
A 25-year-old Japanese man presented at our clinic, because of his removal. Before he visited our clinic, he underwent RIT (administered 131-I dose: 223 Gy) for GD at 23 years old at T thyroid clinic.
He became hypothyroidism soon after 131-I therapy, and had to take levothyroxine (LT4). However, his compliance of taking LT4 was poor. When he visited our clinic, he discontinued to take LT4. His thyroid function was as follows; TSH > 100 µIU/mL, free thyroxine (FT4) 0.97 ng/dL, and TSH receptor antibody (TRAb) (1st-generation assays) 51.2%. Despite severe hypothyroidism, his clinical manifestations were almost all normal and his other laboratory examinations also showed within normal limit. Neck ultrasonography (US) detected atrophic thyroid and a low echoic mass in the left lobe. The diameter of the nodule was 7 mm (Fig.1A). The result of fine needle aspiration biopsy (FNAB) from the nodule was indeterminate, but we did follow-up by neck US, because the size of the nodule was small and he did not hope for an operation. After 9 months, the size of the nodule became 12 mm, then, FNAB was performed again (Fig. 1A). The result of FNAB indicated PTC (Fig. 2A). Total thyroidectomy and lymph node dissection of central compartment was performed. The histological diagnosis was compatible with PTC (Fig. 2B). Figure 3 shows his thyroid function during the follow-up period after RIT, and his thyroid function maintained hypothyroidism because of his poor compliance of taking LT4. We could not detect nodular lesion (Fig. 4A) by US which was done at T thyroid clinic before RIT.
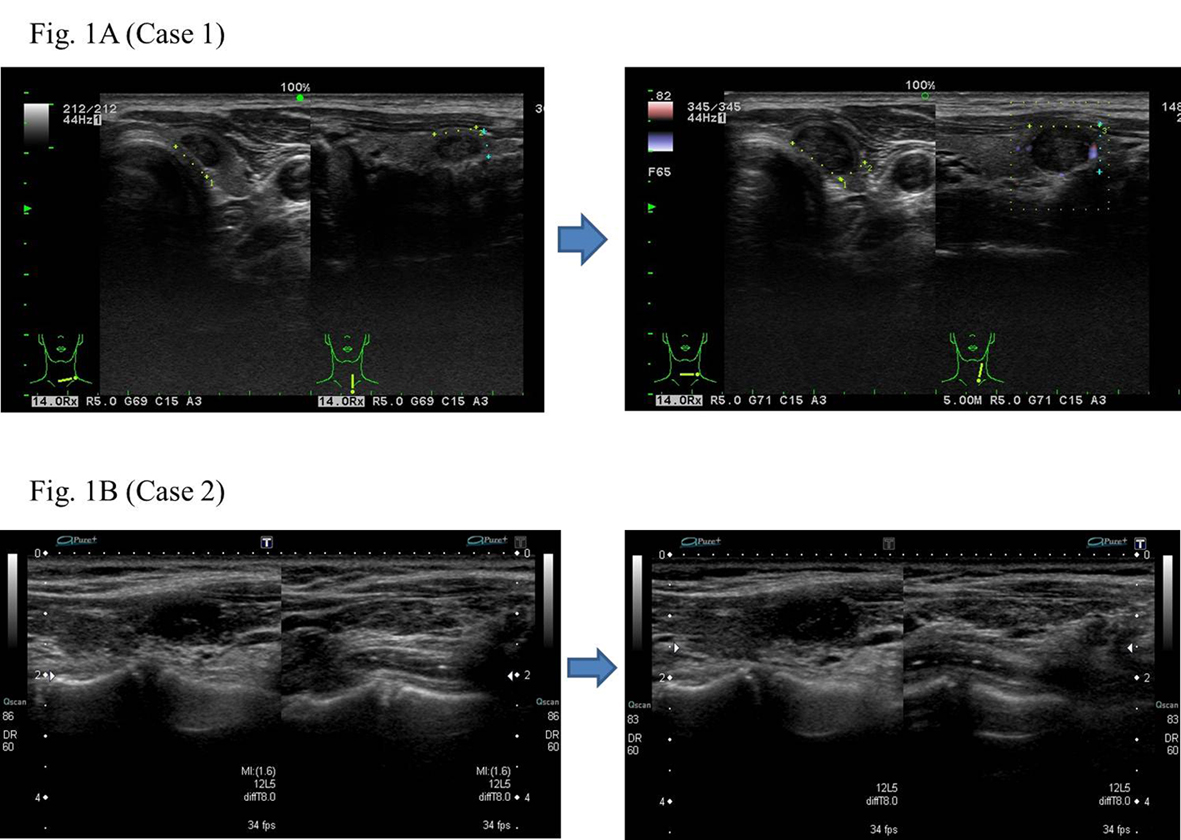 Click for large image | Figure 1. The low echoic nodule in the atrophic thyroid showing relatively rapid growth. |
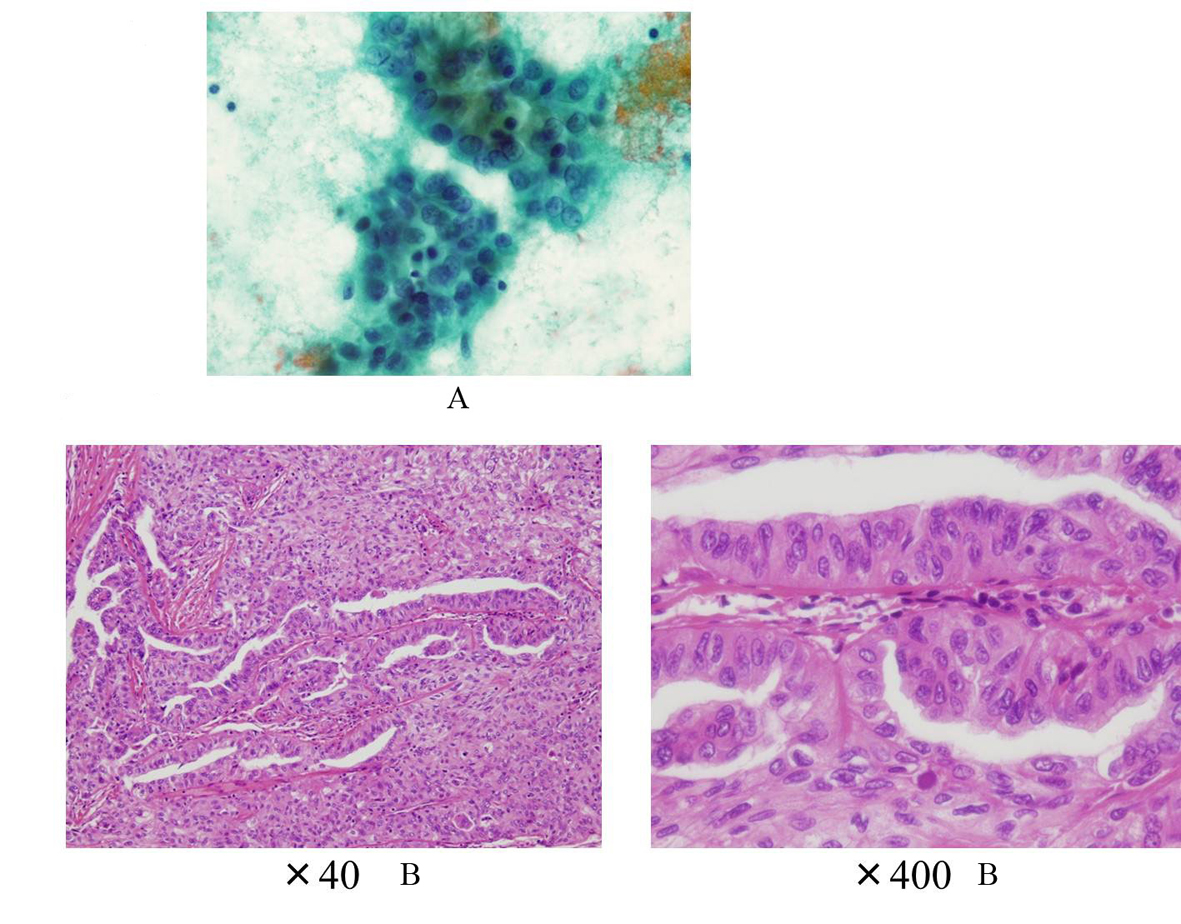 Click for large image | Figure 2. A: The cytology suggesting PTC (Case 1); B: The histology showing PTC without poorly differentiated component (Case 1). |
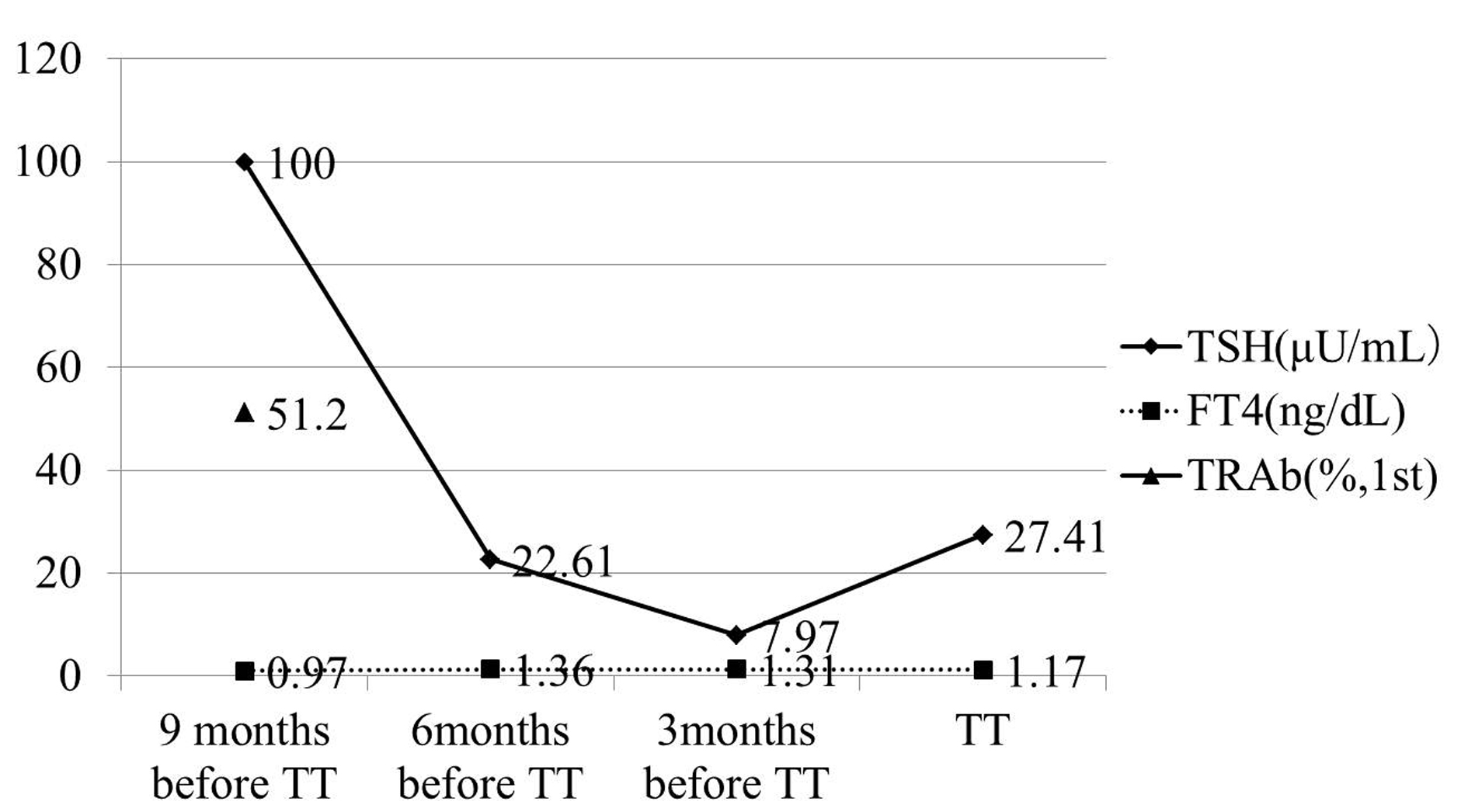 Click for large image | Figure 3. TSH concentration was maintained over normal range, because of bad compliance of taking LT4. TRAb was measured only once, and it was 51.2% (1st-generation). TT: total thyroidectomy. |
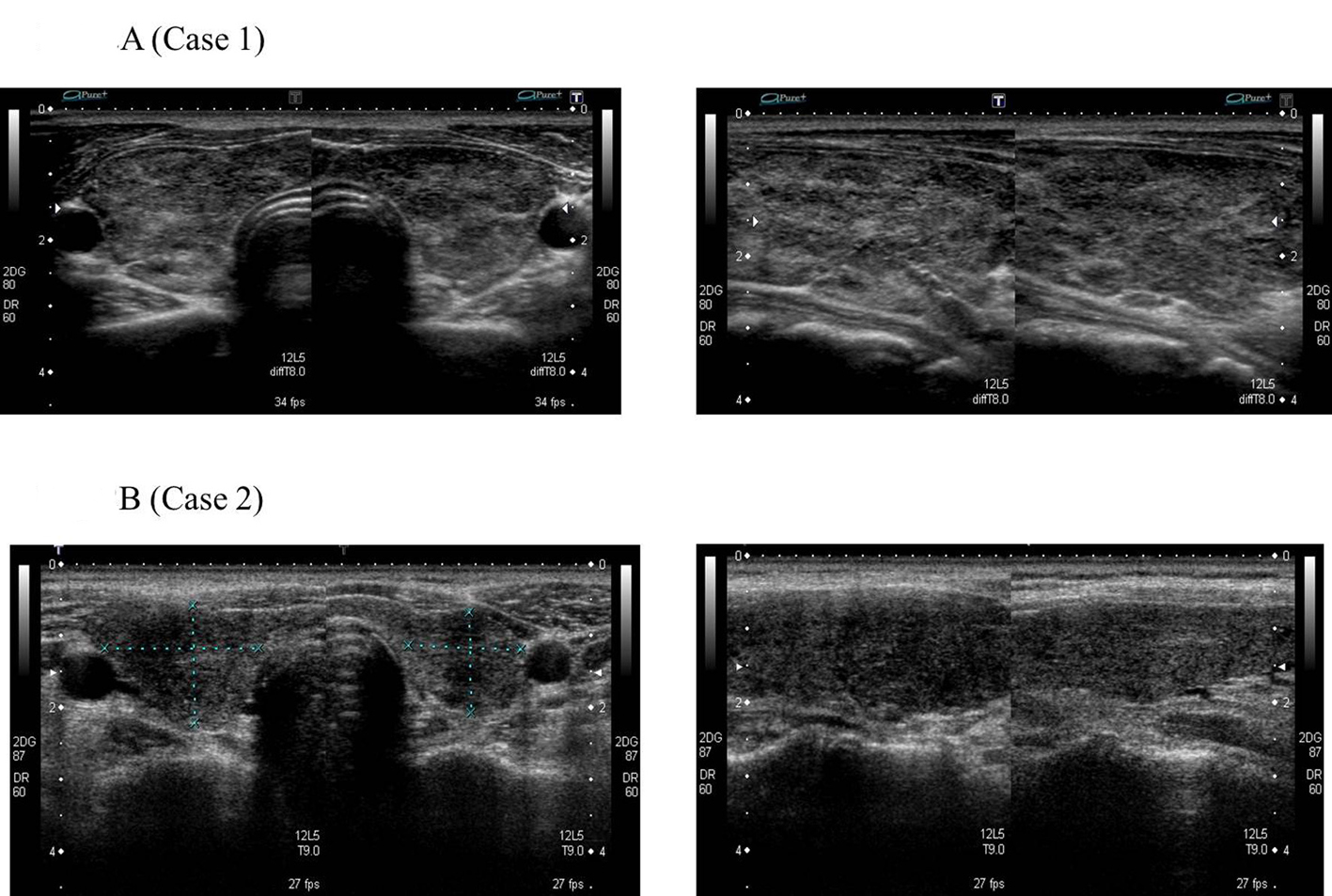 Click for large image | Figure 4. No nodular lesion in thyroid before RIT for GD. |
Case 2
A 40-year-old Japanese woman was referred to our clinic for the surgery of PTC. She also underwent RIT for GD at 34 years old at T thyroid clinic. Administered 131-I dose was 183 Gy and her hyperthyroidism had been cured after RIT. She had to take LT4 because of hypothyroidism, but her compliance of taking LT4 was also poor. Herserum TSH level was often over normal range as shown in Figure 5 after RIT. When she was 39 years old, a low echoic mass was detected in the right atrophic thyroid lobe by neck US and the size of the nodule was 11 mm. After 12 months, the size of the nodule was increased up to 19 mm (Fig. 1B) and swollen lymph nodes were also detected in the central compartment. FNAB from the tumor indicated suggestive of PTC. Then, she was referred to our clinic. When she visited our clinic, her clinical manifestations were almost all normal and her laboratory data were also within normal limit. Her thyroid function was as follows; TSH 4.67 µIU/mL, FT4 1.25 ng/dL, TRAb (3rd-generation assays) 5.0 IU/L, thyroglobulin (Tg) < 0.1ng/mL, and antithyroglobulin antibody (TgAb) 463 IU/mL. Neck US showed a low echoic and irregular shaped mass, and FNAB at our clinic indicated PTC (Fig. 6A). Therefore, total thyroidectomy and lymph node dissection of central compartment was performed. The histological diagnosis was compatible with PTC (Fig. 6B). We also reviewed neck US done at T thyroid clinicbefore RIT, but we could not detect nodular lesion (Fig. 4B).
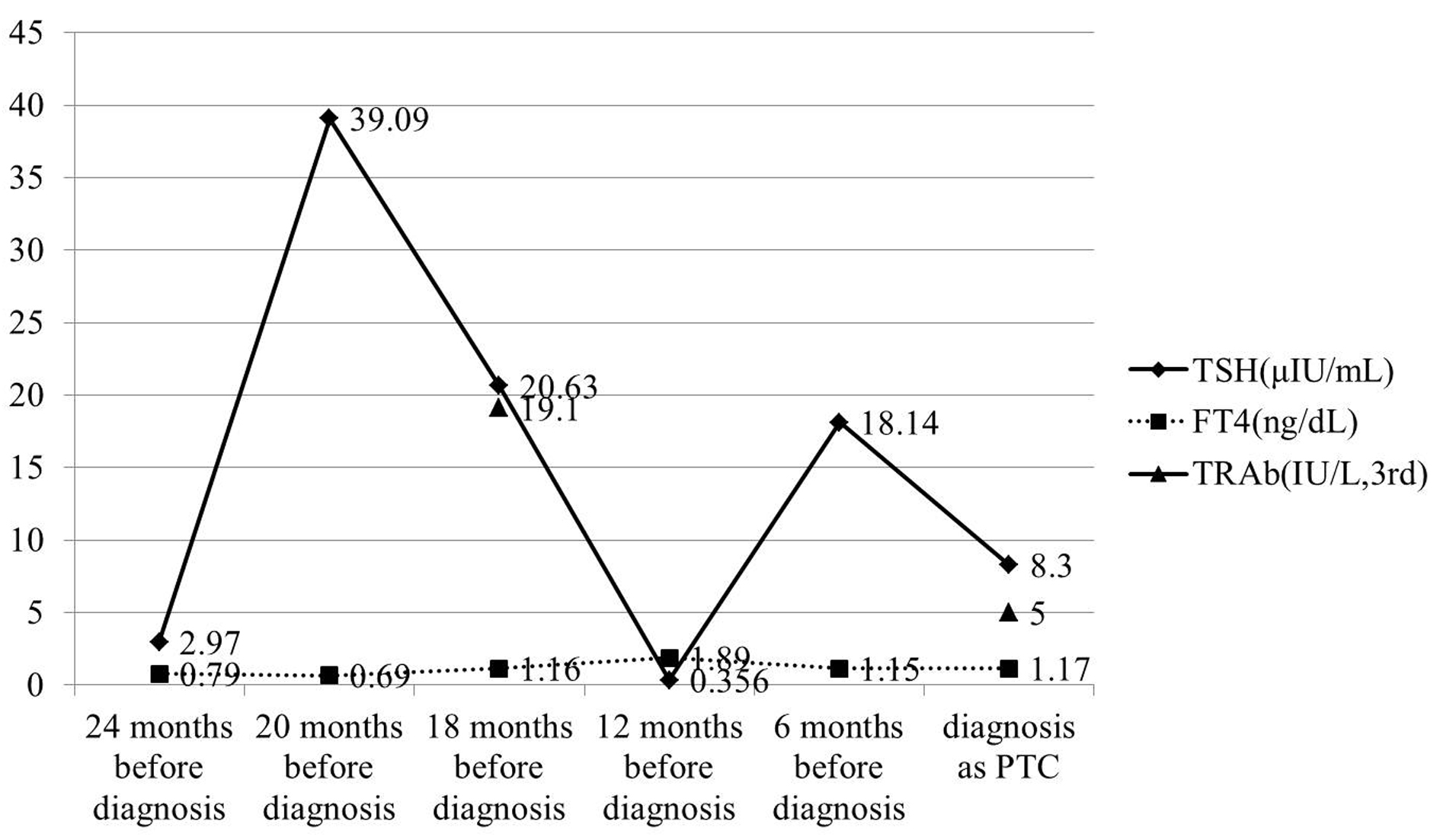 Click for large image | Figure 5. TSH concentration was also maintained over normal range in almost all follow-up period. TRAb (3rd-generation) was measured twice in the follow-up period, and it was gradually decreased. |
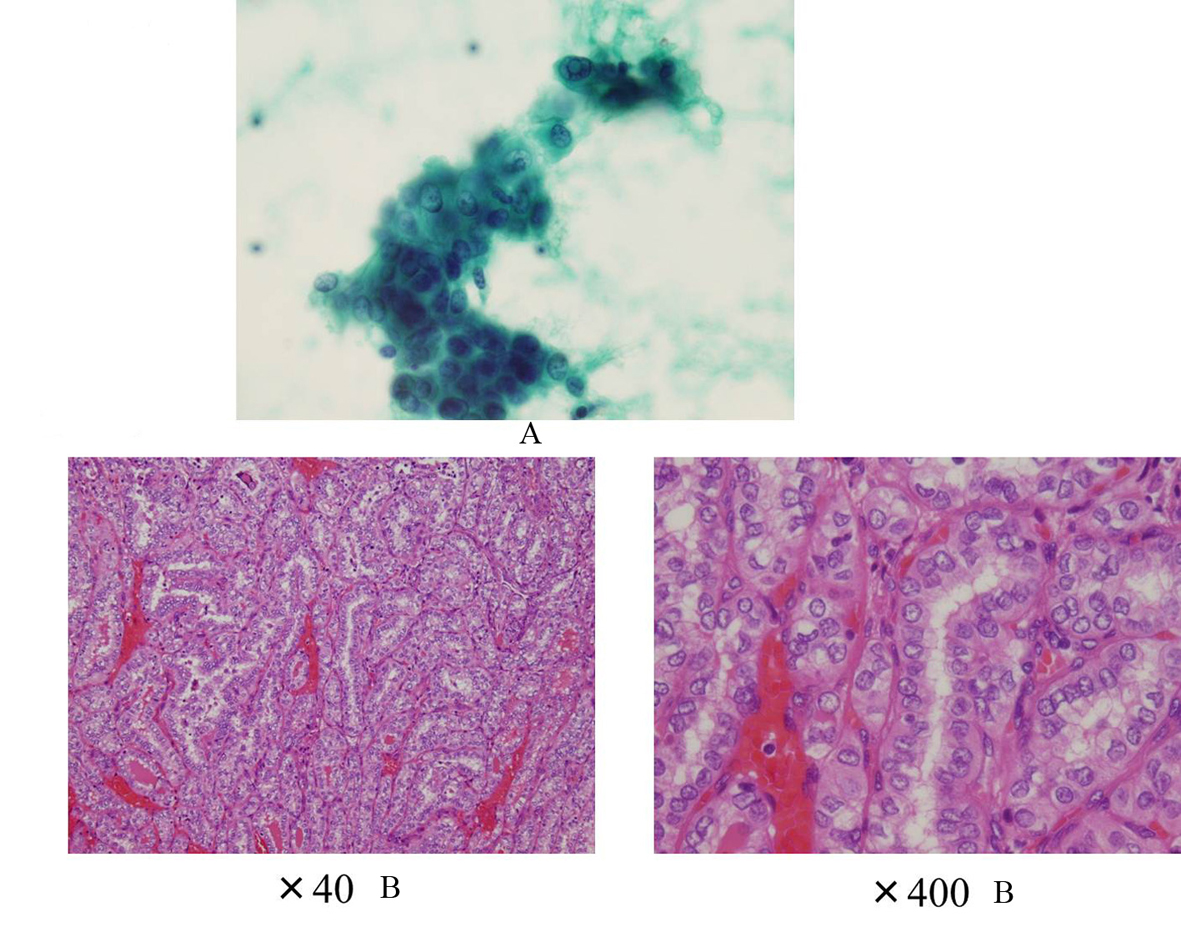 Click for large image | Figure 6. A: The cytology suggesting PTC (Case 2); B: The histology showing PTC without poorly differentiated component (Case 2). |
| Discussion | ▴Top |
In this paper, we reported two cases of PTC after RIT for GD. In general, it is believed that the risk of thyroid cancer is not increased significantly. Ron E et al reported that among adult patients in the Cooperative Thyrotoxicosis Therapy Follow-up Study cohort treated with RIT for GD, a small increase of thyroid cancer mortality was observed. However, this is believed to reflect the nature of the underlying thyroid disease rather than being a consequence of RIT [1]. On the other hand, the increased risk of thyroid cancer after thyroid irradiation in childhood has been recognized [2]. Rivkees SA reported that if relatively high dose of 131-I was administered, the risk of thyroid cancer did not increased because of minimizing the persistence of residual thyroid tissue [3]. In fact, reported four cases of thyroid cancer in children after RIT were all treated with low doses of 131-I [4-9], and high dose of 131-I for children’s and adolescent’s GD would decrease the risks of RIT-induced thyroid tumors [10]. These tendencies were observed not only in children and adolescents, but also in adults. Data from the Cooperative Thyrotoxicosis Therapy Follow-up Study suggested that the amount of residual thyroid tissue after RIT for GD plays an important role in determining the long-term risks of thyroid cancer in adults [11]. In this point of view, we considered that PTC of our two cases was not RIT-induced thyroid cancer, and was already existed micro cancer before RIT, because adequate high dose of 131-I was administered. It is difficult to detect thyroid micro cancers with GD, because the echogenicity of the thyroid of patients with GD is often heterogeneous. The heterogeneous echogenicity of the thyroid of our two patients shown by US made it difficult to detect micro PTC which was considered to be present before RIT.
There are many reports of PTC with GD, and the prognosis of PTC with GD is still controversial [12, 13]. Belfiore et al reported that TSH stimulation by TRAb may play a role in determining the high aggressiveness of thyroid cancer in GD patients [12]. On the other hand, some reports of Japanese populations suggested that PTC with GD in Japanese showed nonaggressive clinical features and good prognosis [13, 14]. Our cases showed relatively rapid growth of the size of PTC. There may be two explanations for the rapid growth. Firstly, in both cases, TSH was maintained in high range during the period before surgery, because of poor compliance of taking LT4. Zofan et al reported the possibility that there exists an increment in tumor size as a function of increment in the TSH level [15]. In addition, in both cases, TRAb was also detected to a certain extent (Fig. 3, 5). We considered that TSH stimulation to micro PTC due to serum TSH and TRAb might contribute to relatively rapid growth. Secondly, after RIT, it was considered that normal thyroid follicles became atrophic, but cancer cells persisted in the thyroid gland. The blood perfusion of PTC after RIT would increase disproportionately more than that before RIT, because the thyroid volume was decreased by RIT and the size of PTC was not affected by RIT. Therefore, we suggested that this disproportionately increased blood perfusion to PTC due to TSH stimulation influenced this relatively rapid growth of PTC. On the other hand, there are some reports of anaplastic thyroid carcinoma after RIT [16-18]. In these cases, RIT was performed for PTC, not for GD. Therefore, it was difficult to reflect these cases to our cases directly. However, in these cases, it was reported that RIT to the differentiated thyroid tumor plays a role in the transformation to an undifferentiated tumor due to p53 gene mutation. When the etiology of our cases was considered, this influence of RIT to preexisting PTC should be discussed. We could not detect undifferentiated or poorly differentiated component in the histology, suggesting that there was no dedifferentiation of preexisting PTC due to RIT in our cases.
We report two cases of rapid growing PTC after RIT for GD and discussed the pathophysiology. Although such occurrence is rare, routine neck US should be continued after RIT for GD, even if there is no tumor in the thyroid before RIT, because the heterogeneous echogenicity of thyroid due to GD makes it difficult to detect preexisting PTC. Further, PTC without poorly differentiated component may grow rapidly as shown in this report.
| References | ▴Top |
- Ron E, Doody MM, Becker DV, Brill AB, Curtis RE, Goldman MB, Harris BS, 3rd, et al. Cancer mortality following treatment for adult hyperthyroidism. Cooperative Thyrotoxicosis Therapy Follow-up Study Group. JAMA. 1998;280(4):347-355.
pubmed doi - Duffy BJ, Jr., Fitzgerald PJ. Cancer of the thyroid in children: a report of 28 cases. J Clin Endocrinol Metab. 1950;10(10):1296-1308.
pubmed doi - Rivkees SA. The use of radioactive iodine in the management of hyperthyroidism in children. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1(3):255-264.
pubmed doi - Kogut MD, Kaplan SA, Collipp PJ, Tiamsic T, Boyle D. Treatment of Hyperthyroidism in Children. Analysis of Forty-Five Patients. N Engl J Med. 1965;272:217-221.
pubmed - Sheline GE, Lindsay S, Mc CK, Galante M. Thyroid nodules occurring late after treatment of thyrotoxicosis with radioiodine. J Clin Endocrinol Metab. 1962;22:8-18.
pubmed - Farbota LM, Calandra DB, Lawrence AM, Paloyan E. Thyroid carcinoma in Graves' disease. Surgery. 1985;98(6):1148-1153.
pubmed - Karlan MS, Pollock WF, Snyder WH, Jr. Carcinoma of the Thyroid Following Treatment of Hyperthyroidism with Radioactive Iodine. Calif Med. 1964;101:196-199.
pubmed - Shore RE. Issues and epidemiological evidence regarding radiation-induced thyroid cancer. Radiat Res. 1992;131(1):98-111.
pubmed doi - Gorman CA, Robertson JS. Radiation dose in the selection of 131I or surgical treatment for toxic thyroid adenoma. Ann Intern Med. 1978;89(1):85-90.
pubmed - Rivkees SA, Sklar C, Freemark M. Clinical review 99: The management of Graves' disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab. 1998;83(11):3767-3776.
pubmed doi - Dobyns BM, Sheline GE, Workman JB, Tompkins EA, McConahey WM, Becker DV. Malignant and benign neoplasms of the thyroid in patients treated for hyperthyroidism: a report of the cooperative thyrotoxicosis therapy follow-up study. J Clin Endocrinol Metab. 1974;38(6):976-998.
pubmed doi - Belfiore A, Garofalo MR, Giuffrida D, Runello F, Filetti S, Fiumara A, Ippolito O, et al. Increased aggressiveness of thyroid cancer in patients with Graves' disease. J Clin Endocrinol Metab. 1990;70(4):830-835.
pubmed doi - Yano Y, Shibuya H, Kitagawa W, Nagahama M, Sugino K, Ito K. Recent outcome of Graves' disease patients with papillary thyroid cancer. Eur J Endocrinol. 2007;157(3):325-329.
pubmed doi - Kikuchi S, Noguchi S, Yamashita H, Uchino S, Kawamoto H. Prognosis of small thyroid cancer in patients with Graves' disease. Br J Surg. 2006;93(4):434-439.
pubmed doi - Zafon C, Obiols G, Baena JA, Castellvi J, Dalama B, Mesa J. Preoperative thyrotropin serum concentrations gradually increase from benign thyroid nodules to papillary thyroid microcarcinomas then to papillary thyroid cancers of larger size. J Thyroid Res. 2012;2012:530721.
pubmed - Maatouk J, Barklow TA, Zakaria W, Al-Abbadi MA. Anaplastic thyroid carcinoma arising in long-standing multinodular goiter following radioactive iodine therapy: report of a case diagnosed by fine needle aspiration. Acta Cytol. 2009;53(5):581-583.
pubmed doi - Kapp DS, LiVolsi VA, Sanders MM. Anaplastic carcinoma following well-differentiated thyroid cancer: etiological considerations. Yale J Biol Med. 1982;55(5-6):521-528.
pubmed - Shingu K, Kobayashi S, Yokoyama S, Fujimori M, Asanuma K, Ito KI, Hama Y, et al. The likely transformation of papillary thyroid carcinoma into anaplastic carcinoma during postoperative radioactive iodine-131 therapy: report of a case. Surg Today. 2000;30(10):910-913.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
