| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 12, Number 6, December 2022, pages 202-208
Conversion From Hypothyroidism to Hyperthyroidism and Back After Anti-SARS-CoV-2 Vaccination
Ushakov Thyroid Clinic, Moscow 109469, Russia
Manuscript submitted August 11, 2022, accepted October 27, 2022, published online December 1, 2022
Short title: Case of Hypothyroidism Conversion
doi: https://doi.org/10.14740/jem833
| Abstract | ▴Top |
Cases presenting with changes in various organs caused by anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination are rare. In particular, after such instances of vaccination, onset of subacute thyroiditis, Hashimoto thyroiditis, and Graves’ disease was observed. We present the case of a 55-year-old woman whose hypothyroidism converted to hyperthyroidism after vaccination with Gam-COVID-Vac (Sputnik V). After the first dose of vaccine, a hormonal metabolism alteration was noted, i.e., conversion from hypothyroidism to euthyroidism. After the second dose of vaccine, onset of hyperthyroidism with thyrotoxicosis was noted without signs of subacute thyroiditis. Hyperthyroidism persisted for several weeks before reverting to hypothyroidism. Assessments did not reveal an increase in the patient’s thyroid-stimulating hormone receptor antibody levels throughout the indicated period. However, after hyperthyroidism reverted to hypothyroidism, a significant increase in the peak systolic velocity of the superior thyroid arteries was observed, which is characteristic of hyperthyroidism. Owing to the known involvement of the autonomic nervous system (ANS) in blood vessel tone regulation, thyroid hormonogenesis, and stress, the ANS-mediated conversion from hypothyroidism to hyperthyroidism in certain individuals after receiving a vaccine, which may serve as a sufficient stimulus for ANS nerve centers, is highly probable. Therefore, diagnostic assessment of ANS condition should be performed before vaccination in patients with chronic thyroid disorders or diseases of other organs or organ systems. If changes in ANS are found, providing preemptive treatment before vaccination is recommended.
Keywords: Hyperthyroidism; Hypothyroidism; SARS-CoV-2; Vaccination; Graves’ disease; Sympathetic nervous system; Thyroid ultrasound; PSV STA
| Introduction | ▴Top |
Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination is being performed worldwide with various agents, but observations show that vaccination not only provides effective immune response but also causes alterations in functions of various organs and body systems [1-5], including the thyroid gland and its hormone production. Onset of subacute thyroiditis, autoimmune thyroiditis, and Graves’ disease after vaccination has been reported previously [6-10].
Such cases are rarely detected and are not typically observed after anti-SARS-CoV-2 vaccination [11-14]. However, all of them require coverage in medical periodical publications and appropriate assessments to gain an insight into their nature. This research methodology will give experts the opportunity to not only be aware of the probability of the condition onset but also get the opportunity to prevent or reduce the reactions caused by vaccination and treat the disease efficiently upon its occurrence.
We present an interesting case of a woman whose hypothyroidism converted to hyperthyroidism after receiving the Gam-COVID-Vac (Sputnik V) vaccine. This patient had been receiving hormone replacement therapy with levothyroxine for several years. Hypothyroidism conversion to hyperthyroidism (Graves’ disease) is known to be a rare phenomenon associated hypothetically with immune changes [15-19]. For instance, this is observed in cases in which the physicians switch between blocking and stimulating thyroid-stimulating hormone receptor (TSHR) antibodies “in nonstandard patients” [15]. However, the case of thyroid hormonal metabolism alteration in our patient was observed at the normal levels of the TSHR antibody.
The objective of this article is to show the characteristics of the key clinical signs and to disclose the probable essence of hypothyroidism conversion to hyperthyroidism after anti-SARS-CoV-2 vaccination.
| Case Report | ▴Top |
Investigations
A female patient (age, 55 years) had been taking levothyroxine for primary hypothyroidism in the last 10 years. The dose was 50 and 75 µg/day in the first 5 years, and 100 µg/day in the last 5 years. The general condition was good throughout this period. The follow-up serum tests performed in November 2020 and February 2021 showed minor hypothyroidism, with thyroid-stimulating hormone (TSH) levels up to 10 µIU/mL above the normal range (Table 1).
 Click to view | Table 1. Thyroid Hormonal Metabolism Alteration in the Female Patient Aged 55 That Resulted From Anti-SARS-CoV-2 Vaccinationa |
In spring 2021, the patient received her first dose of Sputnik V vaccine, and 21 days later she received the second dose. The first dose did not cause any changes in her overall health status. However, the blood test a week after the first vaccine dose (April 13, 2021) showed euthyroidism instead of minor hypothyroidism, which was persistently observed during the last year. Several days after the patient had received the second vaccine dose (on April 28, 2021), the signs of thyrotoxicosis appeared along with increased sleep loss, muscular weakness, fatigue after normal activities, internal tremor, and palpitation episodes. The follow-up blood test conducted after 2 weeks showed reduction in TSH to 0.13 µIU/mL (0.5 - 4.2 µIU/mL). There was no pain or discomfort in the neck and thyroid area. Laboratory investigations show normal erythrocyte sedimentation rate.
The patient stopped taking levothyroxine in mid-May 2021 due to general condition worsening, which resulted in significant improvement and disappearance of symptoms. The serum test in the latter part of June 2021 showed hyperthyroidism (after 6 weeks without a hormonal agent); however, as early as in the latter part of July, severe hypothyroidism (TSH > 30 µIU/mL) with FT4 and FT3 deficiency were noted. Serum TSHR antibodies remained within the normal range throughout spring and summer of 2021, despite the fact that the serum levels of thyroperoxidase antibody were higher than the normal value (939 U/L).
Diagnosis
Recent data showed hypothyroidism (Table 1). Thyroid ultrasound scan (“ultrasound”) in the latter part of July 2021 showed the following: total volume of 17.9 mL (11.6D + 6.3S); minor blood flow increase in the two lobes (greater on the right) in Doppler mode; moderate blood flow increase in the superior thyroid arteries (STAs, greater on the right); lymphocytic infiltration and signs of intralobular regeneration, predominantly on the right wherein proliferative nodules known as “white knight” were detected [19]. Furthermore, approximately 60% of isoechoic and poorly or moderate hypoechoic tissues looked hormonogenic to a different extent (Fig. 1).
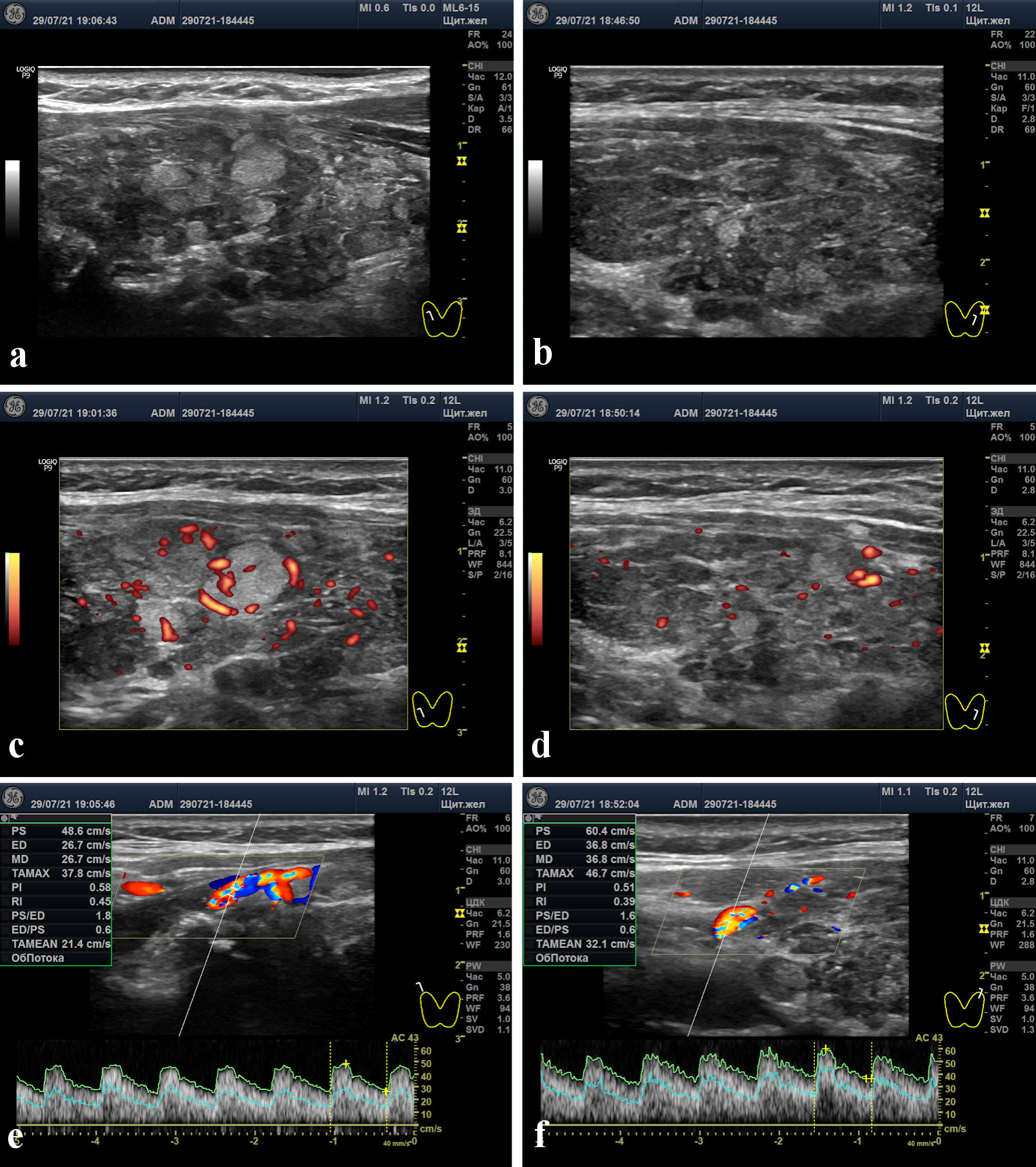 Click for large image | Figure 1. Thyroid ultrasound scans. (a, b) Right and left lobes in gray-scale mode. (c, d) Right and left lobes in power Doppler mode. (e, f) Peak systolic velocities in the right and left superior thyroid arteries (48.6 and 60.4 cm/s, respectively). |
| Discussion | ▴Top |
This report presents an interesting case of reactions to anti-SARS-CoV-2 vaccination, which lead to the conversion of hypothyroidism to hyperthyroidism (without the involvement of TSHR antibodies), persistence of hyperthyroidism for several weeks, and reversion to hypothyroidism. The possibility that the vaccine triggered such a response in the body and the thyroid gland is highly likely. This inference can be drawn not only owing to the fact that this is a known causality but also on the basis of multiple observations about the vaccine’s capability to cause topical (at the injection site), local (in organs including the thyroid gland), and systemic changes [20-23]. In addition, aside from the key reason for the occurrence of such a phenomenon, it is of paramount importance to understand the essence and the underlying mechanisms related to the development of such a condition.
Which systems in the body were affected by the vaccine and what was the biological path of changes in the body, which caused such a strange phenomenon, namely, the development of hyperthyroidism from hypothyroidism under conditions of exhausted thyroid gland over the years? Indeed, hypothyroidism has been characterized by decrease of thyroid function (“underactive thyroid” or “low thyroid”) both since the first publication about it by Dr. W. Gull [24] in 1883 and in more recent times [25]. To understand the essence of this process, it is necessary to thoroughly assess the characteristic clinical signs of the disease and the experience currently known.
Ultrasound data from our patient showed the signs of moderate destruction in both lobes, with lymphocytic infiltration in some large and medium segments. Considering the following points, the question arises as to whether the existing thyroid tissue is capable to produce and release excessive amount of hormones: 1) the amount of macrostructurally complete hormonogenic tissue, comprising approximately 60% of the gland volume; 2) the multiyear period of hormone replacement therapy with levothyroxine in large dosage (100 µg/day); and 3) minor hypothyroidism persistence (TSH about 8 µIU/mL, with normal levels of free thyroxin (FT4) and free triiodothyronine (FT3)) in the last year before vaccination.
Cases of conversion from chronic hypothyroidism, including that compensated with levothyroxine, to hyperthyroidism have been reported in several articles over the past 60 years [26]. However, the authors of these publications sparsely analyzed causes and factors contributing to such a polar hormonal metabolism alteration. They very sparsely described the living conditions, mental strain, acute or chronic diseases, surgical load, or other stresses that overload the nervous system and enhance basal metabolism, which can be additional stimulation of thyroid hormonogenesis. An example of such a rare publication may be a case history reported by Ahmad et al, who detected an inflectional disease of the upper respiratory tract to be a trigger for the conversion from hypothyroidism to hyperthyroidism in a 61-year-old woman [27].
Researchers predominantly focused on the search and confirmation of immune molecular mechanisms triggering Graves’ disease [15, 16]. Such mechanisms, in their opinion, were possible because of the cross effect of autoimmune processes [17], human immunodeficiency virus (HIV) infection, hepatitis C [18], genetic predisposition [27], and medications [28]. However, these hypotheses have weak evidence and remain as assumptions. This is the reason why some authors, aside from considering these hypotheses, express the view that the actual mechanism of hormonal metabolism alteration is not known, and the proposed theories are speculative [17, 18, 26, 29]. However, some observations indicate that in some cases of diffuse hyperthyroidism the level of stimulating TSHR antibodies does not increase [30, 31], and in some hypothyroidism cases the level of blocking TSHR antibodies is normal [32].
The researchers who studied cases showing the conversion of hypothyroidism to hyperthyroidism typically assessed scintigraphy data and the levels of thyroid hormones and antibodies. According to the literature, specialists did not account for the condition of thyroid parenchyma on the ultrasound scans from such patients; they did not assess the amount (in percentage by volume) of its macrostructurally complete hormonogenic tissue. It means that they did not reveal the functional capability of the gland to produce hormones in normal or excessive amount. More importantly, almost none of these researchers attempted to determine the value of blood flow in the gland lobes and the maximum velocity of blood flow in the thyroid arteries.
In our female patient, we found a moderate increase in the peak systolic velocity (PSV) of blood flow in the STAs. Although PSV STA values stay within a range of 10 - 30 cm/s [33-35] in euthyroidism, in our patient these values reached 48 and 60 cm/s on both sides (Fig 1. e, f). What does such a case of blood flow enhancement indicate?
When compared to euthyroidism (assuming the thyroid hormones are normal) and destructive thyroiditis, average PSV STA values in Graves’ disease reached 79 cm/s, and the diagnostic accuracy (cut-off level) was defined in a range between 45 and 67 cm/s [34, 36, 37].
Unfortunately, the specialists treating the patient when she presented with hyperthyroidism did not investigate her PSV STA values. In our clinic, this characteristic was investigated in a time of hypothyroidism, 1 month after the peak of hyperthyroidism period (July 29, 2021). However, we noted that one of the characteristic features of hyperthyroidism was that the PSV STA levels increased up to key values corresponding to Graves’ disease. It is very likely that in the hyperthyroidism period, PSV STA values and blood flow in the thyroid parenchyma were greater.
PSV STA represents the level of autonomic nervous system (ANS) stimulation [38, 39] and indicates the role of peripheral ANS in the activation of the thyroid gland function, with which the blood flow is directly associated [40]. Meanwhile, the direct involvement of conductive autonomic innervation in hormonogenesis within the thyroid gland is natural and evidenced by studies [41-46]. For example, according to Park et al [47], ANS directly affects thyrocytes and increases the production of thyroid hormones independently from TSH and TSHR antibodies. This is the main reason why the PSV value in the thyroid arteries is capable of representing the level of ANS-mediated stimulation of thyroid hormonogenesis.
In both hypo- and hyperthyroidism, an increase in blood flow within the thyroid parenchyma and PSV STA above normal values is often observed [48, 49]. In hypothyroidism, serum FT4 and FT3 are often at normal levels [50, 51], which is indicative of the functional capability of the thyroid gland to provide the level of thyroid hormones necessary for the body in response to stimulation by the nervous system and TSH. At the same time, the facts indicate the leading role of the nervous system in regulating thyroid hormone production [42-46]. Therefore, the excessive ANS-mediated stimulation of the thyroid gland in hypothyroidism can cause hyperthyroidism without the involvement of the immune system.
Can vaccination against SARS-CoV-2 activate ANS up to the levels capable of affecting thyroid function? The Sputnik V vaccine has the same side effects as those observed in other anti-SARS-CoV-2 vaccines, which include pain at the injection site, fever, and muscular pain [20-23]. All these events develop with the involvement of ANS [52-56], and their manifestation may be dependent on ANS activation level.
Therefore, the underlying mechanism of excessive stimulation of the thyroid gland that caused the conversion of hypothyroidism to hyperthyroidism is very likely to be associated with ANS. This system and the thyroid gland are affected by any stress experienced by the body and induced by any adequate trigger [57, 58] including virus or vaccine [59]. In the case of our patient, we can assume that vaccination caused stress, which was a significant individual external influence that consequently affected homeostasis [57, 60], thereby leading to the activation of ANS [58].
Neurocyte activation level in sympathetic nerve centers can vary as affected by irritants [57, 58]. So, if ANS activation significantly increases then the effect on the thyroid gland can rise respectively, and vice versa, if ANS activation decreases then sympathetic stimulation of the thyroid gland reduces. Such changes can contribute to the transition from hypothyroidism to hyperthyroidism and vice versa.
Moreover, the presented case of transition from hypothyroidism to hyperthyroidism and many other cases demonstrate the thyroid compensatory capability to produce not only sufficient but also excessive amounts of hormones. In other words, primary hypothyroidism should not be definitively attributed to complete weakness of the gland.
Such a conclusion can be evidenced by the case of a 49-year-old patient having one thyroid lobe (due to other lobe agenesia) who acquired hypothyroidism with signs of Hashimoto thyroiditis only in this age, and hyperthyroidism in the subsequent 2 years [61]. Currently, 17 similar cases are known [62].
Summing up, what practical conclusion can be made? Despite the importance and the necessity of vaccination against SARS-CoV-2 [20-23], it is imperative to perform a diagnostic assessment of the ANS. This is particularly important for patients having chronic diseases in general and thyroid conditions in particular. In cases where excessive activation of ANS is detected, vaccination should be administered: 1) after achieving adequate suppression of the increased sympathetic tone; or 2) after the course of medication for ANS repair is completed.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient for their anonymized information to be published in this article.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
Abbreviations
TSHR: thyroid-stimulating hormone receptor; TSH: thyroid-stimulating hormone; ANS: autonomic nervous system; FT4: free thyroxin; FT3: free triiodothyronine; PSV: peak systolic velocity; STAs: superior thyroid arteries
| References | ▴Top |
- Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219-226.
doi pubmed - Elboraey MO, Essa E. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132(4):e139-e142.
doi pubmed - Jeet Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, Yadav D, Islam S, et al. Cardiovascular adverse events reported from COVID-19 vaccines: a study based on WHO database. Int J Gen Med. 2021;14:3909-3927.
doi pubmed - Gobel CH, Heinze A, Karstedt S, Morscheck M, Tashiro L, Cirkel A, Hamid Q, et al. Headache attributed to vaccination against COVID-19 (Coronavirus SARS-CoV-2) with the ChAdOx1 nCoV-19 (AZD1222) vaccine: a multicenter observational cohort study. Pain Ther. 2021;10(2):1309-1330.
doi pubmed - Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, Cerny A, Dayer E, Vergani D, Terziroli Beretta-Piccoli B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J Autoimmun. 2021;123:102706.
doi pubmed - Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med (Lausanne). 2021;8:737142.
doi pubmed - Montebello A. Recurrent Graves' disease post SARS-CoV-2 infection. BMJ Case Rep. 2021;14(8):e244714.
doi pubmed - Zettinig G, Krebs M. Two further cases of Graves' disease following SARS-Cov-2 vaccination. J Endocrinol Invest. 2022;45(1):227-228.
doi pubmed - Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sanchez Valadez TI, Jara LJ. Two cases of Graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31(9):1436-1439.
doi pubmed - Oyibo SO. Subacute Thyroiditis After Receiving the Adenovirus-Vectored Vaccine for Coronavirus Disease (COVID-19). Cureus. 2021;13(6):e16045.
doi - Hernan Martinez J, Corder E, Uzcategui M, Garcia M, Sostre S, Garcia A. Subacute thyroiditis and dyserythropoesis after influenza vaccination suggesting immune dysregulation. Bol Asoc Med P R. 2011;103(2):48-52.
- Altay FA, Guz G, Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccin Immunother. 2016;12(4):1033-1034.
doi pubmed - Toft J, Larsen S, Toft H. Subacute thyroiditis after hepatitis B vaccination. Endocr J. 1998;45(1):135.
- Passah A, Arora S, Damle NA, Reddy KS, Khandelwal D, Aggarwal S. Occurrence of Subacute Thyroiditis following Influenza Vaccination. Indian J Endocrinol Metab. 2018;22(5):713-714.
doi pubmed - Kasagi K, Hidaka A, Endo K, Miyamoto S, Takeuchi R, Misaki T, Sakahara H, et al. Fluctuating thyroid function depending on the balance between stimulating and blocking types of TSH receptor antibodies: a case report. Thyroid. 1993;3(4):315-318.
doi pubmed - McLachlan SM, Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid. 2013;23(1):14-24.
doi pubmed - Gonzalez-Aguilera B, Betea D, Lutteri L, Cavalier E, Geenen V, Beckers A, Valdes-Socin H. Conversion to Graves disease from Hashimoto thyroiditis: a study of 24 patients. Arch Endocrinol Metab. 2018;62(6):609-614.
doi pubmed - Watari J, Jassil N. Conversion of hypothyroidism to hyperthyroidism: a rare clinical phenomenon. AACE Clin Case Rep. 2020;6(5):e279-e281.
doi pubmed - Bonavita JA, Mayo J, Babb J, Bennett G, Oweity T, Macari M, Yee J. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193(1):207-213.
doi pubmed - Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-681.
doi - Logunov DY, Dolzhikova IV, Shcheblyakov DV. Data discrepancies and substandard reporting of interim data of Sputnik V phase 3 trial - Authors' reply. Lancet. 2021;397(10288):1883-1884.
doi - Montalti M, Solda G, Di Valerio Z, Salussolia A, Lenzi J, Forcellini M, Barvas E, et al. ROCCA observational study: Early results on safety of Sputnik V vaccine (Gam-COVID-Vac) in the Republic of San Marino using active surveillance. EClinicalMedicine. 2021;38:101027.
doi pubmed - Campana Nacional de Vacunacion contra la COVID-19 14° Informe de vigilancia de seguridad en vacunas AGOSTO de 2021. https://bancos.salud.gob.ar/sites/default/files/2021-09/14o-informe-de-vigilancia-de-seguridad-en-vacunas.pdf. Accessed October 15, 2022.
- Gull WW. On a cretinoid state supervening in adult life in women. Trans Clin Soc Lond. 1873/1874;7:180-185.
- American Thyroid Association. Hypothyroidism. 2017. https://www.thyroid.org/hypothyroidism/. Accessed October 15, 2022.
- Furqan S, Haque NU, Islam N. Conversion of autoimmune hypothyroidism to hyperthyroidism. BMC Res Notes. 2014;7:489.
doi pubmed - Ahmad E, Hafeez K, Arshad MF, Isuga J, Vrettos A. Hypothyroidism conversion to hyperthyroidism: it's never too late. Endocrinol Diabetes Metab Case Rep. 2018;2018(1):18-0047.
doi pubmed - Fan W, Tandon P, Krishnamurthy M. Oscillating hypothyroidism and hyperthyroidism - a case-based review. J Community Hosp Intern Med Perspect. 2014;4(5):25734.
doi pubmed - Sukik A, Mohamed S, Habib MB, Sardar S, Tanous B, Tahtouh R, Mohamed MFH. The Unusual Late-Onset Graves' Disease following Hashimoto's Related Hypothyroidism: A Case Report and Literature Review. Case Rep Endocrinol. 2020;2020:5647273.
doi pubmed - Thomas JS, Leclere J, Hartemann P, Duheille J, Orgiazzi J, Petersen M, Janot C, et al. Familial hyperthyroidism without evidence of autoimmunity. Acta Endocrinol (Copenh). 1982;100(4):512-518.
doi pubmed - Ilicki A, Gamstedt A, Karlsson FA. Hyperthyroid Graves' disease without detectable thyrotropin receptor antibodies. J Clin Endocrinol Metab. 1992;74(5):1090-1094.
doi pubmed - Kasagi K, Hidaka A, Nakamura H, Takeuchi R, Misaki T, Iida Y, Konishi J. Thyrotropin receptor antibodies in hypothyroid Graves' disease. J Clin Endocrinol Metab. 1993;76(2):504-508.
doi pubmed - Macedo TA, Chammas MC, Jorge PT, Pereira de Souza L, Farage L, Pegoraro BL, Pessa SU, et al. Reference values for Doppler ultrasound parameters of the thyroid in a healthy iodine-non-deficient population. Br J Radiol. 2007;80(956):625-630.
doi pubmed - Kim TK, Lee EJ. The value of the mean peak systolic velocity of the superior thyroidal artery in the differential diagnosis of thyrotoxicosis. Ultrasonography. 2015;34(4):292-296.
doi pubmed - Joish UK, Kavitha Y, Reddy RH, Prabhu AS, Kumar MC, Siddharth MC. Doppler indices of superior thyroid artery in clinically euthyroid adults. Indian J Radiol Imaging. 2018;28(1):10-13.
doi pubmed - Uchida T, Takeno K, Goto M, Kanno R, Kubo S, Takahashi S, Azuma K, et al. Superior thyroid artery mean peak systolic velocity for the diagnosis of thyrotoxicosis in Japanese patients. Endocr J. 2010;57(5):439-443.
doi pubmed - Zhao X, Chen L, Li L, Wang Y, Wang Y, Zhou L, Zeng F, et al. Peak systolic velocity of superior thyroid artery for the differential diagnosis of thyrotoxicosis. PLoS One. 2012;7(11):e50051.
doi pubmed - Sundler F, Grunditz T, Hakanson R, Uddman R. Innervation of the thyroid. A study of the rat using retrograde tracing and immunocytochemistry. Acta Histochem Suppl. 1989;37:191-198.
- Abdreshov SN, Akhmetbaeva NA, Atanbaeva GK, Mamataeva AT, Nauryzbai UB. Adrenergic innervation of the thyroid gland, blood and lymph vessels, and lymph nodes in hypothyroidism. Bull Exp Biol Med. 2019;168(2):295-299.
doi pubmed - Ushakov AV. Clinical ultrasound of benign thyroid pathology. Moscow: Ushakov Thyroid Clinic. 2018;216.
- Agipa YI. Endocrine gland nerves and mediators in the regulation of endocrine functions. Moscow: Science. 1981;503.
- Young JB, Burgi-Saville ME, Burgi U, Landsberg L. Sympathetic nervous system activity in rat thyroid: potential role in goitrogenesis. Am J Physiol Endocrinol Metab. 2005;288(5):E861-867.
doi pubmed - Nilsson OR, Karlberg BE. Thyroid hormones and the adrenergic nervous system. Acta Med Scand Suppl. 1983;672:27-32.
doi pubmed - Cardinali DP, Vacas MI, Gejman PV, Pisarev MA, Barontini M, Boado RJ, Juvenal GJ. The sympathetic superior cervical ganglia as "little neuroendocrine brains". Acta Physiol Lat Am. 1983;33(3):205-221.
- Cardinali DP, Stern JE. Peripheral neuroendocrinology of the cervical autonomic nervous system. Braz J Med Biol Res. 1994;27(3):573-599.
- Silva JE, Bianco SD. Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid. 2008;18(2):157-165.
doi pubmed - Park GC, Kim JM, Park HY, Han JM, Shin SC, Jang JY, Jung D, et al. TSH-independent release of thyroid hormones through cold exposure in aging rats. Oncotarget. 2017;8(52):89431-89438.
doi pubmed - Zabolotskaya NV, Strizhakova EM. Application of Doppler ultrasonography for quantitative assessment of blood flow in the thyroid arteries in chronic autoimmune thyroiditis. Ultrasound and Functional Diagnostics. 2009;3:9-20.
- Ishay A, Pollak Y, Chervinsky L, Lavi I, Luboshitzky R. Color-flow doppler sonography in patients with subclinical thyroid dysfunction. Endocr Pract. 2010;16(3):376-381.
doi pubmed - Khan MA, Ahsan T, Rehman UL, Jabeen R, Farouq S. Subclinical Hypothyroidism: Frequency, clinical presentations and treatment indications. Pak J Med Sci. 2017;33(4):818-822.
doi pubmed - Rivolta G, Cerutti R, Colombo R, Miano G, Dionisio P, Grossi E. Prevalence of subclinical hypothyroidism in a population living in the Milan metropolitan area. J Endocrinol Invest. 1999;22(9):693-697.
doi pubmed - Janig W. Systemic and specific autonomic reactions in pain: efferent, afferent and endocrine components. Eur J Anaesthesiol. 1985;2(4):319-346.
- Janig W, Kollmann W. The involvement of the sympathetic nervous system in pain. Possible neuronal mechanisms. Arzneimittelforschung. 1984;34(9A):1066-1073.
- Oka T. Stress-induced hyperthermia and hypothermia. Handb Clin Neurol. 2018;157:599-621.
doi pubmed - Jepson MM, Millward DJ, Rothwell NJ, Stock MJ. Involvement of sympathetic nervous system and brown fat in endotoxin-induced fever in rats. Am J Physiol. 1988;255(5 Pt 1):E617-620.
doi pubmed - Janig W. Autonomic nervous system and inflammation. Auton Neurosci. 2014;182:1-3.
doi pubmed - Kopin IJ. Definitions of stress and sympathetic neuronal responses. Ann N Y Acad Sci. 1995;771:19-30.
doi pubmed - Won E, Kim YK. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr Neuropharmacol. 2016;14(7):665-673.
doi pubmed - Porzionato A, Emmi A, Barbon S, Boscolo-Berto R, Stecco C, Stocco E, Macchi V, et al. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020;287(17):3681-3688.
doi pubmed - Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244-1252.
doi - Ruchala M, Szczepanek E, Skiba A, Czepczynski R, Sowinski J. Graves' hyperthyroidism following primary hypothyroidism due to Hashimoto's thyroiditis in a case of thyroid hemiagenesis: case report. Neuro Endocrinol Lett. 2008;29(1):55-58.
- Faulkner J, Varadharajan K, Choudhury N. A UK reported case of Graves' disease with thyroid hemiagenesis. BMJ Case Rep. 2019;12(8):e228094.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
