| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Case Report
Volume 12, Number 3, June 2022, pages 107-110
Endogenous De Novo Synthesis of Isopropanol Following Severe Non-Diabetic Ketoacidosis in a Patient With Duchenne Muscular Dystrophy
Karl Bihlmaiera, Steffen Gramppa, Carsten Willama, Mario Schiffera, Larissa Herbsta, b
aDepartment of Nephrology and Hypertension, Friedrich-Alexander University Erlangen-Nurnberg (FAU) and University Hospital Erlangen, Ulmenweg 18, 91054 Erlangen, Germany
bCorresponding Author: Larissa Herbst, Department of Nephrology and Hypertension, Friedrich-Alexander University Erlangen-Nurnberg (FAU) and University Hospital Erlangen, Ulmenweg 18, 91054 Erlangen, Germany
Manuscript submitted March 31, 2022, accepted May 20, 2022, published online June 16, 2022
Short title: Endogenous De Novo Synthesis of Isopropanol in DMD
doi: https://doi.org/10.14740/jem804
| Abstract | ▴Top |
In Duchenne muscular dystrophy (DMD), patients seem prone to severe metabolic acidosis following excessive bicarbonate loss due to laxative use or gastrointestinal disorders. In addition, hypoalimentation and fasting can cause life-threatening non-diabetic ketoacidoses with acetonemia in these patients. Isopropanolemia instead is predominantly found following intoxication. Only few reports exist describing isopropanolemia in diabetic or alcoholic ketoacidosis. Here, we report on a patient suffering from DMD who developed life-threatening non-diabetic ketoacidosis, acetonemia, and endogenous de novo synthesis of isopropanol following glucose fasting. We hypothesize isopropanolemia being a byproduct of acetone metabolism, possibly in association with a metabolic predisposition in patients with DMD such as an altered NADH/NAD+ ratio leading to a reduction from acetone to isopropyl alcohol whilst in severe ketoacidosis.
Keywords: Isopropanolemia; Acetonemia; Ketoacidosis; Refeeding syndrome; Duchenne muscular dystrophy
| Introduction | ▴Top |
Survival and quality of life in patients with Duchenne muscular dystrophy (DMD) have significantly improved over the past years. Besides typical disease-related symptoms and complications, DMD patients appear in emergency departments presenting novel symptoms and complications. Here, we report on a life-threatening metabolic ketoacidosis in a DMD patient with severe ketonemia, acetonemia, and, surprisingly, isopropanolemia. Severe metabolic acidosis in adult patients with DMD is rare. It is mainly associated with respiratory or abdominal infections as well as to insufficient caloric intake [1-3], as it is in our case description. In general, isopropanolemia is only detected when isopropanol is orally ingested, inhaled, or transdermally absorbed. Besides intoxication, only diabetic ketoacidoses or chronic alcoholism are metabolic conditions in which elevated serum levels of isopropyl alcohol have been reported to date. Both known metabolic conditions and intoxication could be excluded. DMD seems to provide a third metabolic environment in which isopropanol may be endogenously produced on a non-diabetic and non-alcoholic basis.
| Case Report | ▴Top |
Investigations
A 21-year-old man with known DMD was transferred to the intensive care unit of our university hospital. He had a medical history of community-acquired pneumonia, which had been treated for 7 days with amoxicillin and clavulanic acid. On admission, he was massively hyperventilating and presented a sinus tachycardia of 150/min with a blood pressure of 120/62 mm Hg. Body temperature was 34.8 °C. The patient’s general health-related quality of life was limited by pronounced reduced mobility and the necessity of nocturnal bilevel ventilation. Under antimicrobial therapy, dyspnea did not recede, so he used his home ventilation device permanently without medical supervision. Due to reduced appetite and continuous mechanical ventilation, there was no caloric or fluid intake. Neither emesis nor diarrhea had been reported.
Diagnosis
Blood gas analysis revealed severe metabolic acidosis with pH of 6.9 (normal range 7.35 -7.45), massively decreased bicarbonate levels of 2.2 mmol/L (normal range 22 - 26 mmol/L), and an arterial carbon dioxide partial pressure of 9.3 mm Hg (normal range 35 - 45 mm Hg) in the context of respiratory compensation. Interestingly, lactate levels were normal with 0.7 mmol/L (normal range < 2 mmol/L), but anion gap was elevated at 27 mmol/L (normal range < 10 mmol/L). Potassium levels were elevated to 5.5 mmol/L (normal range 3.5 - 4.5 mmol/L) due to the acidosis, sodium was normal, and chloride was elevated to 116 mmol/L (normal range 98 - 108 mmol/L) in the context of hyperchloremic acidosis. Diabetes had not been reported previously, and blood glucose concentration and HbA1c values were normal (6.2 and 30.2 mmol/L, respectively).
As the anion gap was elevated at 27 mmol/L, blood ketone concentrations were measured and found to be highly elevated at 6.8 mmol/L (normal range < 0.6 mmol/L) leading to the diagnosis of severe ketoacidosis. Urine microscopy ruled out crystalluria. A screening for methanol, salicylates, acetone, isopropyl alcohol, and ethylene glycol revealed highly increased acetone levels at a concentration of 373 mg/L (normal range < 6 mg/L) in consistency with hyperketonemia. Surprisingly, also isopropanol serum levels were highly elevated with 114 mg/dL (normal range < 5 mg/dL) whereat any exposure anamnesis (both patient and his parents) was plausibly negative. Toxicological analyses of urine and serum did not detect further toxins.
Treatment
Besides dyspneic agitation, the mental status was only slightly impaired. We used intermittent non-invasive mechanical ventilation to support the severe diaphragm dysfunction, infused balanced crystalloids and substituted sodium bicarbonate to reduce the respiratory drive. Heart rate, pH, PaCO2 and HCO3- were thereby corrected.
The desiccated patient presented an acute kidney injury (AKI) with serum creatinine levels 14 times over his baseline. Due to his highly reduced muscular mass, his baseline creatinine was 0.06 mg/dL, and maximum serum creatinine was 0.83 mg/dL. Blood urea nitrogen was highly elevated to 37.86 mmol/L (normal range 3.2 - 7.3 mmol/L). Serum osmolality was elevated at 346 mOsmol/kg. Upon treatment using balanced crystalloids renal retention parameters declined rapidly, diuresis increased, and serum sodium and serum osmolality ameliorated guardedly within several days.
Few reports exist describing DMD patients suffering from ketoacidosis after severe undernourishment with complete recovery following glucose infusion [1, 2]. Hence, we initiated low-level parenteral nutrition at 14 kcal/kg body weight/24 h with intravenous glucose infusion at 7 kcal/kg body weight/24 h. This rapidly resolved the fasting state, and ketone levels decreased to 4.4 mmol/L within 12 h and to 1.4 mmol/L after 24 h, respectively.
Following the prehospital antibacterial therapy using amoxicillin/clavulanic acid C-reactive protein was measured at 89.4 mg/L (normal range < 5 mg/L), and leukocytes was at 12.260/µL. Antibiotic therapy was switched to intravenous piperacillin/tazobactam and ciprofloxacin. Leukocytosis and C-reactive protein normalized rapidly. Ciprofloxacin was withdrawn at day 3, piperacillin/tazobactam at day 6.
Serum phosphate levels decreased rapidly due to a refeeding syndrome. Phosphate was substituted and caloric intake was restricted to 7 kcal/kg body weight/24 h. Caloric increment was deliberately performed with 2 kcal/kg body weight/day. Already at 11 kcal/kg body weight/24 h serum phosphate levels re-declined and caloric intake was re-restricted. In the course of several days caloric intake was raised to 21 kcal/kg body weight/24 h, and the patient could be moved to a regular ward for further therapy. Development of refeeding syndrome despite of restricted caloric intake suggested a chronic and preexisting malnourished lifestyle before the onset of pneumonia. At this point gastrostomy was discussed but declined by the patient. Shortly thereafter, he was discharged from hospital.
Follow-up and outcomes
After several weeks relapse of the metabolic acidosis occurred based on pneumonia with pulmonal detection of respiratory syncytial virus and consecutive bacterial superinfection. After admission to the regular ward of our hospital, clinical stabilization and controlled caloric intake the patient obtained an operative gastrostomy to prevent periods of severe hypoalimentation while dependent on intensified bilevel ventilation. One year after final discharge the patient lived in comfort at home and pursued his profession.
| Discussion | ▴Top |
Here, we present a case of severe ketoacidosis with endogenous isopropanolemia in a patient without preexisting diabetes type 1 or 2, chronic alcohol ingestion, or intoxication but in association with DMD.
Severe non-diabetic ketoacidosis in patients suffering from DMD is a rare event [1-3]. To our knowledge, only very few reports exist describing ketoacidoses in DMD patients, whereat most of these cases present metabolic ketoacidosis following intestinal disorders such as acute megacolon, acute cholecystitis, or viral gastroenteritis with consecutive bicarbonate loss due to diarrhea, or laxative use, respectively [1-3]. In total, seven cases were presented in which hypoalimentation seems to be the main cause for metabolic acidosis, since starvation and katabolic metabolism due to acute infectious diseases predispose to ketoacidosis [1-3]. Most patients in the reviewed literature were treated with glucose infusion, partly in combination with insulin. Retrospectively, as other authors mentioned in their reports, glucose substitution without insulin might have been sufficient in these patients. We initiated cautious glucose substitution as described above but had to add insulin per perfusorem for 15 h in order to keep blood glucose levels below 180 mg/dL.
Isopropanolemia is generally linked to poisoning by transdermal absorption, inhalation, or ingestion [4, 5]. Besides intoxication only few reports exist describing isopropanolemia in diabetic ketoacidosis [6-8]. In addition, Lewis et al [9] and Davis et al [10] detected acetone and isopropanol in the blood of autopsy patients not previously exposed to isopropanol. Robertson et al found isopropanol in acetonemic cows [11]. Albeit isopropanol is regularly examined in patients suffering from severe metabolic acidosis with elevated anion gap at our intensive care unit, it has never been detected in our patients.
Usually, ingested isopropanol is rapidly metabolized to acetone via alcohol dehydrogenase (ADH) as well as renally excreted. Acetone is then excreted by kidneys and lungs (Fig. 1a). In this case description, decreased caloric intake led to ketoacidosis [1, 2]. In ketoacidotic patients with DMD, glucose utilization seems highly disordered as intravenous glucose administration is the sufficient therapy. Instead of glucose metabolism energy supply is provided by extensive fatty acid oxygenation. Thereby, ketone bodies as acetoacetate, acetone, and 3-hydroxybutyrate are produced, and reduced nicotinamide adenine dinucleotide (NADH) is generated. Subsequently, severe acidosis, hyperketonemia, and high NADH levels reverse the ADH redox reaction towards the de novo production of isopropyl alcohol by reduction of acetone. In addition, AKI contributes to metabolic acidosis and leads to a decreased renal clearance of isopropyl alcohol and acetone, respectively. Furthermore, diaphragm dysfunction of the exhausted DMD patient impedes effective pulmonal clearance of isopropyl alcohol and acetone. Thus, isopropanol up to toxic blood concentrations can be found in fasting DMD patients (Fig. 1b). Petersen et al [12] detected isopropanol in 260 postmortem analyses with diabetic or alcoholic ketoacidosis or with isopropanol intoxication. Compared with their data, our finding of 114 mg/dL isopropanol is exceptionally elevated compared to non-DMD patients.
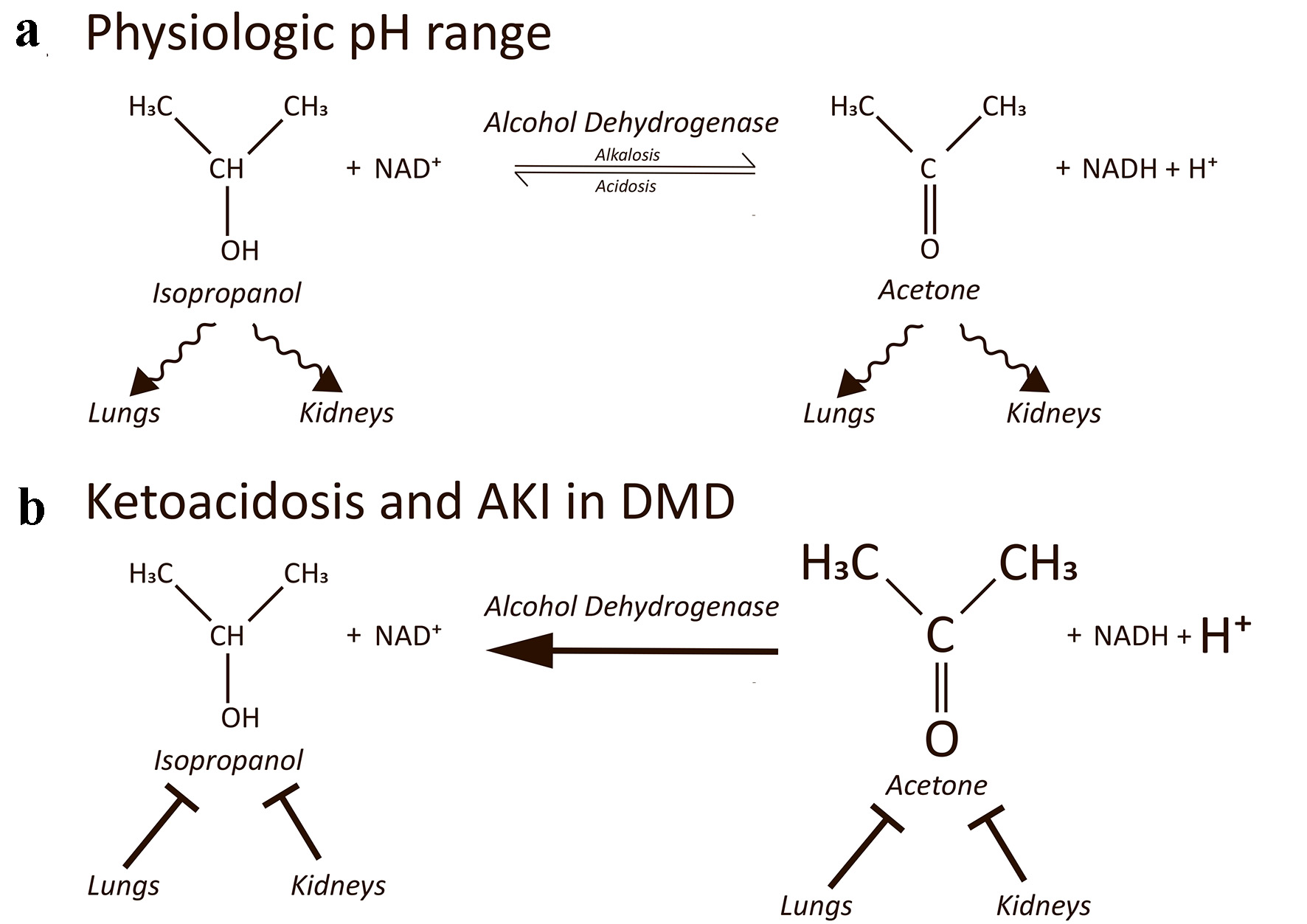 Click for large image | Figure 1. (a) The equilibrium of a redox reaction between isopropyl alcohol and acetone in dependency of NADH and NAD+ is shown. Isopropyl alcohol and acetone are both renally excreted and exhaled by the lungs. Adapted from Jones and Summers [6]. (b) In severe ketoacidosis concentrations of acetone and protons are highly elevated (indicated by increased size). In addition, NADH levels are high as well. The redox reaction predominantly takes place in a reversed manner leading to endogenous de novo reduction of acetone to isopropyl alcohol up to toxic concentrations. Acute kidney injury reduces renal clearance. Diaphragm dysfunction in DMD patients reduces pulmonal clearance. NADH: reduced nicotinamide adenine dinucleotide; DMD: Duchenne muscular dystrophy; |
Furthermore, in patients with type 1 diabetes elevated NADH/NAD+ ratios exist favoring the transition from acetone to isopropyl alcohol. In the presence of ADH, acetone was shown to convert to isopropyl alcohol when NADH levels are high [10]. In addition to diabetic ketoacidosis, only chronic alcoholism is so far known to show high NADH concentrations as an educt of ethanol metabolism [13]. Although mitochondrial dysfunction is well documented in DMD, elevated NADH levels have not been reported to date in DMD, whereat histological analyses with NADH stains show non-specific myofibrillar alterations. Besides diabetes mellitus and chronic alcoholism, we propose patients suffering from DMD to be the third group prone to endogenously produced isopropanol whilst in severe ketoacidosis. It would be interesting to investigate whether altered NADH/NAD+ ratios in addition to high proton concentration and ketonemia drive the ADH in DMD patients to generate isopropanol up to toxic blood levels. In addition, detection of beta hydroxybutyrate may help to decipher endogenous de novo synthesis from intoxication [14].
Learning points
Patients with DMD admitted to hospitals may present severe metabolic acidosis. In addition to regular differential diagnoses metabolic acidosis following hypoalimentation should be taken into consideration. Causal therapy using glucose infusion is rather facile and may be rapidly started in emergency rooms in order to prevent deterioration of metabolic acidosis with production of isopropyl alcohol and consecutive neurologic symptoms following endogenous intoxication. In addition, AKI may be masked by overestimated glomerular filtration rates due to the commonly reduced muscular mass which, in turn, impedes renal elimination of isopropyl alcohol.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient regarding this report.
Author Contributions
KB, SG, CW, and LH discussed the case and treated the patient. KB wrote the manuscript. SG, MS, and LH co-wrote and thoroughly discussed the manuscript.
Data Availability
The data supporting these findings are available from the corresponding author.
| References | ▴Top |
- Doris TE, Bowron A, Armstrong A, Messer B. Ketoacidosis in Duchenne muscular dystrophy: A report on 4 cases. Neuromuscul Disord. 2018;28(8):665-670.
doi pubmed - Lo Cascio CM, Latshang TD, Kohler M, Fehr T, Bloch KE. Severe metabolic acidosis in adult patients with Duchenne muscular dystrophy. Respiration. 2014;87(6):499-503.
doi pubmed - Svart MV, Voss TS, Bayat M, Madsen LR, Andersen LT, Poulsen PL, Moller N. Rare presentations of ketoacidosis: diabetic ketoalkalosis and ketoacidosis secondary to fasting and muscular dystrophy. Clin Diabetes. 2015;33(1):37-39.
doi pubmed - Lacouture PG, Wason S, Abrams A, Lovejoy FH, Jr. Acute isopropyl alcohol intoxication. Diagnosis and management. Am J Med. 1983;75(4):680-686.
doi - Ashurst JV, Nappe TM. Isopropanol Toxicity. In: StatPearls. Treasure Island (FL), 2022.
- Jones AE, Summers RL. Detection of isopropyl alcohol in a patient with diabetic ketoacidosis. J Emerg Med. 2000;19(2):165-168.
doi - Bailey DN. Detection of isopropanol in acetonemic patients not exposed to isopropanol. J Toxicol Clin Toxicol. 1990;28(4):459-466.
doi pubmed - Jones AW, Andersson L. Biotransformation of acetone to isopropanol observed in a motorist involved in a sobriety check. J Forensic Sci. 1995;40(4):686-687.
doi - Lewis GD, Laufman AK, McAnalley BH, Garriott JC. Metabolism of acetone to isopropyl alcohol in rats and humans. J Forensic Sci. 1984;29(2):541-549.
doi pubmed - Davis PL, Dal Cortivo LA, Maturo J. Endogenous isopropanol: forensic and biochemical implications. J Anal Toxicol. 1984;8(5):209-212.
doi pubmed - Robertson A, Thin C, Stirling AM. Isopropyl alcohol in cows suffering from acetonaemia. Nature. 1950;166(4231):954.
doi pubmed - Petersen TH, Williams T, Nuwayhid N, Harruff R. Postmortem detection of isopropanol in ketoacidosis. J Forensic Sci. 2012;57(3):674-678.
doi pubmed - Ritchie JM. The aliphatic alcohols. In: Goodman AG, Gilman LS, Rall TW, Murad F. The pharmacological basis of therapeutics. MacMillan Company. 1985;377.
- Eriksson Hydara Y, Zilg B. Postmortem diagnosis of ketoacidosis: Levels of beta-hydroxybutyrate, acetone and isopropanol in different causes of death. Forensic Sci Int. 2020;314:110418.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
