| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website https://www.jofem.org |
Original Article
Volume 11, Number 5, October 2021, pages 123-133
The Kidney Beans (Phaseolus vulgaris) Pods Extract Affects the Central and Peripheral Serotonergic Systems in Rats With High-Calorie Diet-Induced Obesity
Alona Yurchenkoa, c, Daryna Krenytskaa, Olesya Kalmukovaa, Nataliia Rakshaa, Tetiana Halenovaa, Tetiana Vovka, Olexii Savchuka, Mycola Dzerzhynskya, Ludmyla Ostapchenkoa, Victor Tomchukb
aTaras Shevchenko National University of Kyiv, ESC “Institute of Biology and Medicine”, 64 Volodymyrska Str., Kyiv 01601, Ukraine
bNational University of Life and Environmental Sciences of Ukraine, Kyiv, Ukraine
cCorresponding Author: Alona Yurchenko, Educational and Scientific Center “Institute of Biology and Medicine”, Taras Shevchenko National University of Kyiv, Volodymyrska 64, Kyiv 01601, Ukraine
Manuscript submitted September 10, 2021, accepted September 16, 2021, published online October 31, 2021
Short title: P. vulgaris Affects the Serotonin in Obese Rats
doi: https://doi.org/10.14740/jem650
| Abstract | ▴Top |
Background: Overweight and obesity have become a global epidemic that presents a risk to health. Serotonin is involved in the regulation of many important functions of the body as in the periphery (as a hormone), as well as in the central nervous system as a neurotransmitter, which can be involved in central mechanisms that control food intake. The present work aimed to study the features of serotonin metabolism functioning under the effect of the kidney beans (Phaseolus vulgaris) pods extract in experimental obesity, which was induced by the consumption of high-calorie diet. Phaseolus vulgaris is the plant that possesses plenty of curative and therapeutic properties in particular for the treatment of diabetes mellitus, obesity, and cardiovascular diseases. It has been known that plant extracts, unlike synthetic drugs, can be used practically without toxic effects.
Methods: Experiments were carried out on four groups of white nonlinear male rats (10 rats per group) during a 10-week period. Studies of the serotonergic system functioning were performed using ion-exchange chromatography, spectrofluorimetric and spectrophotometric methods.
Results: The consumption of kidney beans (Phaseolus vulgaris) pods extract has a therapeutic effect on the obesity development in rats, as evidenced by changes in the functioning of the central and peripheral serotonergic systems. Tryptophan and serotonin levels have increased in duodenum mucosa compared to the obese rats, also had shown an increase of the serotonin synthesis enzymes activity (tryptophan hydroxylase and tryptophan decarboxylase) and a simultaneous decrease in the activity of monoamine oxidase. Central tryptophan and serotonin levels, as well as the activity of enzymes of the serotonin synthesis have increased compared to the obese rats while a simultaneous decrease in the activity of monoamine oxidase was observed.
Conclusions: The obtained results indicated a therapeutic effect of the kidney beans (Phaseolus vulgaris) pods extract on the dysfunction of the serotonergic system under obesity development.
Keywords: Obesity; High-calorie diet; Serotonergic system; Kidney beans Phaseolus vulgaris pods extract
| Introduction | ▴Top |
It is generally recognized that overweight and obesity have reached epidemic proportions. The proof lies in the fact that there are over 1 billion overweight adults throughout the world according to many sources including the World Health Organization (WHO) [1]. The main reason for the development of obesity is the energy imbalance between the excess energy input into the body from food and its consumption. Energy balance maintaining requires regulation of many behavioral and physiological processes. These processes are under the control of a number of nerve systems, of which serotonin plays a significant role in the functioning. Serotonin (5-hydroxytryptamine (5-HT)) is a biologically active monoamine. This derivative of an essential amino acid tryptophan is involved in the regulation of a number of important functions of the body as in the periphery, as well as in the central nervous system. In the periphery 5-HT is synthesized by enterochromaffin (EC) cells of the gastrointestinal tract and functions as a hormone. In the central nervous system 5-HT is synthesized by serotonergic neurons of the brain stem and serves as a neurotransmitter associated with mood, behavior, sleep cycles, and appetite [2]. It is known that serotonin cannot pass through the blood-brain barrier so its peripheral and neuronal systems are separated from each other and work separately [3].
Synthesis of 5-HT takes place in two stages, under the influence of two separate enzymatic systems [4]. The first stage of the synthesis is the transformation of tryptophan into the immediate precursor of serotonin (5-hydroxytryptophan) by hydroxylation in the fifth position of the inertial ring. This process takes place with the fast-limiting enzyme tryptophan hydroxylase (TrG) in the presence of a molecular oxygen, Fe2+, and cofactor tetrahydrobiopterin (BH4) [5]. The second stage of the serotonin synthesis is the decarboxylation reaction by the decarboxylase enzyme of aromatic L-amino acids. Tryptophan decarboxylase (TrD) is present in many neurons of the brain, and in addition to the synthesis of serotonin, takes part in the synthesis of adrenaline, noradrenaline and dopamine [6]. Serotonin is synthesized not only in the body of the neuron but also in its axons. Most of the serotonin accumulated in the presynaptic vesicles by vesicular monoamine transporter (VМАТ2). This membrane protein has a high affinity for serotonin, but it protects 5-HT from intracellular catabolism [7]. The serotonergic system is an important link in the regulation of energy balance, and impaired of its functioning may be one of the main factors in the obesity development and concomitant insulin resistance.
Strategies to lose body fat typically involve a combination of dietary changes limiting caloric intake, physical activity, behavioral therapy, pharmacotherapy, and in extreme cases, surgery [8]. Since many prefer a natural approach using dietary means to reverse fat accumulation, the availability and popularity of new dietary regimens and natural dietary supplements have risen dramatically in recent years [9]. Phaseolus vulgaris is the plant that possesses plenty of curative and therapeutic properties that are extensively used in traditional medicine for the treatment of diabetes mellitus, obesity and cardiovascular diseases [10-12]. It has been reported having anti-obesity effects in many studies. For example, the consumption of common beans on mice decreased body weight through a reduction of plasma leptin concentrations [12]. Also, it has been reported that treatments with a standardized Phaseolus vulgaris dry extracts resulted in dose-dependent decreases in daily food intake and body weight [13]. However, in order to understand more about the therapeutic values of this plant in the prevention and treatment of obesity, further investigations are necessary. The purpose of the given study was to study the effect of the aqueous extract from Phaseolus vulgaris pods on the functioning of the central and peripheral serotonergic system in rats under obesity development.
| Materials and Methods | ▴Top |
In vivo experimental procedures
Ethics approval was obtained from the ethics commission of the Taras Shevchenko National University of Kyiv. Our research was conducted in compliance with the standards of the Convention of Bioethics of the Council of Europe in 1997, European Convention for the protection of vertebrate animals that used for experimental and other scientific purposes, the general ethical principles of animal experiments approved by the First National Congress of Bioethics of Ukraine and other international agreements and national legislation in this field. White nonlinear male rats with initial weighing of 195 - 205 g were used in 10-week experiment.
The experiment was carried out at the temperature of 22 ± 3 °C, humidity 60±5%, and light (12 h light/12 h dark) cycle. During the first week of the experiment, all of the animals were fed with standard food and water ad libitum. After that, the animals were randomly divided into four groups (10 rats per group).
The rats of group 1 (control) were fed with standard food during all 10 weeks. The second group (control + Ex) were receiving the standard food and the aqueous extract of Phaseolus vulgaris. The rats of group 3 (HCD) were on a high-calorie diet (38.8% fat, 15.5% protein and 45.7% of carbohydrate, 28.71 kJ/g), which consisted of a standard meal (60%), pork fat (10%), eggs (10%), sugar (9%), peanuts (5%), dry milk (5%), and sunflower oil (1%) [14], and drank water ad libitum for the experimental period. The rats of group 4 (HCD + Ex) were also on a high-calorie diet and water ad libitum, and after 4 weeks of the experiment, they started to receive the aqueous extract of Phaseolus vulgaris (200 mg/kg). One day all the rats received the water extract (with free access) and another day they drank water ad libitum. Food and water consumption were measured daily at the same time (9:00 am to 10:00 am). Body weights were determined once a week. At the end of the experiment (10 weeks) animals were fasted overnight and sacrificed.
Samples preparation
The blood samples were incubated at 37 °C at least 30 min. After that rats’ blood serum was prepared by centrifugation at 1,000 g for 30 min and stored at -20 °C until it was used.
Brain were removed and weighed. Then they were homogenized in 0.4 M perchloric acid at the rate of 5 mL buffer per half of the brain. Samples were centrifuged at 0 °C for 5 min at 800 × g after 60 min of incubation (4 °C). The supernatant was collected and the pH was adjusted to 5 - 6 using 2 M KOH. Then the samples were centrifuged for 5 min at 800 × g at a temperature of 0 °C. The resulting supernatant was used in tryptophan and serotonin measurement.
The duodenum was removed from the body of rats by the dissection of the peritoneum and washed in a petri dish in 0.9% sodium chloride solution. The duodenum mucosa samples were prepared by mechanical separation by a scalpel and homogenization using a glass homogenizer in 10 mM Tris-HCl buffer, pH 7.4, which was labeled with 1 mM ethylenediamine tetraacetic acid (EDTA) and 0.25 M sucrose in a ratio of 1:10 (tissue: buffer). After that the samples were centrifuged for 10 min at 1,500 g. The supernatants were frozen at -20 °C until further use [15].
Preparation of the aqueous kidney beans (Phaseolus vulgaris) pods extract
The aqueous kidney beans (Phaseolus vulgaris) pods extract were prepared according to the next method [16]. In our experiment we have used freshly prepared aqueous solutions of dry kidney beans (Phaseolus vulgaris) pods extract in the concentration of 200 mg/kg.
Biochemical analysis
Serotonin and tryptophan content in the rats brain, duodenum mucosa and blood serum
The tryptophan and serotonin content was determined using ion-exchange chromatography and fluorescence methods [17].
The resulting supernatant was applied to the column of CM-Sepharose (pre-equilibrated 0.01 M Na-phosphate buffer, pH 6.2). Elution was carried with 0.03 M sodium phosphate buffer, pH 6.2. After that, 0.3 mL of 11.6 M HCl was added to 1 mL fraction of serotonin. The serotonin measurements were performed on the Shimadzu spectrophotofluorometer at an excitation wavelength of 295 nm and emission wavelength of 550 nm against a blank sample that contained distilled water instead of the test sample.
Then elution was carried out with 0.01 M sodium phosphate buffer, pH 6.2. To 0.5 mL fraction of tryptophan was added 0.5 mL of a dithiothreitol mixture: ethanol (1 mg dithiothreitol/1 mL of ethanol) and 0.5 mL of ninhydrin reagent (ninhydrin dissolved in a solution of 17.4 M acetic acid and 3 M phosphoric acid in the ratio 3:2). Samples were placed in a boiling water bath for 25 min and then cooled under running water. The tryptophan measurements were performed on the spectrophotofluorometer at an excitation wavelength of 359 nm and emission wavelength of 485 nm against the blank sample that contained distilled water instead of the test sample.
Tryptophan hydroxylase activity in the rats brain and duodenum mucosa
The homogenates were thawed at room temperature and centrifugated at 12,000g for 30 min. To the 0.02 mL of sample was added reaction mixture contained the 500 mM Tris-acetate pH 7.4; 4 mM L-tryptophan; 20 mM dithiothreitol; 1 mM CaCl2; 0.05 mg of catalase. After 15 min of incubation at 37 °C, the reaction was stopped by the addition of 0.01 mL of 6 M perchloric acid. The mixture was centrifuged at 3,000 g for 5 min (4 - 8 °C). To the 0.16 mL of supernatant fraction was added 0.4 mL of 8 M hydrochloric acid. The amount of product, 5-hydroxytryptophan, was measured fluorometrically with a Shimadzu spectrophotofluorometer (excitation 295 nm, emission 540 nm) against the blank sample that contained distilled water instead of the test sample [14].
5-hydroxytryptophan content in the rats brain and duodenum mucosa
The 5-hydroxytryptophan content in the brain was determined using the fluorescence method described previously [14]. The resulting supernatant was added to the 1 N perchloric acid (1:5) and stirred vigorously for 5 min. Samples were centrifuged at 2,000 g for 30 min (4 °C). The resulting supernatant was collected and adjusted to pH 9.5 - 10.5 with the 5 M NaOH solution. Then 2.5 mL of n-heptane saturated with NaCl was added, and intensively stirred for 15 min. For phase separation a sample was centrifuged for 3 min at 500 g (4 °C). The aqueous phase (1 mL) was collected, and 0.3 mL of 11.6 M HCl was added. Measurements were performed on a Shimadzu spectrophotofluorometer (excitation 295 nm, emission 545 nm) against the blank sample that contained distilled water instead of the test sample.
Tryptophan-decarboxylase activity in the rats brain and duodenum mucosa
Determination of tryptophan-decarboxylase activity was carried out by the spectrofluorimetric method described previously [14]. The resulting supernatant was added to the 0.4 M perchloric acid (1:5) and kept for 60 min at 4 °C. Then all the samples were centrifuged for 5 min at 800 g (4 °C). The pH of the supernatant was adjusted to 5 - 6 with 2 M KOH and centrifuged for 5 min at 800 g (4 °C). The supernatant (0.1 mL) was collected and adjusted up to 1 mL by 100 mM Tris-HCl buffer, pH 8.0, containing 5 mM β-mercaptoethanol, 10% glycerol. After that 2 mL of 4 mM NaOH and 5 mL of ethyl acetate were added and samples were mixed on a shaker for 60 s. For phase separation samples were centrifuged for 5 min at 250 g. The determination of tryptophan-decarboxylase activity was performed in the organic phase. Measurements were performed on a Shimadzu spectrophotofluorometer (excitation 280 nm, emission 340 nm).
Monoamine oxidase activity in the rats brain and duodenum mucosa
The resulting supernatant was added to the 0.5 M phosphate buffer pH 7.4 (1:5) and centrifugated at 800 g during 20 min (5 °C). The incubation mixture contained the kynuramine dihydrobromide (100 µg), 0.5 M phosphate buffer pH 7.4 (0.5 mL), homogenate (equivalent to 3.13 mg brain), and de-ionized water to a final volume of 3 mL. The reaction mixtures were incubated at 37 °C for 30 min with shaking. After that to the samples was added 0.6 M perchloric acid followed by centrifugation at 900 g for 10 min (5 °C). The supernatant (1 mL) was mixed with 2 mL of 1 N NaOH and placed in a quartz cuvette. Measurements were performed on a Shimadzu spectrophotofluorometer (excitation 315 nm, emission 380 nm) [14].
Monoamine oxidase activity in the rats blood serum
The incubation mixture contained 2 mL of the distillate water, 0.5 mL of 0.2 M phosphate buffer pH 7.4 and 0.1 mL of 1% bensolamine hydrohloride. The reaction mixtures were incubated at 37 °C for 180 min. After 5 - 7 min 22.5% trichloroacetic acid was added to the samples with the followed centrifugation at 800 g for 5 min. The supernatant was mixed with 0.5 mL of 1% dinitrophenylhydrazine and left at room temperature in the dark. Then the samples were centrifugated at 800 g for 25 min. To the siege it was added 1 - 2 drops of the 2 N NaOH and visualized the brown color. After 2 min 1 mL of the distillate water and 1 mL of the concentrated ethyl alcohol were added. Measurements were performed on a Shimadzu spectrophotometer at 460 nm against the blank sample that contained distilled water [14].
Histochemical analysis for serotonin detection
Formaldehyde-induced fluorescence was used for the histochemical detection of the serotoninergic neurons (Falck-Hillarp technique) [18]. This method is based on the production of fluorophores upon condensation of the biogenic amines with formaldehyde. The technique was partly modified [19]. Condensation was carried out in a fixative containing 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer (FAGLU) [20]. For clearer differentiation of serotonin from other biogenic amines, the fixative contained 0.4% potassium hexacyanoferrate (III), pH 10.0 [21]. After fixation, tissue blocks were dehydrated and embedded in paraffin according to a generally accepted technique with preservation of fluorescence [22]. Slices (5-µm thick, included the hypothalamic nuclei) were prepared from such blocks. The arcuate hypothalamic nucleus borders were identified according to the stereotaxic atlas [23]. Deparaffinized slices were embedded in glycerol and analyzed using a fluorescent microscope, Olympus BX51 (Japan), provided with filters (excitation wavelength, 410 nm; absorption wavelength, 525 nm), a digital camera, Olympus 5050 (Japan), and Olympus DP-Soft 420-480.520 software. The number/density of serotonergic neurocytes and of EC cells (their number within a 75.000 µm2 test area), and number of serotonin-containing vesicles per one neurocyte were measured using ImageJ software (National Institutes of Health, USA). Histochemical evaluation criteria of serotonin-containing in EC cells were scoring as: little-1, mild-2 and marked-3 points.
Statistical analysis
The data of biochemical estimations were reported as mean ± standard error of the mean (SEM) for each group (n = 10). Statistical analyses were performed using analysis of variance (ANOVA). The difference between the parameters was considered statistically significant at P < 0.05.
| Results | ▴Top |
The organometric parameters of obese rats with or without consumption of kidney beans (Phaseolus vulgaris) pods extract
The consumption of a high-calorie diet can lead to the obesity development. In Table 1 the higher levels of body weight, body mass index (BMI) and index Lee in rats that were on a high-calorie diet compared to the intact rats are presented. The weight of adipose tissue (subcutaneous and visceral) was significantly higher in the HCD group than those in the control group. The consumption of kidney beans (Phaseolus vulgaris) pods extract (200 mg/kg) suppressed the increase of the body weight, BMI, and index Lee of the obese rats. So, these parameters were lower in HCD + Ex group of rats compared to the HCD group of rats. Table 1 shows significantly lower weight values of adipose tissues in rats that contemporaneously consumed the high-calorie diet and kidney beans (Phaseolus vulgaris) pods extract compared to the HCD group.
 Click to view | Table 1. The Organometric Parameters of Control and HCD-Induced Obese Rats With or Without Consumption of Kidney Beans (Phaseolus vulgaris) Pods Extract (Ex) |
The parameters of the central and peripheral serotonergic systems in obese rats with or without consumption of kidney beans (Phaseolus vulgaris) pods extract
The level of the tryptophan in duodenum mucosa of the obese rats has been increased (1.72 times) compared to the control group of rats (1.41 ± 0.29 µg/mg of protein, Fig. 1). In the group of rats that were fed with the high-calorie diet and kidney beans (Phaseolus vulgaris) pods extract this parameter was 1.63 times higher compared to the HCD group (0.82 ± 0.20 µg/mg of protein). The research of a key and rate-limiting enzyme of serotonin biosynthesis (tryptophan-hydroxylase activity) in the duodenum mucosa in obese rats has shown a decrease of enzyme activity by 29% in comparison with the values of the control group (Fig. 1). The tryptophan-hydroxylase activity in HCD + Ex group of rats has been increased by 19% compared to the animals that were on high-calorie diet. According to the data presented in Figure 1 the duodenum mucosa 5-hydroxytryptophan content was 1.5 times lower in the obese rats compared to the control group, and 1.5 times higher in HCD + Ex group compared to the animals that ate high-calorie diet. Studies have shown a decrease of the duodenum mucosa tryptophan-decarboxylase activity by 14% in HCD group compared to the control rats and increase of this parameter by 4% in HCD + Ex group compared to the rats that were on high-calorie diet (Fig. 1). The analysis of the duodenum mucosa serotonin level has shown a 2.55-time decrease of this indicator in HCD group compared to the control group (0.97 ± 0.28 µg/mg of protein). The duodenum mucosa serotonin level in HCD + Ex group was 2.51 times higher compared to the obese rats (0.38 ± 0.12 µg/mg of protein, Fig. 1). The duodenum mucosa monoamine oxidase (MAO) activity was higher in rats that were on high-calorie diet by 126% compared to the intact animals. And this indicator was lower in rats that were fed with high-calorie diet and kidney beans (Phaseolus vulgaris) pods extract by 118% compared to the HCD group (Fig. 1).
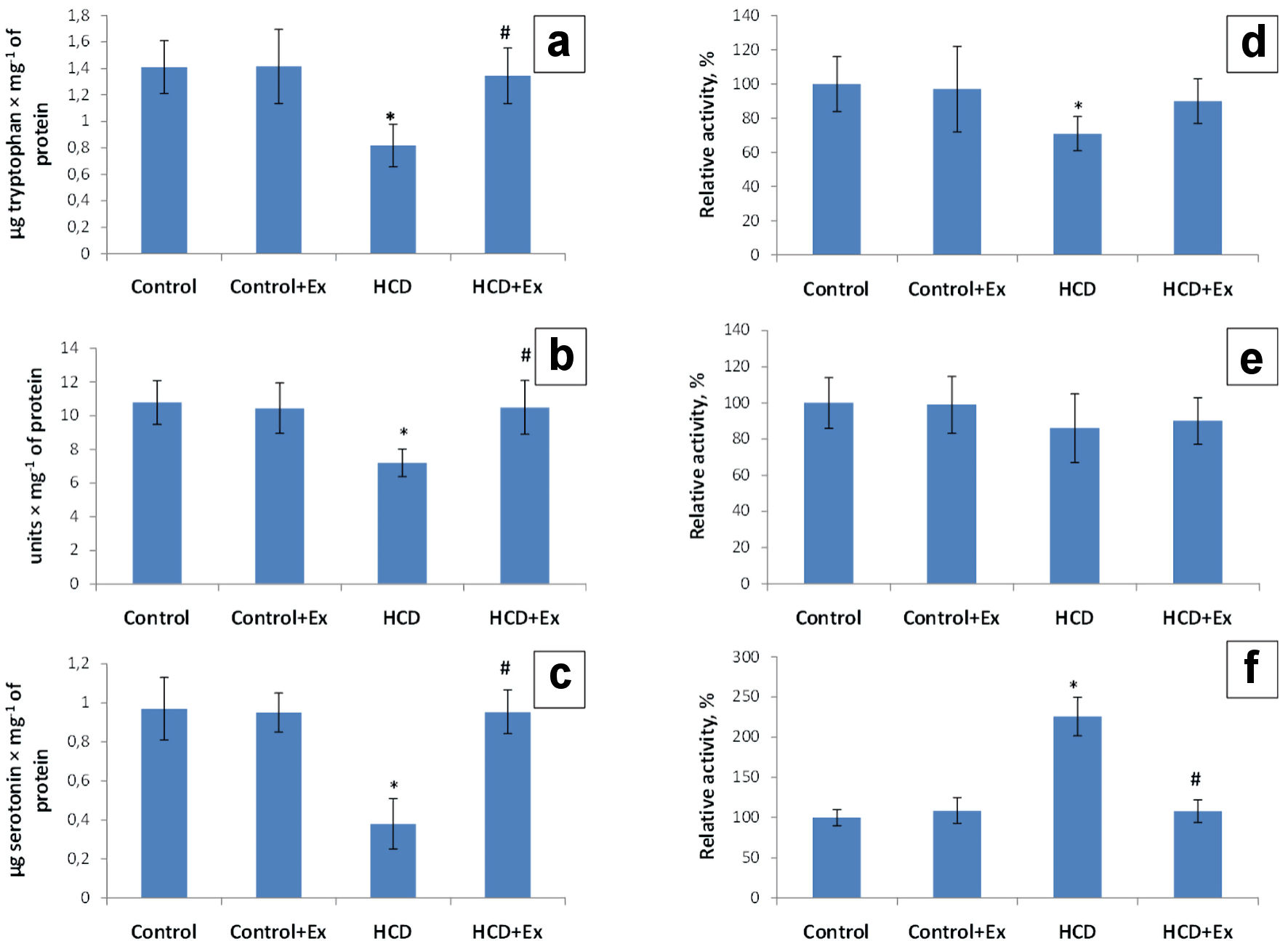 Click for large image | Figure 1. The content of tryptophan (µg/mg of protein) (a), 5-hydroxytryptophan (units/mg of proteins) (b) and serotonin (µg/mg of protein) (c), tryptophan hydroxylase (TPH) activity (d), tryptophan decarboxylase (TPD) activity (e) and monoamine oxidase (MAO) activity (f) in duodenum mucosa in control and HCD-induced obese rats with or without consumption of kidney beans (Phaseolus vulgaris) pods extract (mean ± SEM, n = 10 in each group). *P < 0.05, significant differences compared to the control; #P < 0.05, significant differences compared to the HCD-induced obesity. SEM: standard error of the mean; HCD: high-calorie diet. |
Also, our results showed that the blood serum tryptophan level has been increased (1.3 times) in the obese rats, and 1.6 times in rats that were on the high-calorie diet and consumed the kidney beans (Phaseolus vulgaris) pods extract compared to the control animals (31.9 ± 5.0µg/mL, Fig. 2). The blood serum serotonin level was 1.83 times higher in HCD group compared to the control group (8.99 ± 2.50 µg/mL). This parameter in HCD + Ex group was 1.71 times lower compared to the rats that were on high-calorie diet (16.46 ± 2.92 µg/mL, Fig. 2). According to the data presented in Figure 2 the blood serum MAO activity was lower by 47% in the obese rats compared to the control rats and higher by 37% in HCD + Ex group compared to the obese rats.
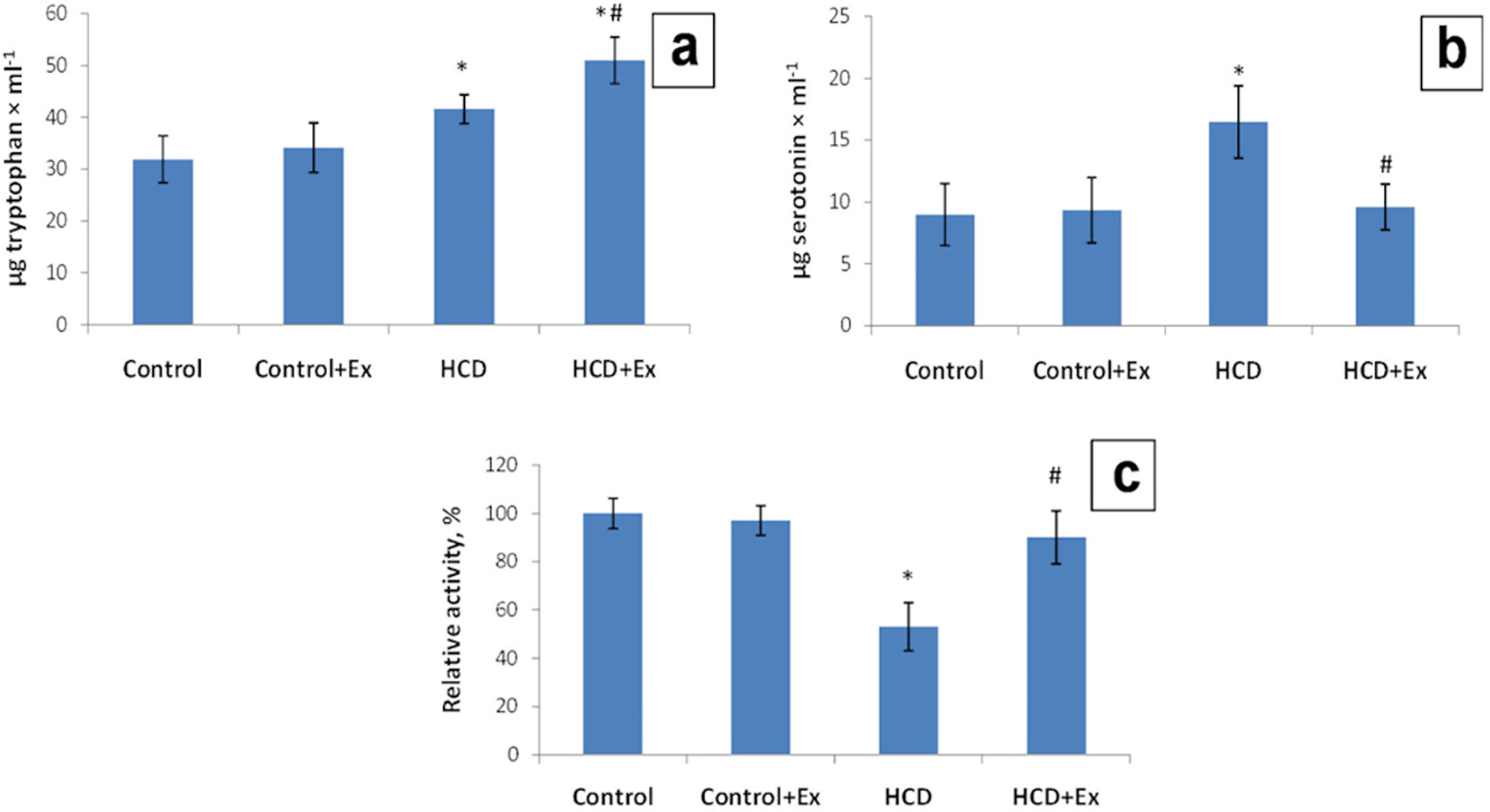 Click for large image | Figure 2. The content of tryptophan (µg/mL) (a), serotonin (µg/mL) (b), and monoamine oxidase (MAO) activity (c) in blood serum in control and HCD-induced obese rats with or without consumption of kidney beans (Phaseolus vulgaris) pods extract (mean ± SEM, n = 10 in each group). *P < 0.05, significant differences compared to the control; #P < 0.05, significant differences compared to the HCD-induced obesity. SEM: standard error of the mean; HCD: high-calorie diet. |
The research of the rats brain tryptophan level of the obese rats and rats that consumed the high-calorie diet and kidney beans (Phaseolus vulgaris) pods extract has shown a decrease of 2.33 and 2.64 times compared to the control group (11.19 ± 3.10 µg/g tissue, Fig. 3). The tryptophan-hydroxylase activity in the brain of rats with the experimental model of obesity has shown a decrease of the enzyme activity by 31% compared with the values of the control group. This parameter in the group of rats that consumed the kidney beans (Phaseolus vulgaris) pods extract in addition to the high-calorie diet was higher by 4% compared to the HCD group of animals (Fig. 3). The level of the rats brain 5-hydroxytryptophan was 1.72 times lower compared to the control rats (57.5 ± 13.1 units/mg of protein) and 1.5 times higher compared to the HCD group (33.5 ± 6.0 units/mg of protein, Fig. 3). Studies also have shown a decrease of the rats brain tryptophan decarboxylase activity by 52% compared to the control rats and an increase of this indicator by 40% compared to the HCD group. According to the data presented in Figure 3 the rats brain serotonin level was 1.42 times lower compared to the control group (0.98 ± 0.20 µg/g tissue). The level of the rats brain serotonin in HCD + Ex group was 1.4 times higher compared to the HCD group (0.69 ± 0.11 µg/g of tissue, Fig. 3). The rats brain MAO activity was higher by 115% in HCD group compared to the control rats, and lower by 92% in HCD + Ex group compared to the HCD group of animals.
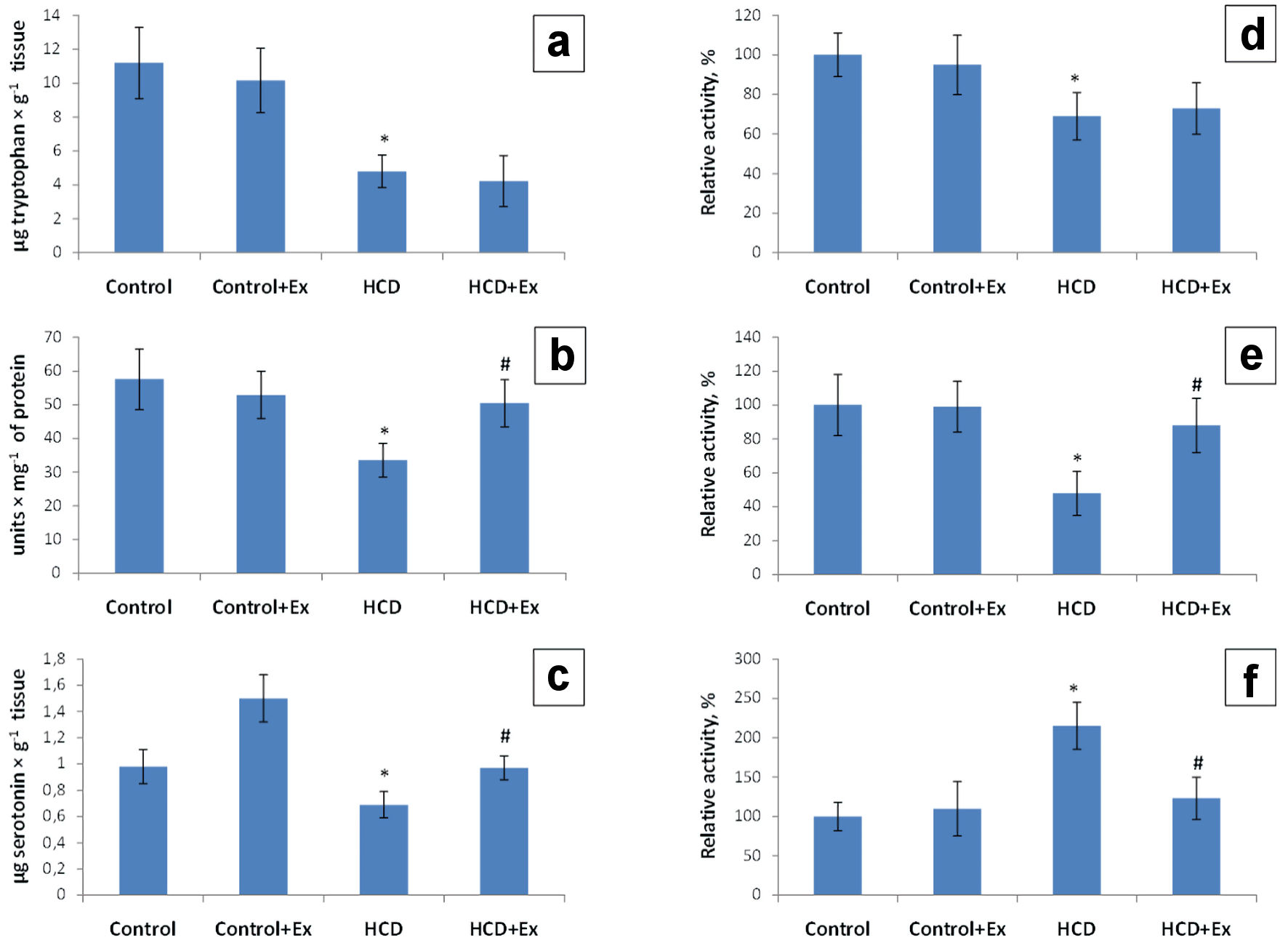 Click for large image | Figure 3. The content of tryptophan (µg/g tissue) (a), 5-hydroxytryptophan (units/mg of proteins) (b) and serotonin (µg/g tissue) (c); tryptophan hydroxylase (TPH) activity (d), tryptophan decarboxylase (TPD) activity (e) and monoamine oxidase (MAO) activity (f) in brain tissue in control and HCD-induced obese rats with or without consumption of kidney beans (Phaseolus vulgaris) pods extract (mean ± SEM, n = 10 in each group). *P < 0.05, significant differences compared to the control; #P < 0.05, significant differences compared to the HCD-induced obesity. SEM: standard error of the mean; HCD: high-calorie diet. |
Histochemical analysis and morphometric parameters of duodenum mucosa and brain of the obese rats with or without consumption of the kidney beans (Phaseolus vulgaris) pods extract
According to the data presented in Figure 4, the amount of the EC cells in the duodenum of HCD + Ex group was significantly higher compared to the rats that were on high-calorie diet; while the same parameter of HCD group was lower compared to the control group (by 48%). The serotonin content of duodenum EC cells was increased somewhat in rats of HCD + Ex group compared to the obese rats.
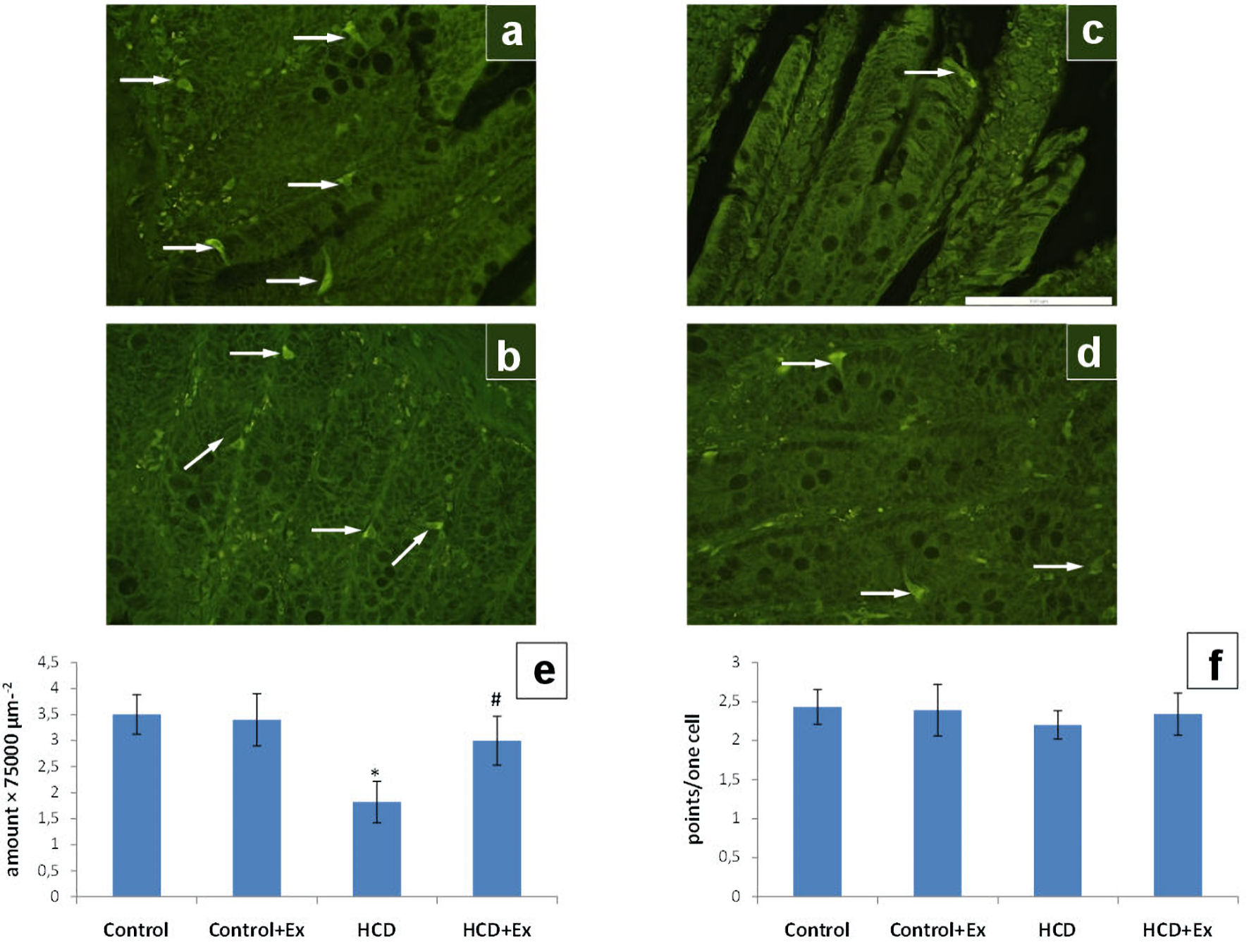 Click for large image | Figure 4. The photomicrographs of the duodenum slices of control (a), control + Ex (b), HCD (c) and HCD + Ex (d) groups (formaldehyde-induced fluorescence, Falck-Hillarp technique, fixation in FAGLU). Enterochromaffin cells are indicated by arrows (scale bar: 100 µm). Results of morphometric analysis of the state of the enterochromaffin cells amount in duodenum (e) and the enterochromaffin cells serotonin content in duodenum (f) (mean ± SEM, n = 10 in each group). *P < 0.05, significant differences compared to the control; #P < 0.05, significant differences compared to the HCD-induced obesity. SEM: standard error of the mean; HCD: high-calorie diet. |
The consumption of the kidney beans (Phaseolus vulgaris) pods extract by obese rats change the number of serotonin-positive neurons in the arcuate nucleus and the number of serotonin vesicles in single neurons (Fig. 5). In HCD group of animals, there was significantly smaller number of serotonergic neurons and number of serotonin-positive vesicles per single neuron, as compared to the control (by 40% and 44%, respectively).
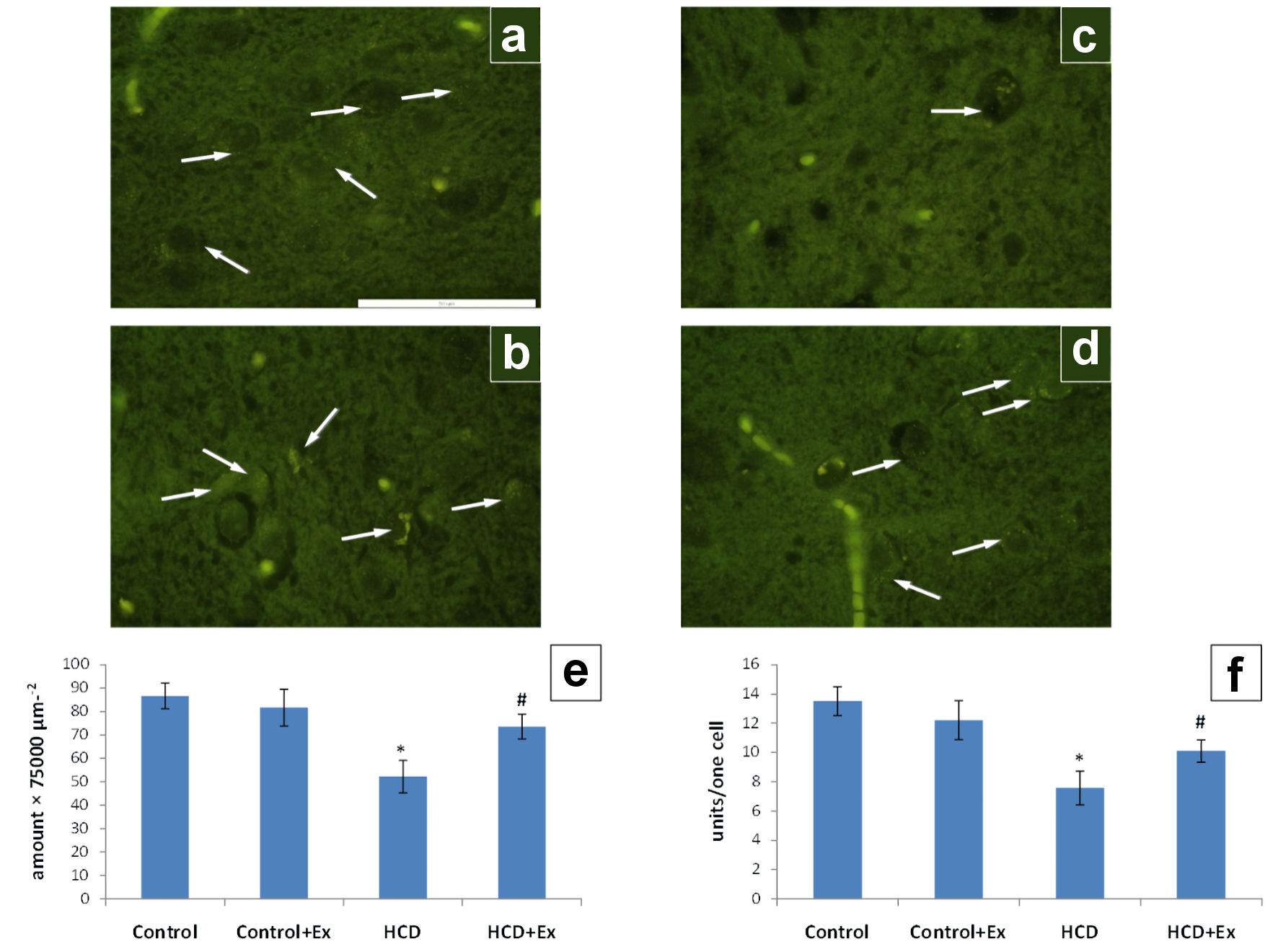 Click for large image | Figure 5. The photomicrographs of the hypothalamic arcuate nucleus slices of control (a), control + Ex (b), HCD (c) and HCD + Ex (d) groups (formaldehyde-induced fluorescence, Falck-Hillarp technique, fixation in FAGLU). Serotonin-positive neurons are indicated by arrows (scale bar: 50 µm). Results of morphometric analysis of the state of the serotonergic neurons in the hypothalamic arcuate nucleus: density of serotonin-positive neurons (e); the number of serotonin-positive vesicles per one neuron of the nucleus (f) (mean ± SEM, n = 10 in each group). *P < 0.05, significant differences compared to the control; #P < 0.05, significant differences compared to the HCD-induced obesity. SEM: standard error of the mean; HCD: high-calorie diet. |
| Discussion | ▴Top |
The results presented in the Table 1 confirm the weight loss properties of the kidney beans (Phaseolus vulgaris) pods extract [12, 13]. The anti-obesity mechanism of Phaseolus vulgaris relies on the inhibition of α-amylase activity. The extract of Phaseolus vulgaris inhibits activity of α-amylase, which could be related with an interfered digestion of complex carbohydrates to simple absorbable sugars. So, carbohydrate-derived calories can be potentially reduced [10, 24]. We found that at the result of the kidney beans (Phaseolus vulgaris) pods extract consumption the rats of HCD + Ex group have significantly lower body weight compared to the obese rats of the HCD group. It was established that the weight of the subcutaneous and visceral adipose tissues in rats that contemporaneously consumed the high-calorie diet and kidney beans was lower compared to the relevant indicators in rats that were on a high-calorie diet. Our results are consistent with the results of different recent studies in this field [25-27]. It has long been known that the serotonergic system plays an important role in the regulation of body energy balance. The nutritional behavior of the body depends on the generation of the signal by neuropeptides in the nuclei of the hypothalamus. In turn, these neuropeptides are closely related to the serotonergic system that controls of their secretion. Effect of serotonin on eating behavior occurs due to direct interaction with NPY/AgRP and POMC/CART neurons, as well as due to interaction with autoreceptors on the presynaptic membrane, which exert an inhibitory effect on the activity of the serotonergic neurons under serotonin exposed [28, 29]. In addition to the inhibitory effect on NPY/AgRP neurons, serotonin is also able to activate the second population of hypothalamus neurons that involved in the regulation of eating behavior (POMC/CART neurons). Serotonin and its agonists binding to the 5-HT2C serotonin receptor are able to activate POMC expression in the arcuate nucleus of the hypothalamus [30, 31].
We studied the metabolism of the serotonergic system under obesity development and simultaneously consumption of the high-calorie diet and kidney beans (Phaseolus vulgaris) pods extract in experimental rats. As the result of our research, the dysfunction of the peripheral and central serotonergic systems in rats with HCD-induced obesity was observed. But after consumption of kidney beans (Phaseolus vulgaris) pods extract all of the investigative parameters have been normalized or even returned to the control values. Based on the results of the previous studies [32-34] we can conclude that dysfunction of the central serotonergic system can play a significant role in the processes that lead to the pancreatic β-cells dysfunction. As a result of the serotonin transmission decrease, the hyperphagia which causes excess glucose and free fatty acids (FFA) arises. It in turn causes insulin hyper secretion and initiates the processes of β-cell depletion [35]. Serotonin is present in the same vesicle with insulin in pancreatic β-cells, and co-secreted when stimulated by glucose [36, 37]. High glucose-stimulated β-cell secretion of serotonin (co-released with insulin) activates α-cell HTR1F and inhibits glucagon secretion in a paracrine manner [38, 39]. The HCD-induced obesity leads to the dysfunction of β-cell’s work in decreasing insulin levels leading to hyperglycemia [40]. The reason that daily oral administration of the kidney beans (Phaseolus vulgaris) pods extract caused a hypoglycemic action might be the increased insulin sensitivity. The extract slowed glucose absorption via α-amylase inhibition and consequently reducing β -cell stimulation [41]. Obesity caused by an imbalance in the serotonin system can cause the development of insulin resistance through the production of FFA and directly through the interaction with the signaling pathways. According to the fact about serotonin and insulin interconnectedness the kidney beans (Phaseolus vulgaris) pods extract may have influence on the serotonergic system normalization through insulin level changes in obese rats. All of these results are confirmed by the histochemical analysis of the serotonergic system. They also showed the normalization of serotonin content in duodenum and brain tissue.
In conclusion, the obtained results indicate a therapeutic effect of the kidney beans (Phaseolus vulgaris) pods extract on the dysfunction of the serotonergic system under obesity development. So, it can influence the restoring of appetite control and the reducing of the body weight. Even though there are several treatments of obesity, such as surgery and drugs, there seems to be no efficient treatment without potential side effects. The greater understanding of the influence of the kidney beans (Phaseolus vulgaris) pods on the obesity development can lead to more effective pharmacotherapies without side effects.
Acknowledgments
None to declare.
Financial Disclosure
This manuscript does not receive any fund.
Conflict of Interest
The authors report no potential conflict of interest relevant to this article.
Informed Consent
Not applicable.
Author Contributions
Alona Yurchenko and Olexii Savchuk were involved in the selection of the study and wrote the manuscript. Olesya Kalmukova and Mycola Dzerzhynsky were involved in histochemical analysis carrying out and wrote the part of the manuscript. Daryna Krenytska, Nataliia Raksha, Tetiana Halenova were involved in the study presentation. Tetiana Vovk, Ludmyla Ostapchenko and Victor Tomchuk interpreted the figures. All authors were involved in the discussion, revision and approval of the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- WHO: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Zucker MB. A study of the substances in blood serum and platelets which stimulate smooth muscle. Am J Physiol. 1944;142:12-26.
doi - Lavrentyeva AV, Sapronova AY, Adamskaya EI, Melnikova VI, Proshlyakova EV, Babichev VN, Ugryumov MV. The brain is one of the most important sources of gonadotropin-releasing hormone in the general circulation of rats during prenatal ontogenesis. Neurochemistry. 2004;21:27-33.
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100(23):13525-13530.
doi pubmed - Eddahibi S, Adnot S. The serotonin pathway in pulmonary hypertension. Arch Mal Coeur Vaiss. 2006;99(6):621-625.
- Shih DF, Hsiao CD, Min MY, Lai WS, Yang CW, Lee WT, Lee SJ. Aromatic L-amino acid decarboxylase (AADC) is crucial for brain development and motor functions. PLoS One. 2013;8(8):e71741.
doi pubmed - Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447(5):636-640.
doi pubmed - Wyatt HR. Update on treatment strategies for obesity. J Clin Endocrinol Metab. 2013;98(4):1299-1306.
doi pubmed - Shao A, Drewnowski A, Willcox DC, Kramer L, Lausted C, Eggersdorfer M, Mathers J, et al. Optimal nutrition and the ever-changing dietary landscape: a conference report. Eur J Nutr. 2017;56(Suppl 1):1-21.
doi pubmed - Celleno L, Tolaini MV, D'Amore A, Perricone NV, Preuss HG. A Dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int J Med Sci. 2007;4(1):45-52.
doi pubmed - Hernandez-Saavedra D, Mendoza-Sanchez M, Hernandez-Montiel HL, Guzman-Maldonado HS, Loarca-Pina GF, Salgado LM, Reynoso-Camacho R. Cooked common beans (Phaseolus vulgaris) protect against beta-cell damage in streptozotocin-induced diabetic rats. Plant Foods Hum Nutr. 2013;68(2):207-212.
doi pubmed - Zhu Z, Jiang W, Thompson HJ. Edible dry bean consumption (Phaseolus vulgaris L.) modulates cardiovascular risk factors and diet-induced obesity in rats and mice. Br J Nutr. 2012;108(Suppl 1):S66-73.
doi pubmed - Carai MA, Fantini N, Loi B, Colombo G, Gessa GL, Riva A, Bombardelli E, et al. Multiple cycles of repeated treatments with a Phaseolus vulgaris dry extract reduce food intake and body weight in obese rats. Br J Nutr. 2011;106(5):762-768.
doi pubmed - Konopelnyuk VV, Karpovets TP, Kot LI, Ostapchenko LI. Biosynthesis of serotonin in the brain of rats under conditions of obesity induced by compatible consumption of high calorie diet and 10% fructose solution as a possible target for obesity prevention. Int J of Health Scien and Res. 2015;5:8.
- Osterloh K, Forth W. Determination of transferrin-like immunoreactivity in the mucosal homogenate of the duodenum, jejunum, and ileum of normal and iron deficient rats. Blut. 1981;43(4):227-235.
doi pubmed - Kyznetsova MY, Makieieva OM, Lavrovska DO, Tymoshenko MO, Sheverova DP, Halenova TI, Savchuk OM, et al. Effect of aqueous extract from phaseolus vulgaris pods on lipid peroxidation and antioxidant enzymes activity in the liver and kidney of diabetic rats. J of Appl Pharm Scien. 2015;5(05):001-006.
doi - Maximenko E, Savchenko V. The level of tryptophan and serotonin in terms of seizure activity in the brain. Journal of V. N. KarazinKharkiv National University Medicine. 2000;494(1):40-43.
- Falck B, Hillarp NA, Thieme G, et al. Fluorescence of catecholamines and related compounds condensed with formaldehyde. J Histochem Cytochem. 1962;10(3):348-354.
doi - Kalmukova OO, Yurchenko AV, Kyryk VM, Nepomnyaschy VM, Savchuk OM, Dzerzhynsky ME. Effects of melatonin administration in different time modes on morphofunctional indices of the hypothalamic serotonergic neurons in obese rats. Neurophysiology. 2018;50:6.
doi - Furness JB, Heath JW, Costa M. Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines and for ultrastructural studies of central nervous tissue. Histochemistry. 1978;57(4):285-295.
doi pubmed - Wreford NG, Singhaniyom W, Smith GC. Microspectrofluorometric characterization of the fluorescent derivatives of biogenic amines produced by aqueous aldehyde (Faglu) fixation. Histochem J. 1982;14(3):491-505.
doi pubmed - Loren I, Bjorklund A, Falck B, Lindvall O. The aluminum-formaldehyde (ALFA) histofluorescence method for improved visualization of catecholamines and indoleamines. I. A detailed account of the methodology for central nervous tissue using paraffin, cryostat or Vibratome sections. J Neurosci Methods. 1980;2(3):277-300.
doi - Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press. 1998.
- Shamki AW, Ali A, Abdulaziz RS. Purification and characterization of amylase inhibitor extracted from white kidney bean (Phaseolus vulgaris). J Cell Plant Sci. 2012;3:17-21.
- Nunez-Aragon PN, Segura-Campos M, Negrete-Leon E, Acevedo-Fernandez JJ, Betancur-Ancona D, Chel-Guerrero L, Castaneda-Corral G. Antihyperglycemic activity and toxicity of protein hydrolysates of three legumes. J Sci Food Agric. 2018.
- Valencia-Mejia E, Batista KA, Fernandez JJA, Fernandes KF. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res Int. 2019;121:238-246.
doi pubmed - Spadafranca A, Rinelli S, Riva A, Morazzoni P, Magni P, Bertoli S, Battezzati A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br J Nutr. 2013;109(10):1789-1795.
doi pubmed - Donovan MH, Tecott LH. Serotonin and the regulation of mammalian energy balance. Front Neurosci. 2013;7:36.
doi pubmed - Nonogaki K, Ohba Y, Sumii M, Oka Y. Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem Biophys Res Commun. 2008;372(1):186-190.
doi pubmed - Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6(5):398-405.
doi pubmed - Zhang Z, Li L, Yang M, Liu H, Boden G, Yang G. Increased plasma levels of nesfatin-1 in patients with newly diagnosed type 2 diabetes mellitus. Exp. and Clin. Endocrin & Diab. 2012;120(02):91-95
- Kuznetsova M, Halenova T, Dovgusha O, Savchuk O. Effect of an aqueous extract of Phaseolus vulgaris pods on blood glucose and body weight in diabetic rats. Bulletin of Taras Shevchenko National University of Kyiv. Series: Problems of Physiological Functions Regulation. 2015;1:12-14.
- Kyznetsova MY, Lavrovska DO, Zhyvolozhnyi AY, Dovgusha OV, Halenova TI, Savchuk OM, Ostapchenko LI. Effect of aqueous extract from phaseolus vulgaris pods on cytokine profile of streptozotocin-induced diabetic rats. Res J Pharm, Biol Chem Sci. 2015;6(1):1511-1520.
- Venkateswaran S, Pari L, Saravanan G. Effect of Phaseolus vulgaris on circulatory antioxidants and lipids in rats with streptozotocin-induced diabetes. J Med Food. 2002;5(2):97-103.
doi pubmed - Bray GA, Lovejoy JC, Smith SR, DeLany JP, Lefevre M, Hwang D, Ryan DH, et al. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr. 2002;132(9):2488-2491.
doi pubmed - Ekholm R, Ericson LE, Lundquist I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7(5):339-348.
doi pubmed - Gylfe E. Association between 5-hydroxytryptamine release and insulin secretion. J Endocrinol. 1978;78(2):239-248.
doi pubmed - Almaca J, Molina J, Menegaz D, Pronin AN, Tamayo A, Slepak V, Berggren PO, et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep. 2016;17(12):3281-3291.
doi pubmed - Ohta Y, Kosaka Y, Kishimoto N, Wang J, Smith SB, Honig G, Kim H, et al. Convergence of the insulin and serotonin programs in the pancreatic beta-cell. Diabetes. 2011;60(12):3208-3216.
doi pubmed - Mudi SR, Akhter M, Biswas SK, Muttalib MA, Choudhury S, Rokeya B, Ali L. Effect of aqueous extract of Aegle marmelos fruit and leaf on glycemic, insulinemic and lipidemic status of type 2 diabetic model rats. J Complement Integr Med. 2017;14(2):.
doi pubmed - Fantini N, Cabras C, Lobina C, Colombo G, Gessa GL, Riva A, Donzelli F, et al. Reducing effect of a Phaseolus vulgaris dry extract on food intake, body weight, and glycemia in rats. J Agric Food Chem. 2009;57(19):9316-9323.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
