| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 10, Number 2, April 2020, pages 49-53
Medical Management of Primary Hyperparathyroidism in Pregnancy: A Case Report and Brief Literature Review
Xinyuan Ninga, Wedad Rahmanb, Rana Malekb, c
aDepartment of Internal Medicine, University of Maryland Medical Center, 22 S. Greene St., Baltimore, MD 21201, USA
bDepartment of Endocrinology, Diabetes, and Nutrition, University of Maryland Medical Center, 22 S. Greene St., Baltimore, MD 21201, USA
cCorresponding Author: Rana Malek, Division of Endocrinology, Diabetes, and Nutrition, University of Maryland Medical Center, 22 S. Greene St., Baltimore, MD 21201, USA
Manuscript submitted January 17, 2020, accepted February 10, 2020
Short title: Medical Management of PHPT in Pregnancy
doi: https://doi.org/10.14740/jem624
| Abstract | ▴Top |
There is no established standard of care for the medical management of primary hyperparathyroidism in pregnancy for those patients who are not surgical candidates. We present a case of primary hyperparathyroidism in the third trimester that was managed with cinacalcet and a literature review on the various modalities for the medical management of primary hyperparathyroidism in pregnancy. The primary aim of this case report is to document a case of hyperparathyroidism in pregnancy that was managed medically and to perform a brief systematic review of the literature available on the medical management of primary hyperparathyroidism in pregnancy. The secondary aim is to contribute to the literature available on the use of cinacalcet in pregnancy. A 37-year-old woman with untreated primary hyperparathyroidism presented at 32 weeks of gestation with hypercalcemia that was not amenable to surgical intervention. We treated her with increasing doses of cinacalcet with improvement in her serum calcium until developing pre-eclampsia which prompted emergent cesarean delivery of the infant. The neonate developed respiratory distress after delivery but did not develop hypocalcemia after birth. The neonate became transiently hypercalcemic in the setting of calcium gluconate infusions given to prevent hypocalcemia. The patient underwent surgical removal of a parathyroid adenoma and required calcium supplementation for 1 month afterwards. Hypercalcemic crisis during pregnancy is associated with significant maternal and fetal morbidity. There is limited information regarding the medical management of primary hyperparathyroidism due to the lack of high-powered studies and prospective studies owing to the relative rarity of the condition. No serious adverse maternal events were reported for either bisphosphonate or cinacalcet use. Adverse neonatal events include transient hypocalcemia of the infant with cinacalcet use and possibly low birth weight, infantile hypocalcemia, and shortened gestational periods with bisphosphonate use.
Keywords: Hyperparathyroidism non-surgical; Hyperparathyroidism pregnancy; Medical management hyperparathyroidism; Bisphosphonates pregnancy; Cinacalcet pregnancy
| Introduction | ▴Top |
Primary hyperparathyroidism (PHPT) is an endocrine disorder that affects women more commonly than men. In pregnancy, it is a rare phenomenon and was first described by Hunter and Bull in 1931 [1]. A large number of cases during pregnancy go undiagnosed due to non-specific symptoms of fatigue, nausea, vomiting, difficulty concentrating and achiness. Furthermore, due to physiological decrease in albumin levels during pregnancy, serum calcium may appear falsely normal which makes this diagnosis particularly difficult.
Although pregnancy may develop uneventfully, complications to both mother and fetus have been reported even in cases of mild hyperparathyroidism. Maternal complications occur at a rate of 67% and include hyperemesis gravidarum, intrauterine-growth retardation (IUGR), pre-eclampsia, pre-term labor, nephrolithiasis, pancreatitis or hypercalcemic crisis. Fetal complications occur at 80% and are associated with a 30% mortality rate. Neonates are at risk for tetany and, in some cases, death [2]. If PHPT is detected in a woman of child-bearing age, surgery to remove affected gland(s) should ideally take place either pre-conception or in the second trimester to minimize risks to both mother and fetus. However, there is no clear consensus for medical management of PHPT in pregnancy in cases where surgery is not feasible. Here we present a case of PHPT in a woman in her third trimester of pregnancy.
| Case Report | ▴Top |
A 37-year-old Nigerian woman (G1P0) with human immunodeficiency virus (HIV) and hypertension was admitted at 32 weeks of gestation with hypertensive crisis and hypercalcemia. She was diagnosed with PHPT 1 year prior to presentation by her primary care physician but did not follow up with an endocrinologist at the time. Her pregnancy course was complicated by recurrent admissions for hypertensive crises and hypercalcemia. Initial laboratory evaluation (Table 1) revealed elevated serum calcium of 12.3 mg/dL (8.7 - 10.2 mg/dL), ionized calcium of 1.49 mmol/L (1.15 - 1.29 mmol/L), parathyroid hormone (PTH) level of 135 pg/mL (15 - 65 pg/mL), parathyroid-related hormone level of 3.0 (0 - 3.4), vitamin 1,25 level of 103 pg/mL (19 - 79 pg/mL) and alkaline phosphatase level of 255 U/L (38 - 126 U/L). Home medications included oral furosemide 20 mg twice daily. Initial management with intravenous fluids and oral furosemide failed to lower calcium levels below 12.0 mg/dL.
 Click to view | Table 1. Initial Maternal Laboratory Data Adapted From Medical Chart Through Electronic Medical Record |
Due to the known detrimental effects of maternal hypercalcemia on fetal and maternal health, the decision was made to start Sensipar 30 mg at 32 weeks of gestation after reviewing similar cases in literature. However, she developed nausea, a common side effect of the drug, soon after discharge from the hospital. Symptoms were initially manageable with ondansetron but worsened after her dose was increased to 60 mg at a follow-up visit. A week after discharge, she developed lower abdominal cramping and was readmitted to the hospital due to concern for pre-term labor. Initial evaluation revealed that she was in hypertensive crisis and hypercalcemic yet again, with a serum calcium of 12.3 mg/dL. During this time, she was at 34 weeks of gestation. She was subsequently treated with intravenous fluids, intravenous loop diuretic furosemide, and Sensipar was increased to 90 mg with scheduled ondansetron for nausea relief. She remained on Sensipar of 90 mg until delivery as she was unable to tolerate further up-titration due to intractable nausea and vomiting. Endocrine surgery was consulted to evaluate the patient for possible third trimester parathyroidectomy due to the concern that persistent hypercalcemia was exacerbating hypertension; however, the risk of surgery in the third trimester was considered too great. Calcium levels improved slightly on this regimen but remained above 11.5 mg/dL, so oral phosphate was added to decrease intestinal calcium absorption. After these medical measures, patient’s serum calcium down-trended (Fig. 1). By the next day, the patient started to display signs of pre-eclampsia and results of fetal non-stress test (NST) were not reassuring (non-reactive). Labor was induced and she was delivered of a female infant via cesarean section at 34 weeks and 5 days of gestation. The infant had Apgar scores of 1, 4 and 7 at 1, 5 and 10 min, respectively, and developed respiratory distress soon after birth necessitating neonatal intensive care unit (NICU) monitoring.
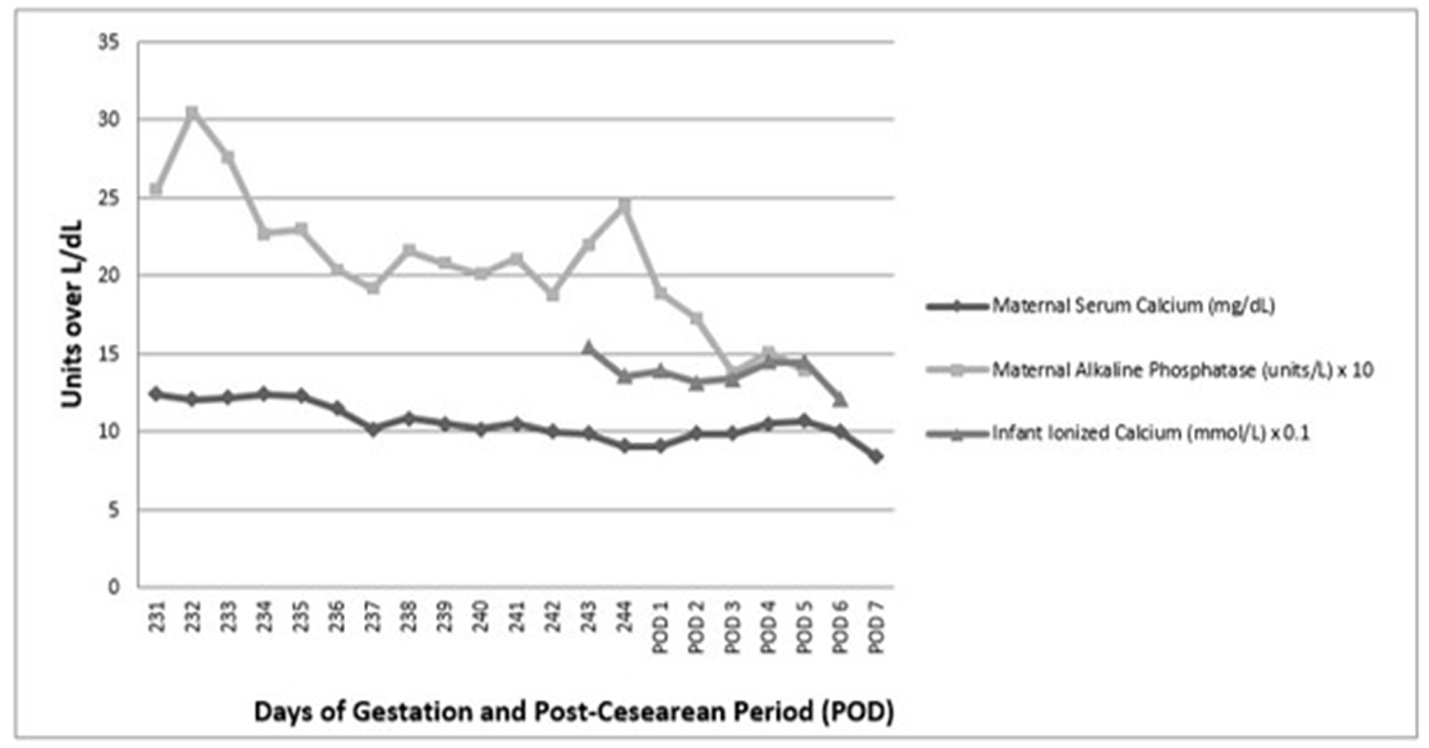 Click for large image | Figure 1. Trend of mother’s calcium, alkaline phosphatase, and neonate’s ionized calcium. Adapted from patient chart through electronic medical record. |
Due to immediate concern for fetal hypocalcemia, she was also started on an intravenous infusion calcium gluconate. Feeding commenced with parenteral peripheral nutrition (PPN) which did not contain calcium supplementation. Subsequent laboratory evaluation revealed fetal hypercalcemia with ionized calcium levels of 1.36 mmol/L (1.15 - 1.29 mmol/L) (Table 2). Hypercalcemia in the infant was self-limiting and resolved after cessation of calcium infusion (Fig. 1). The patient’s alkaline phosphatase levels normalized within a week and she subsequently was able to undergo removal of right inferior parathyroid adenoma 11 days after delivery. Intra-operative PTH decreased from 146 to 23 (Fig. 1). Post-operatively, she required calcium supplements for 1 month.
 Click to view | Table 2. Infant Laboratory Data Immediately After Birth Adapted From Medical Chart Through Electronic Medical Record |
| Discussion | ▴Top |
Maternal-fetal calcium homeostasis
Calcium homeostasis in pregnancy is the result of a delicate interplay between multiple hormones released from the maternal, placental, and fetal circulations. During pregnancy, there is an increased demand for calcium from the fetus for adequate bone mineralization. Calcium accumulation in a fetus by term is around 28 - 30 g, with the bulk of the acquisition occurring during the third trimester. Calcium ion regulation across maternal circulation to fetal circulation is not PTH mediated. Instead calcium transport through the placenta is largely parathyroid hormone-related protein (PTHrP) mediated which stimulates active transport of calcium ions into the fetus via placenta at a gradient of 1:1.4. This is evidenced by fetal cord blood measurements which show a 0.5 - 1 mEq/L higher concentration of calcium compared to the mother. There is also a compensatory two-fold increase in intestinal calcium absorption from the mother through increased levels of vitamin D 1,25, prolactin and placental lactogen [3].
PTHrP increases in the first trimester and is critical in maintaining the maternal-fetal-calcium gradient and plays a role in milk production and labor onset. PTHrp is released from the breasts, deciduas, placenta, fetal membranes and umbilical cord. In a study done in 1996, PTHrP gene deletion in mice resulted in fetal hypocalcemia and reduced maternal-fetal placental calcium gradient [4].
An imbalance in this delicate system, as is the case in maternal hyperparathyroidism, puts the fetal calcium balance at risk. Maternal hypercalcemia suppresses fetal parathyroid gland function and development and can lead to neonatal hypocalcemia which can result in tetany or death in severe cases. In contrast, as placental PTHrP also mediates increased shunting of maternal to fetal calcium, loss of placental PTHrP in the post-partum period may worsen maternal hypercalcemia [5].
Literature review on treatment
Treatment of choice for severe or symptomatic hypercalcemia in pregnancy due to PHPT is parathyroidectomy in the early second trimester [6]. Surgery may also be attempted in the first or third trimester; however, this must be individualized to the patient due to risks associated with incomplete organogenesis, higher pre-term labor, and increased fetal mortality [7]. In situations where the patient is a poor surgical candidate or when hypercalcemia is mild and asymptomatic, medical management is indicated.
Medical management of hypercalcemia associated with PHPT in pregnancy is complicated by several factors and general lack of data. Most treatment starts with conservative therapy which includes rehydration usually with loop diuretics to promote renal excretion of calcium. This therapy has limited efficacy, and when used alone, would be most appropriate in asymptomatic patients with calcium level < 11. Otherwise, a calcium-lowering agent should be used in conjunction with conservative management in pregnant patients with calcium levels > 11 who are not surgical candidates [8].
Calcium-lowering agents include calcitonin, cinacalcet, and bisphosphonates. Calcitonin is a hormone secreted by the parafollicular cells of the thyroid gland which inhibit osteoclast activity in the bone thereby decreasing calcium levels. There have been case reports of calcitonin use in pregnancy with no associated adverse fetal events and calcitonin has negligible passage through the placental barrier. However, calcium-lowering effects of calcitonin are moderate (maximum calcium decrease 0.3 - 0.5 mmol/L) and tachyphylaxis generally occurs after 24 - 48 h of use. However, calcitonin does not cross the placenta and is generally considered safe for use in pregnancy [9-11].
Cinacalcet is a member of drug class type II calcimimetics. It binds to and allosterically activates calcium-sensing receptors on the chief cell of the parathyroid tissue which increases sensitivity to calcium that results in negative regulation on PTH production [12]. Calcium-sensing receptors are also present in the placenta and cinacalcet use during pregnancy may alter placental function and potentially induce fetal or neonatal hypocalcemia and hypoparathyroidism. Animal studies done in pregnant rats and rabbits show that cinacalcet does cross the placenta; however, there is no evidence of embryonic or fetal toxicity. Regardless, it is classified as a category C drug in pregnancy [13, 14]. There are some case studies of cinacalcet use in pregnancy to treat PHPT with evidence of fetal hypocalcemia after delivery.
In several case reports, the mothers were treated with varying doses of cinacalcet, ranging from 15 to 240 mg/day, at various gestational ages. Two cases used cinacalcet in conjunction with calcitonin to decrease maternal calcium levels as cinacalcet monotherapy did not achieve adequate reduction in serum calcium levels. The first described case in literature by Horjus et al that used combined cinacalcet and calcitonin therapy proposed that cinacalcet monotherapy may not be useful for severe PHPT [15, 16]. The other three case reports used cinacalcet alone to decrease maternal calcium [17-19]. Four case reports did report some neonatal hypocalcemia after birth which required oral calcium supplementation; however, no other adverse effects were noted in the infants. No adverse events for the mothers were reported with cinacalcet use in pregnancy; however, nausea was a limiting factor in the ability to up-titrate dosing as was the case in our patient. Given that four cases reported neonatal hypocalcemia, it is unclear if cinacalcet use promotes fetal hypoparathyroidism as maternal hypercalcemia also contributes to fetal calcium-sensing receptor modulation.
Bisphosphonates are synthetic non-hydrolysable pyrophosphate analogues that bind to bone hydroxyapatite and are released by osteoclasts during bone breakdown. Bisphosphonates are then taken up into the osteoclasts where they inhibit intracellular enzymatic functions necessary for bone resorption. Bisphosphonates are excreted renally and are not metabolized; thus, the half-life of bisphosphonates ranges from 1 to 10 years depending on the rate of bone resorption [20]. There is limited data on the effect of bisphosphonates in pregnancy; however, it is currently classified as a category C drug [21]. In one animal study, bisphosphonates given to pregnant rats were found to cross the placenta and were associated with decreased fetal weight and retarded bone growth [22]. In a separate study, where pregnant rats were exposed to pamidronate at a high dose 10 to 15 mg/kg/day, the dams experienced hypocalcemia and protracted parturition; however, teratogenic effects were not seen until the rats were given daily doses ten times higher than the recommended therapeutic human dose. These teratogenic effects included embryolethality and marked skeletal retardation [23].
Between three review articles, they covered several cases of neonates born to mothers who had been exposed to bisphosphonates before conception or during pregnancy. The most commonly used bisphosphonates were oral alendronate and intravenous pamidronate and duration of bisphosphonate use ranged from 5 years to 6 months prior to pregnancy or was started during pregnancy. In one article, the authors reviewed 78 cases of fetuses whose mothers had been exposed to bisphosphonates before conception or during pregnancy. The majority of mothers in this review article did not experience serious adverse effects; however, there were cases of shortened gestational age, low neonatal birth weight and transient hypocalcemia of the newborns. There were also a few reported cases of spontaneous abortions and congenital anomalies; however, there were several confounding factors involved given the underlying maternal diseases that required bisphonate use [24]. A second review article reviewed 51 cases of exposure to bisphosphonates before or during pregnancy. None of these cases were identified to have skeletal abnormalities or congenital defects in the infants [25]. In one case by Vujasinovic-Stupar et al, they followed a case of pregnancy associated spinal osteoporosis treated with bisphosphonates for 12 years and found no adverse effects on the two infants who were exposed in utero. Both children, aged 5 and 6 years, had normal bone density [26]. Finally, in a multicenter cohort prospective trial by Levy et al, they described 21 women who had used bisphosphonates during or less than 3 months prior to pregnancy and 21 matched comparison group women who did not have exposure to known teratogens. The primary endpoint was neonatal outcomes including major birth defects and the secondary endpoint was pregnancy outcomes including live births, spontaneous abortions, and therapeutic abortions. They concluded that they did not find any significant difference between the two groups in either outcome [27].
Conclusions
Hypercalcemic crisis during pregnancy is associated with significant maternal and fetal morbidity. Mothers with uncontrolled hyperparathyroidism have increased risk of fetal hypoparathyroidism and hypocalcemia. Infants should be monitored for signs of hypocalcemia and tetany for 4 weeks after birth and may require calcium supplementation for up to 6 weeks after birth. Based on the literature available, the treatment of choice is parathyroidectomy in the second trimester. Medical therapy of hypercalcemia due to PHPT in pregnancy has several limitations. Calcitonin is well tolerated; however, use is limited by tachyphylaxis. Additionally, use is only associated with moderate decreases in serum calcium. Cinacalcet is not well tolerated and unlikely to be effective monotherapy in reducing serum calcium in severe hyperparathyroid cases. Several cases described cinacalcet use in conjunction with intravenous fluid, loop diuretics, and calcitonin. Additionally, use is also associated with neonatal hypocalcemia requiring oral supplementation. The use of bisphosphonates in pre-conception or in any trimester has not shown adverse effects on neonatal bone production as of yet. There are very few prospective studies on bisphosphonate use in pregnancy and no prospective studies on cinacalcet use in pregnancy. This highlights the need for long-term prospective high-powered studies of bisphosphonates and cinacalcet given their increased use. However, this is again limited due to the relative rarity of hyperparathyroidism in pregnancy and thus there may not be enough cases to complete a reasonably high-powered study. In this event, clinical experience with systematic reviews and case studies may be the next viable option.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
WR and XN conceived the manuscript, and XN generated all figures and tables and wrote the manuscript with contributions from RM.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Som M, Stroup JS. Primary hyperparathyroidism and pregnancy. Proc (Bayl Univ Med Cent). 2011;24(3):220-223.
doi pubmed - Kelly TR. Primary hyperparathyroidism during pregnancy. Surgery. 1991;110(6):1028-1033; discussion 1033-1024.
- Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832-872.
doi pubmed - Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A. 1996;93(26):15233-15238.
doi pubmed - Sato K. Hypercalcemia during pregnancy, puerperium, and lactation: review and a case report of hypercalcemic crisis after delivery due to excessive production of PTH-related protein (PTHrP) without malignancy (humoral hypercalcemia of pregnancy). Endocr J. 2008;55(6):959-966.
doi pubmed - Kaplan EL, Burrington JD, Klementschitsch P, Taylor J, Deftos L. Primary hyperparathyroidism, pregnancy, and neonatal hypocalcemia. Surgery. 1984;96(4):717-722.
- Tachamo N, Timilsina B, Dhital R, Lynn T, Magaji V, Gabriely I. Primary hyperparathyroidism in pregnancy: successful parathyroidectomy during first trimester. Case Rep Endocrinol. 2018;2018:5493917.
doi pubmed - Diaz-Soto G, Linglart A, Senat MV, Kamenicky P, Chanson P. Primary hyperparathyroidism in pregnancy. Endocrine. 2013;44(3):591-597.
doi pubmed - Rey E, Jacob CE, Koolian M, Morin F. Hypercalcemia in pregnancy - a multifaceted challenge: case reports and literature review. Clin Case Rep. 2016;4(10):1001-1008.
doi pubmed - Patel S, Lyons AR, Hosking DJ. Drugs used in the treatment of metabolic bone disease. Clinical pharmacology and therapeutic use. Drugs. 1993;46(4):594-617.
doi pubmed - Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):795-826.
doi pubmed - Poon G. Cinacalcet hydrochloride (Sensipar). Proc (Bayl Univ Med Cent). 2005;18(2):181-184.
doi pubmed - Rubin MR, Silverberg SJ. Use of Cinacalcet and (99m)Tc-sestamibi Imaging During Pregnancy. J Endocr Soc. 2017;1(9):1156-1159.
doi pubmed - Hyperparathyroidism 1.1. Secondary. Full prescribing information. Available: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/sensipar/sensipar_pi_hcp_english.pdf.
- Horjus C, Groot I, Telting D, van Setten P, van Sorge A, Kovacs CS, Hermus A, et al. Cinacalcet for hyperparathyroidism in pregnancy and puerperium. J Pediatr Endocrinol Metab. 2009;22(8):741-749.
doi pubmed - Gonzalo Garcia I, Robles Fradejas M, Martin Macias MLA, Biain Ciganda A, Bustinza Beaskoetxea Z, Ruiz Perez E, Fernandez Matia G, et al. Primary hyperparathyroidism in pregnancy treated with cinacalcet: a case report. J Obstet Gynaecol. 2018;38(1):132-134.
doi pubmed - Vera L, Oddo S, Di Iorgi N, Bentivoglio G, Giusti M. Primary hyperparathyroidism in pregnancy treated with cinacalcet: a case report and review of the literature. J Med Case Rep. 2016;10(1):361.
doi pubmed - Nadarasa K, Bailey M, Chahal H, Raja O, Bhat R, Gayle C, Grossman AB, et al. The use of cinacalcet in pregnancy to treat a complex case of parathyroid carcinoma. Endocrinol Diabetes Metab Case Rep. 2014;2014:140056.
doi pubmed - Horton WB, Stumpf MM, Coppock JD, Lancaster L, Dalkin AC, Liu Z, Chisholm CA, et al. Gestational primary hyperparathyroidism due to ectopic parathyroid adenoma: case report and literature review. J Endocr Soc. 2017;1(9):1150-1155.
doi pubmed - Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75-85.
doi - McNicholl DM, Heaney LG. The safety of bisphosphonate use in pre-menopausal women on corticosteroids. Curr Drug Saf. 2010;5(2):182-187.
doi pubmed - Minsker DH, Manson JM, Peter CP. Effects of the bisphosphonate, alendronate, on parturition in the rat. Toxicol Appl Pharmacol. 1993;121(2):217-223.
doi pubmed - Graepel P, Bentley P, Fritz H, Miyamoto M, Slater SR. Reproduction toxicity studies with pamidronate. Arzneimittelforschung. 1992;42(5):654-667.
- Stathopoulos IP, Liakou CG, Katsalira A, Trovas G, Lyritis GG, Papaioannou NA, Tournis S. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones (Athens). 2011;10(4):280-291.
doi pubmed - Djokanovic N, Klieger-Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy? J Obstet Gynaecol Can. 2008;30(12):1146-1148.
doi - Vujasinovic-Stupar N, Pejnovic N, Markovic L, Zlatanovic M. Pregnancy-associated spinal osteoporosis treated with bisphosphonates: long-term follow-up of maternal and infants outcome. Rheumatol Int. 2012;32(3):819-823.
doi pubmed - Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, Moretti M, et al. Pregnancy outcome following in utero exposure to bisphosphonates. Bone. 2009;44(3):428-430.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
