| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Editorial
Volume 9, Number 5, October 2019, pages 117-119
Dipeptidyl Peptidase-4 Inhibitor Versus Sodium-Glucose Cotransporter-2 Inhibitor in the Management of Type 2 Diabetes
Hidekatsu Yanai
Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, 1-7-1 Kohnodai, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted September 6, 2019, accepted October 1, 2019
Short title: DPP4i Versus SGLT2i in T2DM
doi: https://doi.org/10.14740/jem609
Currently, dipeptidyl peptidase-4 inhibitors (DPP4i) and sodium-glucose cotransporter-2 inhibitors (SGLT2i) are frequently used to treat type 2 diabetes. In this issue (Journal of Endocrinology and Metabolism, 2019), Matsuba et al compared the effects of treatment with sitagliptin (DPP4i) for 24 weeks in the ASSET-K study [1-7] with those with ipragliflozin (SGLT2i) for 24 weeks in the ASSIGN-K study [8-10] and found different effects of these two drugs on clinical parameters [11]. At the baseline, the patients treated by SGLT2i were younger and showed higher body mass index (BMI) and blood pressure as compared with patients treated with DPP4i, indicating that the patients treated by SGLT2i were more likely to have metabolic syndrome than patients treated with DPP4i. A significantly higher incidence of hypertension and dyslipidemia in patients treated by SGLT2i as compared with patients treated with DPP4i supported this hypothesis. HbA1c values were similar in both groups at the baseline.
The difference between two groups in effects on HbA1c was not significant at 12 or 24 weeks [11]. BMI showed a significantly larger decrease with SGLT2i treatment than DPP4i treatment throughout most of the study period [11]. The mean blood pressure also showed a significantly larger decrease with SGLT2i treatment than DPP4i treatment [11]. The decrease of the estimated glomerular filtration rate (eGFR) after 24 weeks was significantly larger in patients treated with DPP4i than those receiving SGLT2i [11].
I previously reported possible anti-atherosclerotic effects beyond glucose lowering [12] (Fig. 1). SGLT2i reduces renal glucose reabsorption and decreases plasma glucose, in an insulin-independent manner, which induces reduction of body weight. Reduction of body weight, improvement of insulin sensitivity and osmotic diuresis decrease blood pressure. Our previous studies also demonstrated that SGLT2i significantly reduced body weight and blood pressure [13, 14].
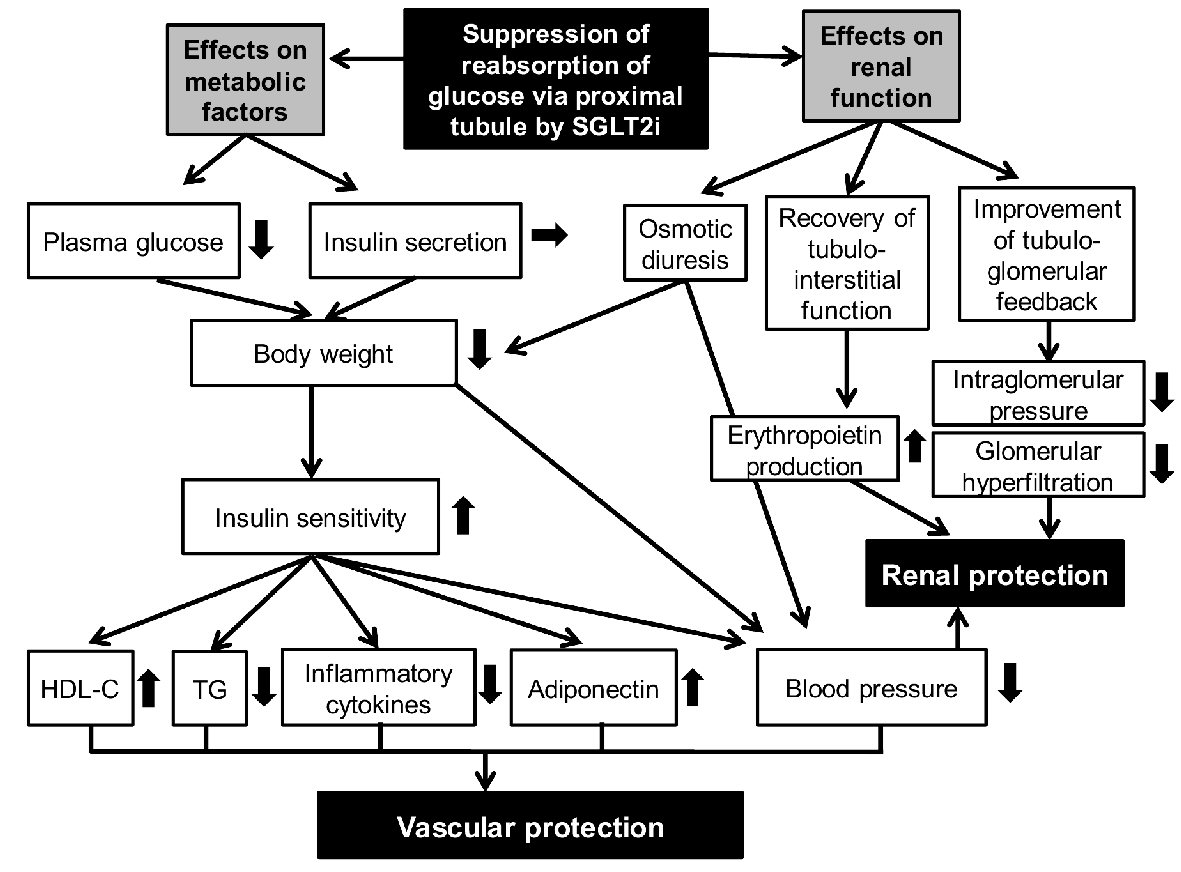 Click for large image | Figure 1. Putative mechanisms for vascular and renal protection by SGLT2i. SGLT2i: sodium-glucose cotransporter-2 inhibitors. |
The EMPA-REG OUTCOME trial showed that empagliflozin significantly reduced incident or worsening of nephropathy, progression to macroalbuminuria, doubling of serum creatinine level accompanied by eGFR of ≤ 45 mL/min/1.73 m2 and initiation of renal replacement therapy [15]. The CANVAS program also demonstrated that canagliflozin reduced progression to macroalbuminuria, and 40% reduction in eGFR, renal replacement therapy, or renal death [16]. Very recently, CREDENCE trial showed that canagliflozin significantly reduced the relative risk of renal failure in patients with type 2 diabetes and albuminuric chronic kidney disease [17]. The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34%, and the relative risk of end-stage kidney disease was lower by 32% in patients treated with canagliflozin. Recent accumulated high level of evidences showed a significant renal protective effect of SGLT2i.
Various underlying mechanisms may contribute to renal protection by SGLT2i [18-20] (Fig. 1). Sano et al mentioned that SGLT2i reduces the overload of the proximal tubules and improves tubulointerstitial hypoxia, inducing the recovery of erythropoietin production by fibroblasts [21]. They concluded that increased hematocrit during SGLT2i therapy indicates the recovery of tubulointerstitial function in diabetic kidney [21]. We think that elevated erythropoietin may be a possible mechanism for the renal protective effect of SGLT2i. Chronic treatment with recombinant human erythropoietin exerted renal protective effects beyond hematopoiesis in streptozotocin-induced diabetic rat [22]. Erythropoietin protected mouse podocytes from damage by advanced glycation end-products [23]. Furthermore, erythropoietin ameliorated podocyte injury in advanced diabetic nephropathy in the db/db mouse [24].
Suppression of reabsorption of glucose via proximal tubule by SGLT2i may improve tubulo-glomerular feedback [20], which may reduce intraglomerular pressure and glomerular hyperfiltration. Reduction of blood pressure, serum urate levels and utilization of ketone bodies by failing kidney may also contribute to renal protection by SGLT2i [19].
Acknowledgments
None to declare.
Financial Disclosure
Author has no financial disclosure to report.
Conflict of Interest
The author declares that he has no conflict of interest concerning this article.
| References | ▴Top |
- Maeda H, Kubota A, Tanaka Y, Terauchi Y, Matsuba I, ASSET-K Study group. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95(1):e20-22.
doi pubmed - Kubota A, Maeda H, Kanamori A, Matoba K, Jin Y, Minagawa F, Obana M, et al. Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J Clin Med Res. 2012;4(5):309-313.
doi pubmed - Kubota A, Maeda H, Kanamori A, Matoba K, Jin Y, Minagawa F, Obana M, et al. Efficacy and safety of sitagliptin monotherapy and combination therapy in Japanese type 2 diabetes patients. J Diabetes Investig. 2012;3(6):503-509.
doi pubmed - Kanamori A, Matsuba I. Factors associated with reduced efficacy of sitagliptin therapy: analysis of 93 patients with type 2 diabetes treated for 1.5 years or longer. J Clin Med Res. 2013;5(3):217-221.
doi pubmed - Maeda H, Kubota A, Kanamori A, Tanaka Y, Terauchi Y, Matsuba I, ASSET-K Study Group. Long-term efficacy and safety of sitagliptin in the treatment of Japanese Type 2 diabetes (ASSET-K1) to a target of HbA1c <7%. J Endocrinol Invest. 2013;36(8):568-573.
doi pubmed - Umezawa S, Kubota A, Maeda H, Kanamori A, Matoba K, Jin Y, Minagawa F, et al. Two-year assessment of the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes: Post hoc analysis of the ASSET-K study. BMC Endocr Disord. 2015;15:34.
doi pubmed - Yuasa S, Sato K, Takai M, Ishikawa M, Umezawa S, Kubota A, Maeda H, et al. Factor analysis of changes in hemoglobin A1c after 12 months of sitagliptin therapy in patients with type 2 diabetes. J Clin Med Res. 2016;8(6):461-471.
doi pubmed - Iizuka T, Iemitsu K, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, et al. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes: interim outcome of the ASSIGN-K study. J Clin Med Res. 2016;8(2):116-125.
doi pubmed - Iemitsu K, Iizuka T, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, et al. Factors influencing changes in hemoglobin A1c and body weight during treatment of type 2 diabetes with ipragliflozin: interim analysis of the ASSIGN-K study. J Clin Med Res. 2016;8(5):373-378.
doi pubmed - Iemitsu K, Kawata T, Iizuka T, Takihata M, Takai M, Nakajima S, Minami N, et al. Effectiveness of ipragliflozin for reducing hemoglobin a1c in patients with a shorter type 2 diabetes duration: interim report of the ASSIGN-K study. J Clin Med Res. 2017;9(9):793-801.
doi pubmed - Matsuba I, Iemitsu K, Kawata T, Iizuka T, Takihata M, Takai M, Nakajima S, et al. Sitagliptin versus ipragliflozin for type 2 diabetes in clinical practice. J Endocrinol Metab. 2019;9(5):151-158.
doi - Yanai H, Katsuyama H, Hamasaki H, Adachi H, Moriyama S, Yoshikawa R, Sako A. Sodium-Glucose cotransporter 2 inhibitors: possible anti-atherosclerotic effects beyond glucose lowering. J Clin Med Res. 2016;8(1):10-14.
doi pubmed - Katsuyama H, Hamasaki H, Adachi H, Moriyama S, Kawaguchi A, Sako A, Mishima S, et al. Effects of sodium-glucose cotransporter 2 inhibitors on metabolic parameters in patients with type 2 diabetes: a chart-based analysis. J Clin Med Res. 2016;8(3):237-243.
doi pubmed - Yanai H, Hakoshima M, Adachi H, Kawaguchi A, Waragai Y, Harigae T, Masui Y, et al. Effects of six kinds of sodium-glucose cotransporter 2 inhibitors on metabolic parameters, and summarized effect and its correlations with baseline data. J Clin Med Res. 2017;9(7):605-612.
doi pubmed - Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334.
doi pubmed - Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
doi pubmed - Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
doi pubmed - Yanai H, Katsuyayama H. A possible mechanism for renoprotective effect of sodium-glucose cotransporter 2 inhibitor: elevation of erythropoietin production. J Clin Med Res. 2017;9(2):178-179.
doi pubmed - Koguchi A, Adachi H, Yanai H. The Application of Sodium-Glucose Cotransporter 2 Inhibitors to Chronic Kidney Disease Stage 4. J Clin Med Res. 2017;9(12):1029-1031.
doi pubmed - Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587-597.
doi pubmed - Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844-847.
doi pubmed - Toba H, Sawai N, Morishita M, Murata S, Yoshida M, Nakashima K, Morita Y, et al. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2009;612(1-3):106-114.
doi pubmed - Ruester C, Franke S, Bondeva T, Wolf G. Erythropoietin protects podocytes from damage by advanced glycation end-products. Nephron Exp Nephrol. 2011;117(1):e21-30.
doi pubmed - Loeffler I, Ruster C, Franke S, Liebisch M, Wolf G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. Am J Physiol Renal Physiol. 2013;305(6):F911-918.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
