| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 7, Number 2, April 2017, pages 68-71
Diabetic Sclerodactyly Mimicking Limited Sclerosis: Case Report and Review of the Literature
Wais Afzala, c, Malahat Movahediana, Bhupinder B. Singha, Katerina Tellera, Kelly Cervellioneb, Richard W. Pinskera
aDepartment of Internal Medicine, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA
bDepartment of Clinical Research, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA
cCorresponding Author: Wais Afzal, Department of Internal Medicine, Jamaica Hospital Medical Center, 8900 Van Wyck Expressway, Jamaica, NY 11418, USA
Manuscript accepted for publication April 13, 2017
Short title: Diabetic Sclerodactyly
doi: https://doi.org/10.14740/jem394e
| Abstract | ▴Top |
Diabetes mellitus can be associated with a variety of musculoskeletal conditions. These conditions mostly occur in longstanding and poorly controlled diabetes and are the result of progressive alterations in tissues. Timely recognition of these conditions is of paramount clinical importance as diabetes control helps in preventing progressive deformity and disability, pain control, and maintaining reasonable quality of life. In addition, some of these conditions can be addressed with available treatment modalities. We present a 43-year-old female with a longstanding history of type 1 diabetes mellitus admitted for diabetic ketoacidosis. She had a significant prior history of musculoskeletal conditions. Physical examination findings of her hands revealed thick and tight skin, sclerodactyly, and “prayer sign”. Appropriate workup to rule out systemic and limited sclerosis was performed and was negative.
Keywords: Diabetes mellitus; Musculoskeletal complications; Sclerodactyly
| Introduction | ▴Top |
Diabetes mellitus (DM) can be associated with a variety of musculoskeletal conditions. These conditions most often occur in longstanding diabetics, particularly in patients with poor glycemic control. Progressive alterations in connective tissue due to glycosylation of proteins, microvasculature and peripheral nerve damage, along with collagen deposition in the skin and periarticular structures are the most likely explanations for the pathogenesis of musculoskeletal manifestations in DM [1]. Although these conditions are encountered in the general population, the frequency is higher in diabetic patients, particularly those with prolonged history of DM [2]. It is of paramount importance that clinicians be vigilant about recognizing these conditions during routine care for diabetic patients. Evidence indicates that diabetes control helps in preventing progressive deformity and disability, pain control, and maintaining reasonable quality of life in diabetic patients. In addition, certain treatment modalities are available for some of these conditions.
We present a 43-year-old female with a history of type 1 DM since age 4 who was admitted for diabetic ketoacidosis. She had significant prior history of musculoskeletal conditions. On physical examination, the skin of the palmar and dorsal aspect of both of her hands was found to be thick and tight, limiting the mobility of her hand joints. In addition, she had sclerodactyly of bilateral fingers, and “prayer sign” was evident. She stated that these findings had evolved over the course of the last several years affecting her daily activities. Appropriate workup to rule out systemic and limited sclerosis was performed and showed negative results. This case illustrates that longstanding DM can be associated with a variety of musculoskeletal findings including those mimicking systemic or limited sclerosis.
| Case Report | ▴Top |
A 43-year-old Hispanic female with history of type 1 DM since age 4 presented with nausea and vomiting. In the morning of the admission day, she experienced fatigue, dry mouth, and loss of appetite. Because of her anorexia and poor oral intake, she turned off her insulin pump. Later in the day, her condition deteriorated, and she developed nausea and vomited several times. Fingerstick glucose 1 h before calling the emergency medical services was above 300 mg/dL (normal range 70 - 110 mg/dL). As said, she was diagnosed with type 1 DM at age 4 and had since been on different insulin products and regimens. After failing several regimens, she started using an insulin pump 4 years prior and regularly followed with her endocrinologist. Her other medical conditions included peripheral neuropathy and osteoarthritis of bilateral hand joints. Also, she had a left shoulder surgery for limited mobility 3 years prior, surgery for bilateral carpal tunnel syndrome 2.5 years prior, and corticosteroid injections in right second metacarpophalangeal and left third metacarpophalangeal joints for trigger fingers 5 months prior. Beside the insulin pump, she was taking aspirin 81 mg daily and pregabalin 150 mg three times every day. She was not taking any herbal remedies. She used to smoke 5 - 10 cigarettes daily for about 10 years but stopped 2 years prior. She drank occasionally, but denied any recreational drug use.
Physical examination revealed a well-developed, well-nourished overweight female in no acute distress with normal vital signs. Head and neck examination showed dry oral mucous membranes. Heart, lung and abdominal examinations were unremarkable. Neurologically, she was alert, awake and oriented with no focal deficits except decreased sensation to pinprick on her feet and lower legs. Upper extremities indicated prominent distal interphalangeal joints (Herberden’s nodes) bilaterally. Skin of palmar and dorsal aspect of both hands showed significant thickness and tightness as well as sclerodactyly and “prayer sign” (Fig. 1a, b). The range of motion of her hand joints was limited due to tight skin bilaterally. No ulcerations of fingertips, focal tenderness or nail pitting were present. Pulses were intact on both sides. No rashes were observed. Moreover, she denied Raynaud’s phenomenon.
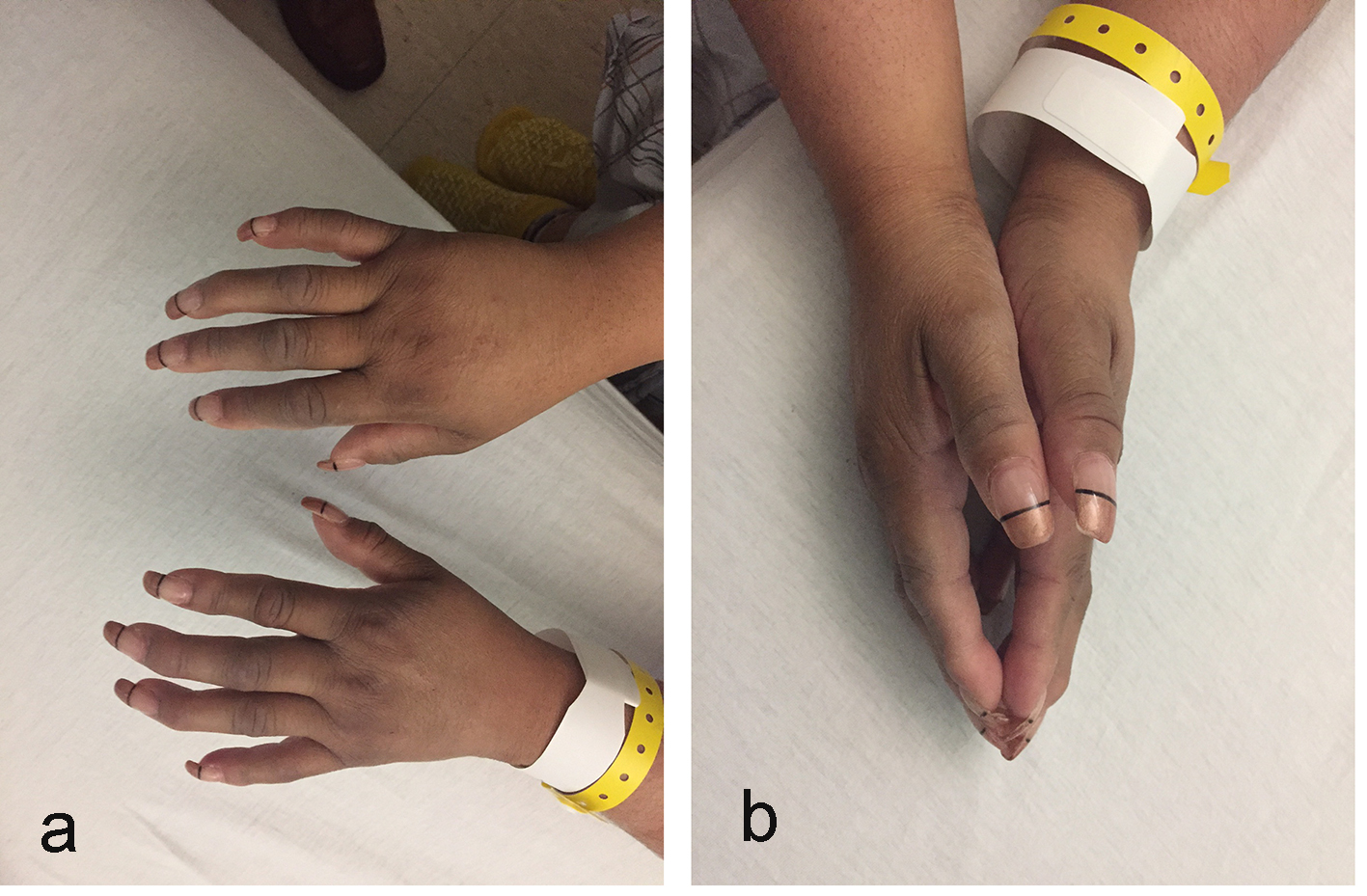 Click for large image | Figure 1. (a, b) Hands with thick and tight skin, sclerodactyly, and “prayer sign”. |
Laboratory workup indicated hyperglycemia of 399 mg/dL, hyperkalemia of 5.7 mEq/L (reference range of 3.5 - 5.1 mEq/L), anion gap of 25 mEq/L (reference range of 8 - 12 mEq/L), beta-hydroxybutyrate level of 6.53 mmol/L (reference range of 0.02 - 0.27 mmol/L), and arterial blood pH of 7.23 (reference range of 7.35 - 7.45). Urine analysis was significant for glycosuria and ketonuria. She was admitted to the medical intensive care unit (ICU) for the management of diabetic ketoacidosis (DKA), and treatment with intravenous fluid and insulin infusion was initiated. The following day the anion gap closed and her clinical status improved. She tolerated oral diet and was started on neutral protamine Hagedorn (NPH) insulin. She was transferred to the regular floor for continuity of care.
Given her prior history of musculoskeletal conditions and significant physical examination findings of her hands, she was evaluated by a rheumatologist and physical examination findings were confirmed. The patient stated that those findings had evolved over the course of last several years. She was never diagnosed with connective tissue diseases and had no family history of such. X-rays of her hands were performed and indicated arthritic changes involving the distal interphalangeal joints with the prominence of soft tissues. Mild arthritic changes of the bilateral radiocarpal joint were observed. No erosive changes were seen. The overall picture was consistent with osteoarthritis. Erythrocyte sedimentation (ESR) and C-reactive protein (CRP) levels were within the reference ranges. Connective tissue disease panel was negative (Table 1).
 Click to view | Table 1. Connective Tissue Disease Panel |
Results were discussed with the patient, and she was told that her prior history of musculoskeletal conditions and significant physical examination findings of her hands were explainable by her longstanding history of DM. She was also told that, to date, no standardized treatment for her hand findings existed, and only symptom-based treatment could be offered. She was advised that meticulous management of her blood sugar levels might help delay the progression of these conditions. Her insulin pump was adjusted, and she was told to follow up with her endocrinologist.
| Discussion | ▴Top |
DM can be associated with a variety of musculoskeletal manifestations that can affect the hands, shoulders, feet, spine and muscles. Commonly encountered conditions include diabetic cheiroarthropathy (limited joint mobility), flexor tenosynovitis (trigger finger), Dupuytren contractures, diabetic sclerodactyly, carpal tunnel syndrome, adhesive capsulitis (frozen shoulder), calcific periarthritis, reflex sympathetic dystrophy, Charcot (neuropathic) arthropathy, diabetic muscle infarction and diffuse idiopathic skeletal hyperostosis (DISH) [1]. The frequency of these conditions is higher in patients with a longtime history of DM and, in particular, those with poor glycemic control [2]. Our patient had a prolonged history of DM since age 4. She experienced a significant prior history of musculoskeletal conditions including left shoulder surgery for limited mobility due to frozen shoulder, surgery for bilateral carpal tunnel syndrome, and corticosteroid injections in right second metacarpophalangeal and left third metacarpophalangeal joints for trigger fingers. These were most likely secondary to her longtime history of diabetes.
In the majority of patients, a comprehensive history and physical examination will discern these conditions and imaging techniques such as magnetic resonance imaging (MRI) may be utilized as an adjunct for ascertaining diagnosis in ambiguous scenarios. The advent of sonography in the diagnosis and management of musculoskeletal diseases, including those seen in patients with diabetes, has emerged as a useful modality for detection of various musculoskeletal conditions [3]. The merits of sonography in these circumstances are real time availability, lack of radiation exposure, and cost-effectiveness. The main limitation of sonography is the lack of effectiveness in the detection of osseous lesions. The physical findings in our patient were consistent with skin tightening, thickening of palmar and dorsal aspects of her hands, sclerodactyly and “prayer sign”. The mobility of the small joints of her hands was very limited. These findings were so apparent that the utility of any imaging modalities for obtaining further details was precluded.
From the pathophysiologic perspective, the combination of diabetes-induced vascular and neurological changes, as well as alteration in the milieu of connective tissue, are the possible mechanisms behind the musculoskeletal complications of DM. Progressive changes in connective tissue due to glycosylation of proteins, microvasculature injury, and peripheral nerve damage, and collagen depositions in the skin and periarticular structures are the most likely explanations for the pathogenesis of musculoskeletal manifestations in DM [1]. Strikingly, the histopathological picture of diabetes-induced musculoskeletal findings, in particular, those concerning the hands, is very similar to scleroderma [4]. Microscopic examination of diabetes-induced hand findings indicates thick dermis, an increment in cross-linked collagen in the reticular dermis, and small amounts of mucin [5]. Similarly in scleroderma, there is thickening of the dermis and excessive production of fibrillar collagen along with changes in the architecture of connective tissue components [6]. This illustrates resemblance of the two distinct clinical entities at the histological level.
Diabetic sclerodactyly, which is also known as pseudoscleroderma diabeticorum, can clinically imitate scleroderma. The initial step in the differentiation of diabetic sclerodactyly from scleroderma is to obtain a proper history and physical examination. Diabetic sclerodactyly shows significant skin thickening, and in its severe form may impair the mobility of joints, which is also seen in scleroderma. Nevertheless, typical tapering, ulceration of fingertips, calcinosis and dystrophy with necrosis of fingernails are never seen in diabetic sclerodactyly. Moreover, Raynaud’s phenomenon may be seen in scleroderma but almost never in diabetic sclerodactyly. Raynaud’s phenomenon can be noted in more than 90% of patients with scleroderma [7]. Nevertheless, 4% of the general population may report this entity as an isolated phenomenon [8]. Capillaroscopy is the objective examination method that might assist with differentiation of scleroderma from diabetic sclerodactyly [9]. This modality would not indicate any pathologic findings in a patient with diabetic sclerodactyly but would be seen in scleroderma [9]. Our patient denied a history of Raynaud’s phenomenon. Moreover, physical examination did not indicate any fingertip ulceration, necrosis or nail dystrophy. We did not perform capillaroscopy.
The next step is to look for the presence of antinuclear antibodies (ANA). These antibodies are directed against nuclear antigens. ANA is reportedly seen in around 85% of patients with scleroderma [10]. However, 5% of healthy individuals are reportedly positive for this antibody, which is usually in low titers [11]. There are different subtypes of ANA, and those concerning scleroderma are anti-Scl-70 and anti-centromere antibodies. Connective tissue disease panel including anti-Scl-70 and anti-centromere antibodies was reported as negative. Absence of Raynaud’s phenomena, lack of digital ulceration and necrosis, as well as the negativity of connective tissue disease panel, effectively excluded scleroderma in our patient.
Musculoskeletal and skin conditions associated with DM do not increase the risk of overall mortality but are indeed associated with progressive deformity, disability, pain, and overall poor quality of life [2]. It is of paramount importance that clinicians be familiar with these conditions and look for them as part of the general care of diabetic patients. Disease control and available treatment options, including surgery in certain scenarios, can be offered on an as needed basis.
Conclusion
A variety of musculoskeletal and skin conditions can be seen in patients with DM. These conditions mostly occur in patients with longstanding and poorly controlled diabetes. The combination of diabetes-induced vascular and neurological damage and alteration in the milieu of connective tissue is the possible mechanism behind the musculoskeletal complications of DM. It is of paramount importance that clinicians be vigilant about these conditions as part of their routine care of diabetics. Diabetes control helps in preventing progressive deformity and disability, pain control, and maintaining overall quality of life in diabetic patients. Certain treatment modalities are available for some of these conditions, which may be offered as needed. These conditions do not affect the mortality in diabetes patients, but increase the morbidity and interfere with the quality of life.
Disclosure
The manuscript, patient data, and imaging are reproduced in accordance with Jamaica Hospital Medical Center policies approved by the internal IRB.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
| References | ▴Top |
- Kim RP, Edelman SV, Kim DD. Musculoskeletal Complications of Diabetes Mellitus. Clin Diab. 2001;19(3):132-135.
- Merashli M, Chowdhury TA, Jawad AS. Musculoskeletal manifestations of diabetes mellitus. QJM. 2015;108(11):853-857.
doi pubmed - Park M, Park JS, Ahn SE, Ryu KN, Park SY, Jin W. Sonographic Findings of Common Musculoskeletal Diseases in Patients with Diabetes Mellitus. Korean J Radiol. 2016;17(2):245-254.
doi pubmed - Gruson LM, Franks A, Jr. Scleredema and diabetic sclerodactyly. Dermatol Online J. 2005;11(4):3.
pubmed - Yosipovitch G, Loh KC, Hock OB. Medical pearl: Scleroderma-like skin changes in patients with diabetes mellitus. J Am Acad Dermatol. 2003;49(1):109-111.
doi pubmed - Verrecchia F, Laboureau J, Verola O, Roos N, Porcher R, Bruneval P, Ertault M, et al. Skin involvement in scleroderma - where histological and clinical scores meet. Rheumatology (Oxford). 2007;46(5):833-841.
doi pubmed - Cappelli L, Wigley FM. Management of Raynaud Phenomenon and Digital Ulcers in Scleroderma. Rheum Dis Clin North Am. 2015;41(3):419-438.
doi pubmed - Wigley FM, Flavahan NA. Raynaud's Phenomenon. N Engl J Med. 2016;375(6):556-565.
doi pubmed - Fabri M, Hunzelmann N. Differential diagnosis of scleroderma and pseudoscleroderma. J Dtsch Dermatol Ges. 2007;5(11):977-984.
doi pubmed - Jacobsen S, Halberg P, Ullman S, Van Venrooij WJ, Hoier-Madsen M, Wiik A, Petersen J. Clinical features and serum antinuclear antibodies in 230 Danish patients with systemic sclerosis. Br J Rheumatol. 1998;37(1):39-45.
doi pubmed - Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, Gordon T, et al. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum. 1997;40(9):1601-1611.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
