| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 6, Number 6, December 2016, pages 183-186
Teriparatide Prevented Near Atypical Femur Fracture by Reducing Cortical Thickening in a Symptomatic Patient With Prolonged Use of Oral Bisphosphonate: A Case Report
Jaspinder Kaura, b, Luis Chaveza, Issac Sachmechia, Paul Kima
aDepartment of Internal Medicine, Icahn School of Medicine at Mount Sinai, Queens Hospital Center, Jamaica, NY 11432, USA
bCorresponding Author: Jaspinder Kaur, Department of Internal Medicine, Icahn School of Medicine at Mount Sinai, Queens Hospital Center, Jamaica, NY 11432, USA
Manuscript accepted for publication November 10, 2016
Short title: Teriparatide-Induced Reduction in Cortical Thickening
doi: https://doi.org/10.14740/jem382w
| Abstract | ▴Top |
Long-term bisphosphonate (BP) treatment is associated with adverse event of atypical femur fracture (AFF) due to their long-lasting unique bone-seeking properties, and subsequent uptake by osteoclasts results in sustained inhibition of bone resorption even after their cessation. We report a case of postmenopausal women who presented with pain and severe femoral cortical thickening on alendronate for 10 years, but with no signs of obvious radiolucent fracture line on radiograph and bone marrow edema on magnetic resonance imaging representing impending AFF. Teriparatide (TPP) was started after discontinuation of BP which showed resolution of pain and reduction of femoral cortical thickness. We recommend biannual radiographs follow-up for evaluation of cortical thickening in patients taking BP along with the review of bone mineral density, and consideration of treatment with TPP if cortical thickening is present.
Keywords: Atypical femur fracture; Bisphosphonate; Cortical thickening; Osteoporosis; Teriparatide
| Introduction | ▴Top |
Bisphosphonates (BPs) are antiresorptive agents that inhibit the osteoclastic bone destruction and suppress the bone volume loss in osteoporosis. However, the prolonged BP use may lead to oversuppression of bone remodeling, impaired ability to repair skeletal microcracks, and increased skeletal fragility which can lead to atypical femoral fractures (AFFs) [1]. These fractures first appear as focal or diffuse radiographic cortical thickening in the lateral cortex of the subtrochanteric or femoral shaft region. As the fracture propagates, a radiolucent fracture line as an incomplete AFF may appear and extend medially, ultimately displacing and becoming a complete AFF. FDA suggested to discontinue BP after 3 - 5 years of use among low risk patients (e.g., younger patients without a fracture history and with bone mineral density approaching normal), while patients at increased risk for fractures (e.g., older patients with a history of fractures and bone density remaining in osteoporotic range) may benefit from continued BP therapy [2].
| Case Report | ▴Top |
A 70-year-old postmenopausal Guyanese female with past medical history of type 2 diabetes mellitus and hypertension was diagnosed with near osteoporosis corresponding to vertebral and femoral neck T scores of -2.4 SD (0.896 g/cm2) and -1.6 SD (0.755 g/cm2), respectively. She was given 70 mg weekly of alendronate and Oscal 500/200-D3 twice a day for 10 years; however, she presented with progressive right thigh and low back pain with difficulty in ambulation. She denies any history of low energy fall, previous pathological fracture, corticosteroids and postmenopausal estrogen use. She never smoked cigarettes or drank alcohol excessively. On physical exam, no muscle weakness and point tenderness was noticed. Her laboratory tests showed normal serum calcium 9.3 mg/dL (8.5 - 10.5), serum alkaline phosphate 81 units/L (30 - 115), thyroid-stimulating hormone 1.11 mIU/mL (0.34 - 5.6), serum protein electrophoresis with normal distribution pattern, and 25 hydroxy vitamin D levels 40 ng/mL (30 - 100). A dual energy X-ray absorptiometry bone mineral density (BMD) confirmed the presence of vertebral osteoporosis (0.905 g/cm2; T-score: -2.5 SD) and worsening of femoral neck T score (-2.0; 0.763 g/cm2). The radiograph of symptomatic right femur revealed severe/diffuse cortical thickening of the lateral aspect of the mid shaft with no obvious radiolucent fracture line. Magnetic resonance imaging (MRI) of right lower extremity was ordered to look further for impending underlying fracture. It showed focal cortical thickening along lateral aspect of right femur with no obvious periosteal or bone marrow edema indicating impending AFF. Hence, a diagnosis of near AFF state with focal cortical thickening and no other signs of AFF secondary to severe alendronate suppression of bone turnover was made.
BP was discontinued, and she was started on once daily subcutaneous injection of TPP (Forteo®, 600 μg/2.4 mL). She was followed every 3 months with femur radiographs. During treatment course, her right thigh and low back pain gradually subsided. In addition, repeat BMD of her lumbar vertebrae revealed significant improvement with T score of -0.4 (1.128 g/cm2), and femoral neck showed stable T score (-2.1; 0.749 g/cm2) (Table 1). Radiograph of her symptomatic right femur revealed reduction in cortical thickening from 12.2 to 11.3 mm after a full 2-year course of TPP (Fig. 1).
 Click to view | Table 1. Timeline Measurement of Vertebral and Femoral Neck Bone Mineral Density (BMD) and T Score |
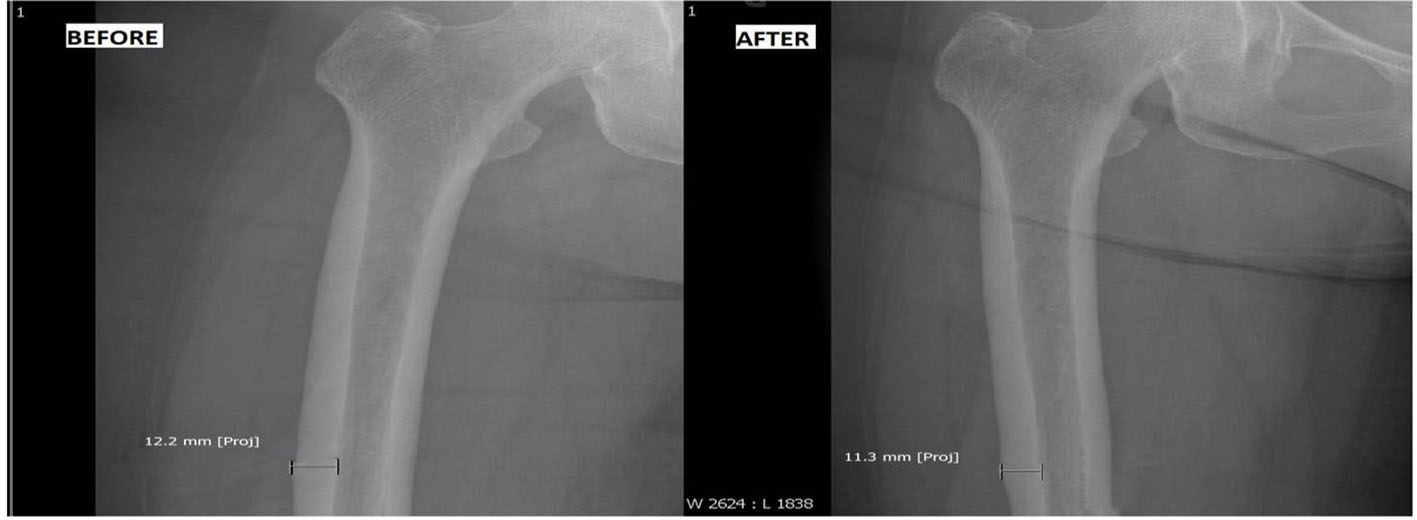 Click for large image | Figure 1. Before: cortical thickening of 12.2 mm before teriparatide use; after: reduction of cortical thickening to 11.3 mm after teriparatide use. |
| Discussion | ▴Top |
Several cases have been reported in the literature where TPP had been used to treat incomplete or complete AFF (Table 2) [3-8]; however, none of them showed the effect of TPP on reducing the cortical thickening. We hereby presented a case of preventing near AFF with clinical symptoms; cortical thickness; no obvious radiographic radiolucent line and bone marrow edema by reducing cortical thickness with TPP use.
 Click to view | Table 2. List of the Cases Where Teriparatide (TPP) Was Used to Treat Complete or Incomplete Atypical Femur Fracture (AFF) Caused by Prolonged Use of Bisphosphonate (BP) |
The American Society for Bone and Mineral Research (ASBMR) task force has defined the major and minor features of the AFF and has clarified the features that distinguish AFFs from ordinary osteoporotic femur fractures in 2013 [1]. The patients developing AFF were usually female dominant and younger than the patients with typical osteoporotic femoral fractures. The median duration of use of BP therapy was 7 years. Approximately 70% of patients with AFF had a history of prodromal groin or thigh pain; 28% had bilateral fractures and bilateral radiographic abnormalities; and 26% had delayed healing [9]. It has also been reported that the risk of AFF among women increased progressively with the duration of BP use with an annual absolute risk of 11 fractures per 10,000 person-years of use. As compared with risedronate, alendronate users had a relatively higher risk for AFF, which might be related to a higher antiresorptive effect and long-lasting skeletal accumulation of alendronate with recommended doses [10]. After BP discontinuation, the risk diminished by 70% per year since the last use. Other additional risk factors include a history of a low energy fracture, the use of glucocorticoid therapy for more than 6 months, use of other antiresorptive drugs in addition to BPs (estrogen, raloxifene, and calcitonin), active rheumatoid arthritis, and a level of serum 25-hydroxyvitamin D less than 16 ng/mL [9].
TPP (rhPTH 1 - 34) is an osteoanabolic agent that stimulates the differentiation, proliferation, and activity of osteoblasts, restores the synthesis of the bone matrix, increases the life span of osteoblasts and osteocytes, and inhibits their apoptosis [11]. Cortical bone quality is a function of material properties and structural architecture. Recently, Orlov et al (2015) conducted a prospective study on 69 postmenopausal women with osteoporosis who were treated with TPP with a primary outcome on cortical thickness. They reported significant decrease in cortical thickness on TPP therapy with increase in trabecular area, thickness and number, and no decrease in estimated bone strength. However, the clinical implication of reducing cortical thickening in terms of decreasing fracture risk is not well understood and recommended further studies [12]. This might be explained by increase in cortical porosity through reopening of remodeling space with the removal of older, more mineralized bone and replacement with newer less mineralized bone during treatment with TPP.
Our patient showed a relatively greater improvement in vertebral BMD and T score than femoral heads on TPP therapy as compared to alendronate. Similarly, Keaveny et al (2007) revealed that both BP and TPP positively affect predicted vertebral strength through their effects on average BMD, but the magnitudes of the effects were quite different. TPP improves vertebral strength by altering the distribution of vertebral density through increasing the strength in the trabecular compartment, hence had a five-fold greater percentage increase in the strength/density ratio than BPs [13].
We demonstrated the reduction of cortical thickening with use of TPP therapy in a symptomatic patient having severe femoral cortical thickening which is considered one of the major features for AFF which can later lead to a complete fracture [14]. However, there is still debate about the management of how to screen lateral cortical thickening in patients on long-term BP. It has been reported that an acute/varus angle of the femoral neck and a narrow center-edge angle were also associated with the development of AFFs in long-term BP users [15]. We suggest to screen biannually for cortical thickening of femur and other potential risk factors with X-ray in a patient with prolonged use of oral BP along with the review of BMD, and consider the use of TPP therapy to prevent AFF by reversing cortical thickening.
Conclusion
To the best of our knowledge, this is the first case reported in the literature to prevent clinically symptomatic near AFF due to prolonged use of BP by TPP-induced reduction in cortical thickening. Further studies will be necessary to better understand the implications of reduced cortical thickening on complete AFF risk. We recommend biannual radiographs follow-up for evaluation of cortical thickening in patients taking BP along with the review of BMD, and consideration of treatment with TPP if cortical thickening is present.
Consent
A patient gave her written informed consent for this case report.
Financial Support
None.
Author Contributions
All the authors have equally contributed towards this case report.
Conflicts of Interest
None.
| References | ▴Top |
- Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1-23.
doi pubmed - Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis - where do we go from here? N Engl J Med. 2012;366(22):2048-2051.
doi pubmed - Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96(6):1627-1632.
doi pubmed - Carvalho NN, Voss LA, Almeida MO, Salgado CL, Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J Clin Endocrinol Metab. 2011;96(9):2675-2680.
doi pubmed - Mastaglia S, Aguilar G, Rossi E. Rapid resolution with teriparatide in delayed healing of atypical fracture associated to longterm bisphosphonate use. J Bone Miner Res. 2012;27(Suppl 1):S1.
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J. 2012;8(2):103-110.
doi pubmed - Tsuchie H, Miyakoshi N, Nishi T, Abe H, Segawa T, Shimada Y. Combined Effect of a Locking Plate and Teriparatide for Incomplete Atypical Femoral Fracture: Two Case Reports of Curved Femurs. Case Rep Orthop. 2015;2015:213614.
doi - Huang HT, Kang L, Huang PJ, Fu YC, Lin SY, Hsieh CH, Chen JC, et al. Successful teriparatide treatment of atypical fracture after long-term use of alendronate without surgical procedure in a postmenopausal woman: a case report. Menopause. 2012;19(12):1360-1363.
doi pubmed - Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use. N Engl J Med. 2010;362(19):1848-1849.
doi pubmed - Schilcher J, Koeppen V, Aspenberg P, Michaelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;371(10):974-976.
doi pubmed - Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357(9):905-916.
doi pubmed - Orlov S, Ridout R, Tile L, Kapral M, Cardew S, Werb MR, Sandler SD, et al. Effects of Teriparatide on Bone Microarchitecture in Postmenopausal Women with Osteoporosis. Presented at Endocrine Society's 97th Annual Meeting and Expo. March 5-8, 2015. San Diego.
- Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22(1):149-157.
doi pubmed - Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93(8):2948-2952.
doi pubmed - Taormina DP, Marcano AI, Karia R, Egol KA, Tejwani NC. Symptomatic atypical femoral fractures are related to underlying hip geometry. Bone. 2014;63:1-6.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
