| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 6, Number 5, October 2016, pages 154-157
The Caveats of Corticotropin Stimulation Test in Diagnosing Secondary Adrenal Insufficiency: Case Reports and Literature Review
Ekaterina Manuylovaa, Laura M. Calvia, Catherine Hastingsb, G. Edward Vatesb, Maryanne Stahlecker-Ettera, Kenneth Foxxb, Ismat Shafiqa, c
aDepartment of Endocrinology, Diabetes and Metabolism, University of Rochester, Elmwood Ave., Box 693, Rochester, NY 14642, USA
bDepartment of Neurosurgery, University of Rochester, 2180 South Clinton Ave., Rochester, NY 14618, USA
cCorresponding Author: Ismat Shafiq, Department of Endocrinology, Diabetes and Metabolism, University of Rochester, Elmwood Ave., Box 693, Rochester, NY 14642, USA
Manuscript accepted for publication August 17, 2016
Short title: CST for Secondary Adrenal Insufficiency
doi: http://dx.doi.org/10.14740/jem366w
| Abstract | ▴Top |
Corticotropin stimulation test (CST) is commonly used to diagnose secondary adrenal insufficiency. We present two patients who underwent transsphenoidal pituitary surgery for pituitary macroadenoma. Both patients had additional pituitary hormone deficiencies before and after the surgery. The patients were maintained on glucocorticoid (GC) replacement for at least 3 months after the surgery. In the remote follow-up period, they underwent conventional CST with resultant cortisol levels above 18 μg/dL. This led to discontinuation of GC treatment. Few months later, both patients developed clinically evident adrenal insufficiency. Providers should be cautious interpreting the results of CST in patients with pituitary disorders. The 250-μg CST with standard cortisol cutoff has low sensitivity and can give falsely reassuring results. Thus, it is prudent to use a higher cortisol threshold to define intact hypothalamic-pituitary-adrenal axis.
Keywords: Cortisol; Adrenal insufficiency; Corticotropin stimulation test
| Introduction | ▴Top |
Adrenal insufficiency (AI) can be primary, due to defect in the adrenal glands, or secondary, due to diminished ACTH secretion from the pituitary gland. Secondary AI is observed in the settings of a mass pressing on the normal pituitary gland, for example, pituitary macroadenoma, Rathke’s cyst, or craniopharyngiomas. It is also seen as a result of radiation treatment to the pituitary area, inflammation, or after surgical intervention in the sella turcica. Transsphenoidal pituitary surgery (TSS) is the first line treatment for most symptomatic pituitary adenomas [1]. The incidence of new AI after TSS is variable depending on the study, ranging from 0.8% to 18.5% [2-6]. The predictors of new pituitary insufficiency are the size of adenoma [3, 4] and preexisting one or more hormonal deficiency [4]. Among the dynamic procedures, corticotropin stimulation test (CST) is often used to assess hypothalamic-pituitary-adrenal (HPA) axis. Here we report two cases of a falsely reassuring standard dose CST at 3 and 6 months after surgery in patients with other pituitary hormone deficiencies.
| Case Reports | ▴Top |
Case 1
A 70-year-old man with prior history of hypertension, hyperlipidemia and coronary artery disease presented with progressive visual loss. Visual field testing demonstrated bilateral hemianopsia and optic atrophy. Magnetic resonance imaging (MRI) of the brain revealed a 4.3 cm pituitary adenoma pressing on the optic chiasm and displacing the optic nerves laterally (Fig. 1a). His preoperative random cortisol was 7.4 μg/dL, and ACTH was 33 pg/mL. He had central hypogonadism based on morning total testosterone of 107 ng/dL (193 - 740 ng/dL), SHBG of 45 nmol/L, and low LH of at 1.6 mIU/mL (1.7 - 8.6 mIU/mL). Other laboratory evaluation revealed central hypothyroidism with low free T4 of 0.7 ng/dL (0.9 - 1.7 ng/dL) and inappropriately normal TSH of 1.63 μIU/mL (0.27 - 4.2 μIU/mL). His prolactin was elevated at 19.9 ng/mL (4.0 - 15.2 ng/mL) and IGF-1 was 162 ng/mL (60 - 220 ng/mL) (Table 1).
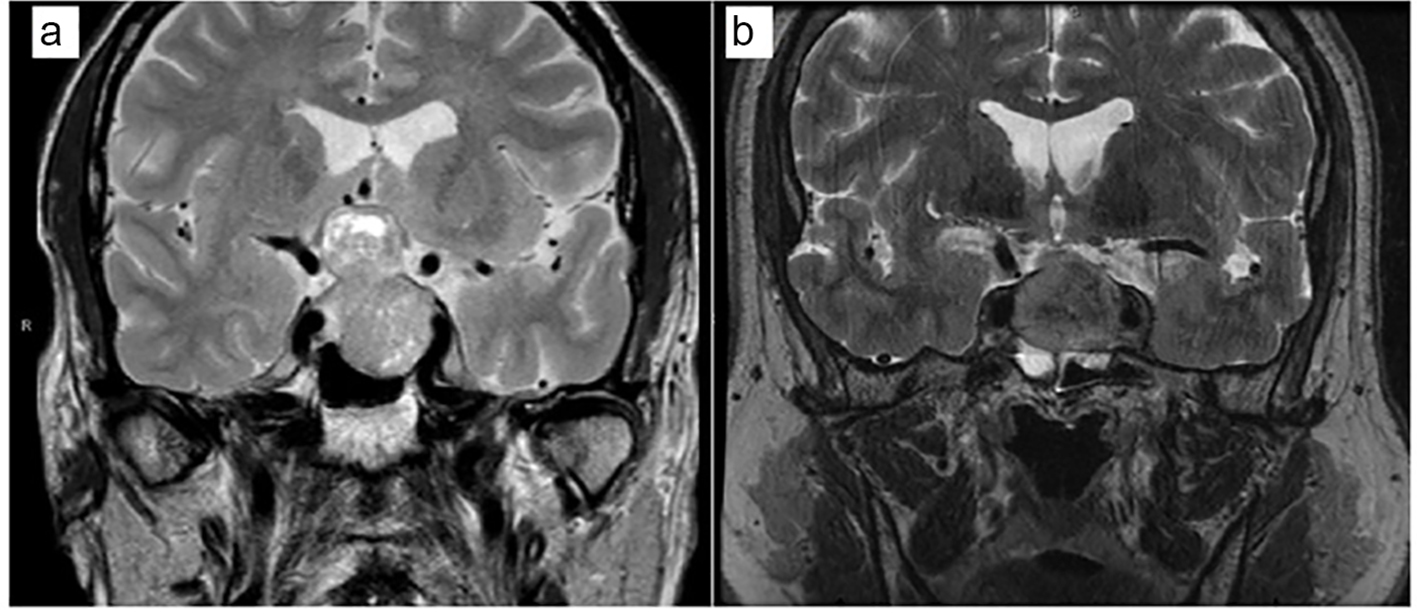 Click for large image | Figure 1. An MRI of the brain at the time of presentation. Coronal T2-weighted images. (a) Case 1. (b) Case 2. |
 Click to view | Table 1. Laboratory Findings Before and After the Surgery |
The patient underwent TSS. Pathology was consistent with non-functional pituitary adenoma. Intraoperatively, he received hydrocortisone 100 mg due to hypotension and continued on glucocorticoid (GC) upon discharge. Three months postoperatively, his thyroid function normalized (free T4 1.0 ng/dL; TSH 1.76 μIU/mL). However, he had persistent hypogonadism (morning testosterone level 32 ng/dL) requiring testosterone replacement. His morning cortisol levels ranged from 6.8 to 8.2 μg/dL after holding GC for 24 h. He underwent 250-μg CST 3 months postoperatively, which resulted in the cortisol levels rising to 18.6 and 22.8 μg/dL at 30 and 60 min, respectively. The patient was educated about symptoms of AI, and GC replacement was subsequently discontinued. Ten months later, he was re-admitted with sudden onset of nausea and altered mental status. His relatives reported progressive fatigue, shortness of breath, decreased appetite and weight loss over several weeks prior to admission. Laboratory evaluation demonstrated sodium 118 mmol/L (133 - 145 mmol/L), morning cortisol 4.9 μg/dL (6.2 - 19.4 μg/dL), TSH 1.5 μIU/mL and free T4 0.9 ng/dL. He was started on GC replacement with the quick resolution of his symptoms. Head MRI at 3 months after the pituitary surgery and at the time of presentation revealed a stable 1.1 cm residual pituitary lesion.
Case 2
A 59-year-old man with no prior history presented to an emergency department with a sudden onset of severe frontal headache, accompanied by nausea, vomiting, double vision, and photophobia. A pituitary MRI showed a 2.9 cm lesion in the sellar and suprasellar regions (Fig 1b). He underwent urgent transsphenoidal surgery; pathology showed extensive acute infarction consistent with pituitary apoplexy. His random preoperative lab work revealed TSH of 6.19 μIU/mL (0.27 - 4.2 μIU/mL), free T4 of 0.8 ng/dL (0.9 - 1.7 ng/dL), prolactin of 1.7 ng/mL (4.0 - 15.2 ng/mL), LH of 2.1 mIU/mL (1.7 - 8.6 mIU/mL), random total testosterone of 9 ng/dL (193 - 740 ng/dL), and IGF-1 of 237 ng/mL (68 - 245 ng/mL) (Table 1). Perioperatively, he received stress-dose GCs and was discharged on GC replacement. During follow-up, morning cortisol levels after holding GC for 24 h ranged from 5.0 to 8.1 μg/dL. His testosterone level remained low, but he refused testosterone treatment. In addition, he elected to discontinue GC replacement. Thus, 6 months postoperatively, a 250-μg CST was performed with cortisol levels 18.1 and 22.9 μg/dL at 30 and 60 min, respectively. He was educated about symptoms of AI, and GC replacement was discontinued. Four months later, he presented to his primary care doctor with complaints of joint pain, muscle aches, leg swelling and 20 lb weight loss. His morning cortisol level was 3.0 μg/dL (normal 4.0 - 22.0 μg/dL), morning total testosterone was 44 ng/dL (241 - 927 ng/dL), LH was 1.2 mIU/mL (1.6 - 15.2 mIU/mL), TSH was 3.97 μIU/L (0.35 - 4.94 μIU/L) and free was T4 0.54 ng/dL (0.7 - 1.48 ng/dL), altogether, indicating adrenal insufficiency, central hypothyroidism and hypogonadism. Upon GC treatment, his symptoms quickly resolved. He was also started on testosterone and thyroid replacement. An MRI scan at the time of presentation with adrenal insufficiency showed no evidence of residual or recurrent mass.
| Discussion | ▴Top |
CST is used to assess HPA axis. It is performed by intravenous or intramuscular injection of corticotropin analog with measurement of cortisol at baseline, 30 and 60 min. Synthetic corticotropin analog, tetracosactide, has 1 - 24 amino acids of the endogenous ACTH and mimics its action. CST is a test of choice for diagnosis of primary adrenal insufficiency [7]. However, for secondary adrenal insufficiency, insulin tolerance test (ITT) and overnight metyrapone suppression test are gold standards. These tests are not commonly available, thus many providers rely on CST as an alternative.
The adrenal cortex relies on the trophic effect of pulsatile ACTH secretion to maintain its integrity. In patients with secondary AI, in the absence of ACTH, the zona fasciculata atrophies within 4 - 6 weeks. This leads to impaired ability to produce cortisol in response to exogenous corticotropin [8]. Given the lag period between the onset of diminished ACTH secretion and the atrophy of the adrenal gland, CST can be falsely normal if performed early in the course of the disease. Also, in patients with partial ACTH deficiency and intact adrenal glands, corticotropin analog can lead to substantial cortisol release and resultant levels above 18 μg/dL.
Experts disagree on the dosing of corticotropin and the cortisol value cutoffs for the diagnosis of secondary AI. A conventional high dose (HD) CST refers to use of 250-μg tetracosactide, while a low dose (LD) CST refers to 1-μg tetracosactide. A peak cortisol level equal or above 18 μg/dL, at any time point, is considered an adequate response [9]. LD CST provides higher sensitivity than HD CST [10-12]. This is explained by the degree of adrenal stimulation with corticotropin. The adrenal cortex is maximally stimulated at the corticotropin levels of 70 - 80 pg/mL [13]. HD CST results in measurable plasma corticotropin levels > 13,000 pg/mL at 10 min and > 150 pg/mL at 60 min. Thus, for 1 h, the adrenal glands are maximally stimulated [14], and cortisol levels continue to rise after 30 min, with the highest at 60 min. LD CST also leads to supra-physiological plasma level of corticotropin (up to > 1,900 pg/mL), but only within 30 min after injection [14]. Therefore, cortisol levels peak at 30 min and later decrease [14]. Although LD CST appears to be more sensitive, the test has technical challenges. The 250-μg vial of corticotropin has to be diluted which can result in inaccurate dosing and a variable cortisol response.
HD CST is not very sensitive for secondary AI. In one study sensitivity of the test was only 57% [15]. Therefore, many authors suggest raising cortisol cutoff level to 20 - 22 μg/dL [16, 17] or even higher [14, 18]. At the cortisol cutoff of 20 μg/dL, the sensitivity increases to 83% [19]. Furthermore, Kazlauskaite et al calculated that the normal HPA axis is best predicted when cortisol is above 30 μg/dL at 30 min [18]. There is a strong correlation between cortisol levels among LD and HD CST at different time points [7, 14, 20, 21]. For this reason, when adjusted cortisol cutoffs are used, both tests provide equal sensitivity [14, 15, 22, 23]. Mayenknecht et al found that cortisol cutoff of 19.4 μg/dL (30 min) for LD, and 22.5 μg/dL (30 min) and 26.3 μg/dL (60 min) for HD CTS resulted in about 94% sensitivity in patients with pituitary disorders [14].
Neither LD nor HD CST reaches 100% sensitivity with acceptable specificity level. For this reason, an interpretation of CST must rely on other factors, including morning cortisol levels, other pituitary hormone deficiencies as well as clinical suspicion.
Conclusion
CST is commonly used to evaluate HPA axis. Low dose 1-μg CST is more sensitive, for central adrenal insufficiency at the cortisol cutoff of 18 μg/dL. High dose 250-μg CST has low sensitivity and can give falsely reassuring results with standard cortisol cutoff. Thus, for HD CST, a higher cortisol threshold should be considered to determine intact HPA axis, especially in the settings of other pituitary hormone deficiencies. Some authors suggest cortisol levels of 22 μg/dL at 30 min and 30 μg/dL at 60 min. In the two presented cases, raising the cortisol threshold to 22 μg/dL at 30 min for HD CST would have prevented discontinuation of GC replacement and further complications.
Acknowledgments
We thank all medical personnel at University of Rochester for the timely evaluation and treatment of the presented patients. We are grateful to Barbara Morabito for help with manuscript preparation.
Conflicts of Interest
The authors declare that they have no conflicts of interest concerning this article.
Author Contributions
EM performed the review of cases and wrote the first draft. IS, MS and LC reviewed the draft and made corrections. EV, KF, and CH participated in the patients’ care.
| References | ▴Top |
- Miller BA, Ioachimescu AG, Oyesiku NM. Contemporary indications for transsphenoidal pituitary surgery. World Neurosurg. 2014;82(6 Suppl):S147-151.
doi pubmed - Berker M, Hazer DB, Yucel T, Gurlek A, Cila A, Aldur M, Onerci M. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary. 2012;15(3):288-300.
doi pubmed - Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas - a study on 721 patients. Acta Neurochir (Wien). 2004;146(1):27-35.
doi pubmed - Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J, Wang C, et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63(4):709-718; discussion 718-709.
- Dusick JR, Fatemi N, Mattozo C, McArthur D, Cohan P, Wang C, Swerdloff RS, et al. Pituitary function after endonasal surgery for nonadenomatous parasellar tumors: Rathke's cleft cysts, craniopharyngiomas, and meningiomas. Surg Neurol. 2008;70(5):482-490; discussion 490-481.
- Gleeson HK, Walker BR, Seckl JR, Padfield PL. Ten years on: Safety of short synacthen tests in assessing adrenocorticotropin deficiency in clinical practice. J Clin Endocrinol Metab. 2003;88(5):2106-2111.
doi pubmed - Ospina NS, Al Nofal A, Bancos I, Javed A, Benkhadra K, Kapoor E, Lteif AN, et al. ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2016;101(2):427-434.
doi pubmed - Stewart PM, Corrie J, Seckl JR, Edwards CR, Padfield PL. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet. 1988;1(8596):1208-1210.
doi - Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335(16):1206-1212.
doi pubmed - Broide J, Soferman R, Kivity S, Golander A, Dickstein G, Spirer Z, Weisman Y. Low-dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. J Clin Endocrinol Metab. 1995;80(4):1243-1246.
doi - Tordjman K, Jaffe A, Grazas N, Apter C, Stern N. The role of the low dose (1 microgram) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab. 1995;80(4):1301-1305.
doi - Tordjman K, Jaffe A, Trostanetsky Y, Greenman Y, Limor R, Stern N. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin Endocrinol (Oxf). 2000;52(5):633-640.
doi - Oelkers W, Boelke T, Bahr V. Dose-response relationships between plasma adrenocorticotropin (ACTH), cortisol, aldosterone, and 18-hydroxycorticosterone after injection of ACTH-(1-39) or human corticotropin-releasing hormone in man. J Clin Endocrinol Metab. 1988;66(1):181-186.
doi pubmed - Mayenknecht J, Diederich S, Bahr V, Plockinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83(5):1558-1562.
doi pubmed - Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204.
doi pubmed - Inder WJ, Hunt PJ. Glucocorticoid replacement in pituitary surgery: guidelines for perioperative assessment and management. J Clin Endocrinol Metab. 2002;87(6):2745-2750.
doi pubmed - Agha A, Tomlinson JW, Clark PM, Holder G, Stewart PM. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2006;91(1):43-47.
doi pubmed - Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, Choi CH, et al. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245-4253.
doi pubmed - Orme SM, Peacey SR, Barth JH, Belchetz PE. Comparison of tests of stress-released cortisol secretion in pituitary disease. Clin Endocrinol (Oxf). 1996;45(2):135-140.
doi - Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):151-156.
doi - Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987.
doi - Suliman AM, Smith TP, Labib M, Fiad TM, McKenna TJ. The low-dose ACTH test does not provide a useful assessment of the hypothalamic-pituitary-adrenal axis in secondary adrenal insufficiency. Clin Endocrinol (Oxf). 2002;56(4):533-539.
doi - Courtney CH, McAllister AS, Bell PM, McCance DR, Leslie H, Sheridan B, Atkinson AB. Low- and standard-dose corticotropin and insulin hypoglycemia testing in the assessment of hypothalamic-pituitary-adrenal function after pituitary surgery. J Clin Endocrinol Metab. 2004;89(4):1712-1717.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
