| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 5, Number 4, August 2015, pages 267-271
18F-Choline PET/CT as a New Tool for Functional Imaging of Non-Proliferating Secreting Neuroendocrine Tumors
Bernies van der Hiela, d, Marcel P. M. Stokkelb, Wieneke A. Buikhuisenb, Hans Janssenc, Marie-Louise F. van Velthuysenc, Robert J. Rhodiusb, Wouter V. Vogela
aDepartment of Nuclear Medicine, the Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital (NKI-AVL), Amsterdam, the Netherlands
bDepartment of Thoracic Oncology, the Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital (NKI-AVL), Amsterdam, the Netherlands
cDepartment of Pathology, the Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital (NKI-AVL), Amsterdam, the Netherlands
dCorresponding Author: Bernies van der Hiel, Department of Nuclear Medicine, the Netherlands Cancer Institute, Antoni van Leeuwenhoek Hospital (NKI-AVL), Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands
Manuscript accepted for publication August 14, 2015
Short title: 18F-Choline PET/CT for Secreting Tumors
doi: http://dx.doi.org/10.14740/jem300w
| Abstract | ▴Top |
Choline is an essential component for the formation of new cell membrane and the current understanding is that increased choline uptake in cancer lesions is explained by - and roughly correlates with - cellular proliferation.18F-fluoromethylcholine, a radiolabeled PET-tracer, is increasingly used for the detection of proliferating cancers with PET/CT (choline PET), for example for restaging of recurrent prostate carcinoma. However, clinical findings have suggested that choline uptake may not always be related to proliferation. We present three cases with a carcinoid of the lung with high uptake of radiolabeled choline on PET/CT and a low Ki-67 proliferation index as demonstrated by immunostaining. Although the mechanism behind the enhanced uptake in well-differentiated neuroendocrine tumors is not yet fully understood, for clinical practice this finding means that not every highly choline-avid lesion represents an aggressive cancer; the diagnosis of a functional neuroendocrine tumor should be considered as well.
Keywords: Neuroendocrine tumor; Tumor proliferation; Cell secretion; 18F-fluoromethylcholine; PET/CT
| Introduction | ▴Top |
Molecular imaging with 18F-fluoromethylcholine PET/CT (choline PET) is increasingly used for the detection and (re-)staging of proliferating cancers, most commonly prostate cancer. Choline is an essential component for the formation of new cell membrane components and the current understanding is that malignancy-induced upregulation of choline kinase leads to the incorporation and trapping of choline in the tumor cell membrane. Therefore, increased choline uptake in cancer lesions is explained by - and roughly correlates with - cellular proliferation [1]. However, we present the histopathological analysis of a choline-positive secreting differentiated neuroendocrine tumor in three patients with very low proliferation rate, proving that high choline uptake in tumors can also be induced by other biological processes. This finding contributes to better understanding of the tumor biology behind the recently demonstrated applicability of choline PET for imaging of benign secreting tumors, such as parathyroid adenomas [2, 3].
| Case Reports | ▴Top |
Case 1
A 70-year-old man was referred to our hospital for a cT2bNxM0 Gleason 4 + 4 = 8 adenocarcinoma of the prostate, with an initial PSA of 24.9 ng/mL. He underwent a laparoscopic radical prostatectomy combined with a lymph node dissection. Histopathological evaluation revealed a pT3bN1Mx Gleason 10 adenocarcinoma of the prostate, with metastases in one of seven resected lymph nodes. The patient received additional radiotherapy of the prostate bed. PSA responded with a decline to 0.9 ng/mL, but increased to 1.2 ng/mL 7 weeks later. Progressive disease was suspected and a choline PET was ordered to restage the patient. The PET images showed no signs of local recurrence but revealed three choline-avid parailiac lymph nodes suspicious for lymph node metastases. The largest node is indicated in Figure 1 with a green arrow.
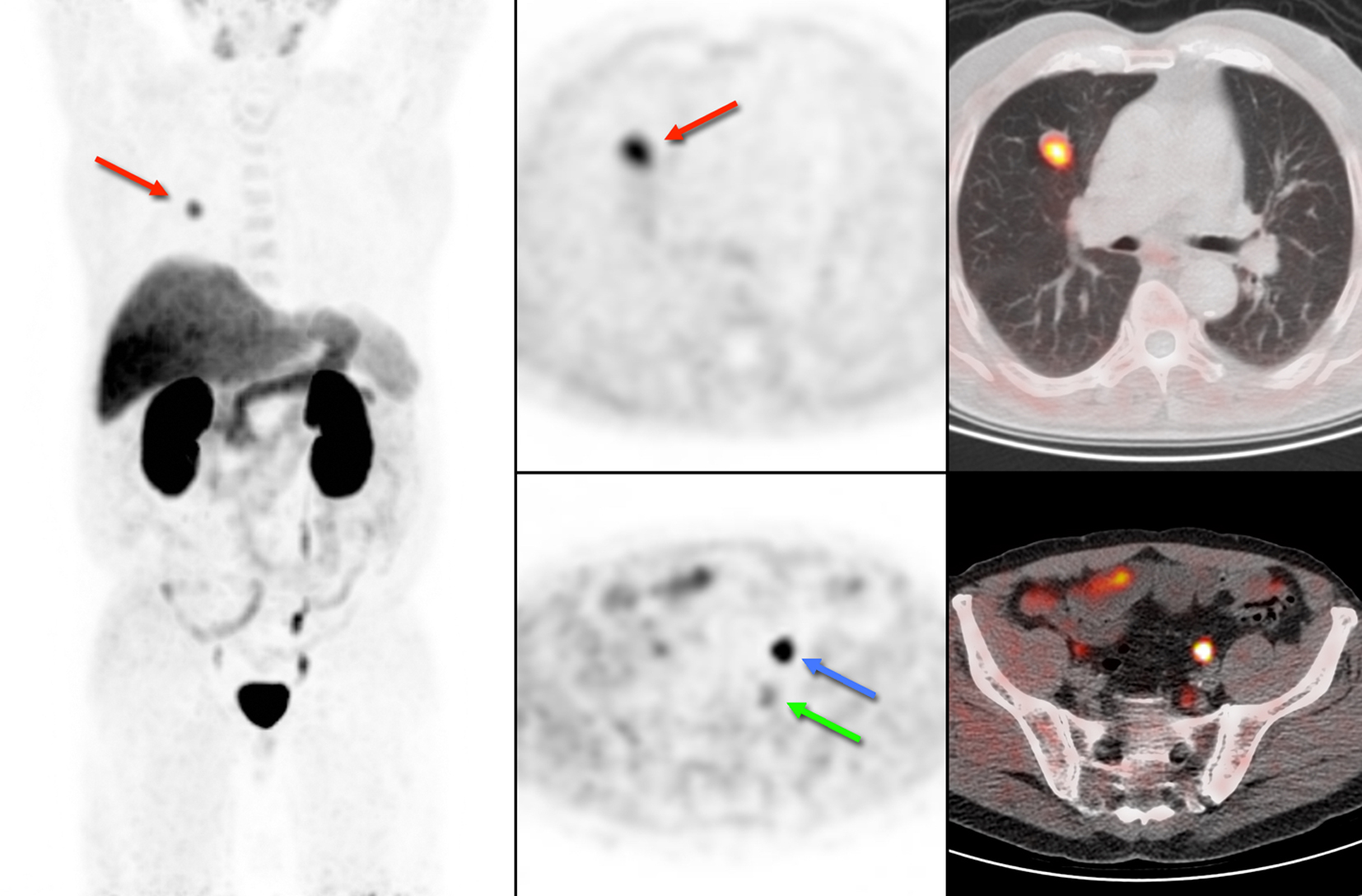 Click for large image | Figure 1. 18F-choline PET/CT of case 1. Physiological uptake of 18F-choline is seen in the salivary glands, liver, spleen and kidneys. The blue arrow shows excretion of radioactive urine in the left ureter and the green arrow indicates pathological uptake in a lymph node. In addition, we encountered an unexpected lung lesion in the right upper lobe with high uptake of 18F-choline (red arrow). |
In addition, we encountered an unexpected lesion in the upper lobe of the right lung, with a maximum diameter of 2 cm on low-dose CT and intense uptake of 18F-choline (Fig. 1, red arrow). Based on the high choline metabolism a highly proliferative malignant process was suspected. However, fine needle aspiration of the pulmonary nodule revealed well-differentiated tumor cells of neuroendocrine origin. There were no signs of metastases in regional lymph nodes, and the patient underwent resection of the right upper lobe. Histopathological examination of the resected specimen confirmed the diagnosis of a typical carcinoid (Fig. 2A), with good vascularization, no necrosis, and hardly any mitotic activity (< 2 per 2 mm2) or Ki-67 immunostaining (< 1%, Fig. 2B). Immunostaining for chromogranin, a general marker for neuroendocrine tumors, was 100%.
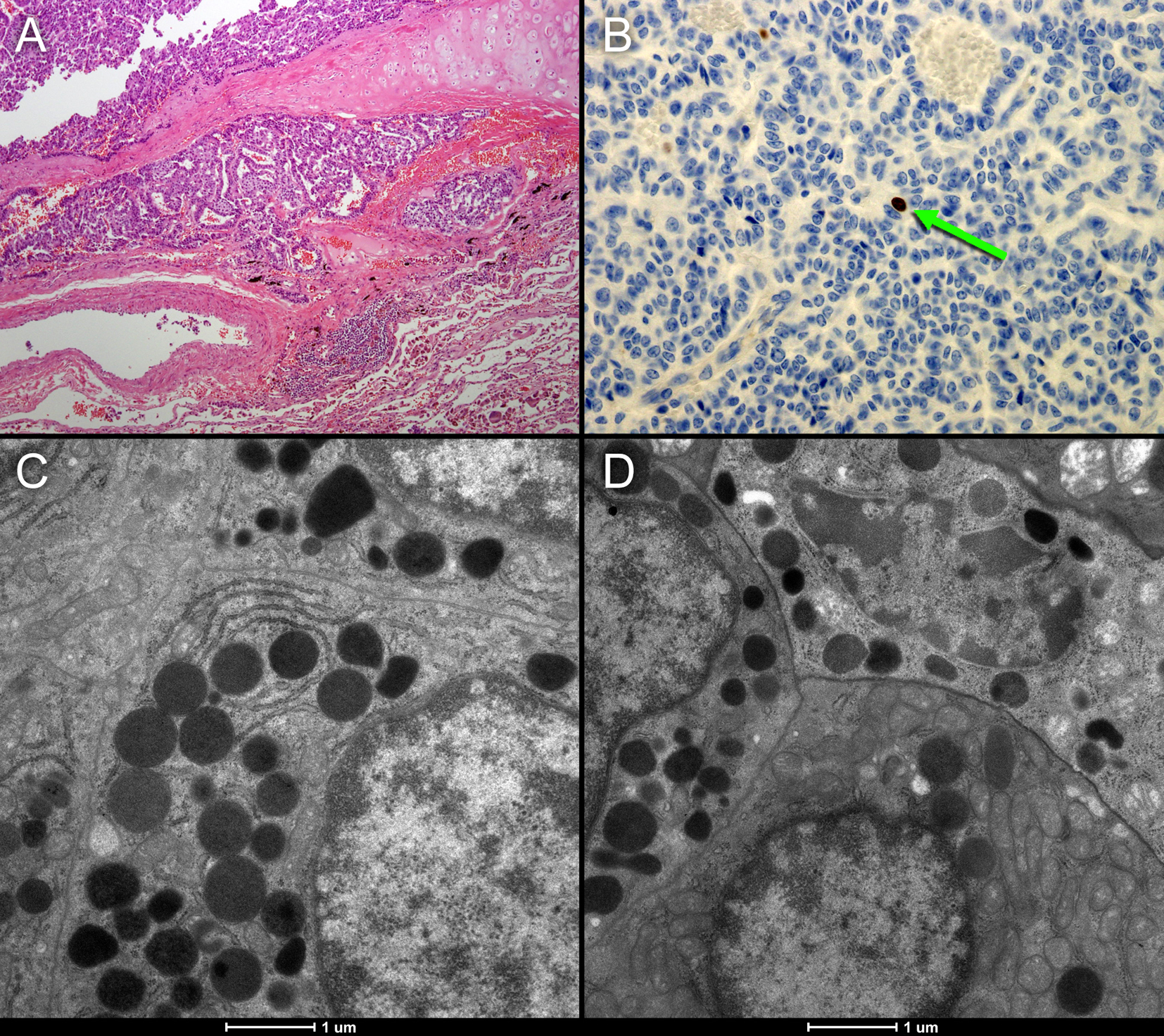 Click for large image | Figure 2. (A, B) Histopathological sample of the resected neuroendocrine tumor of case 1. The tumor shows good vascularization and hardly any necrosis (A). MIB1 immunostaining representing Ki-69 mitotic activity (B: brown colored cells) shows that there is hardly any mitotic activity (< 2 per 2 mm2). The electron microscope images (C and D) of the neuroendocrine tumor show numerous voluminous electron dense vesicles in the cytoplasm (black circles). |
Case 2
A 73-year-old man presented with a 3 cm tumor in the right lung and biopsy revealed a typical carcinoid. Subsequently, the patient was referred for 68Gallium-DOTATATE (68Ga-DOTATATE) PET revealing intense uptake in the tumor, but no metastasis (Fig. 3a). Two weeks after this scan, choline PET was performed to assess whether this tumor also demonstrated increased uptake as found in case 1. The scan revealed comparable intense uptake in the primary tumor and also no metastasis (Fig. 3b). Excision of the lesion revealed an atypical carcinoid, with good vascularization, no necrosis and mitotic activity of 6 per 2 mm2. Ki-67 was 1-5%, and chromogranin staining was 100%.
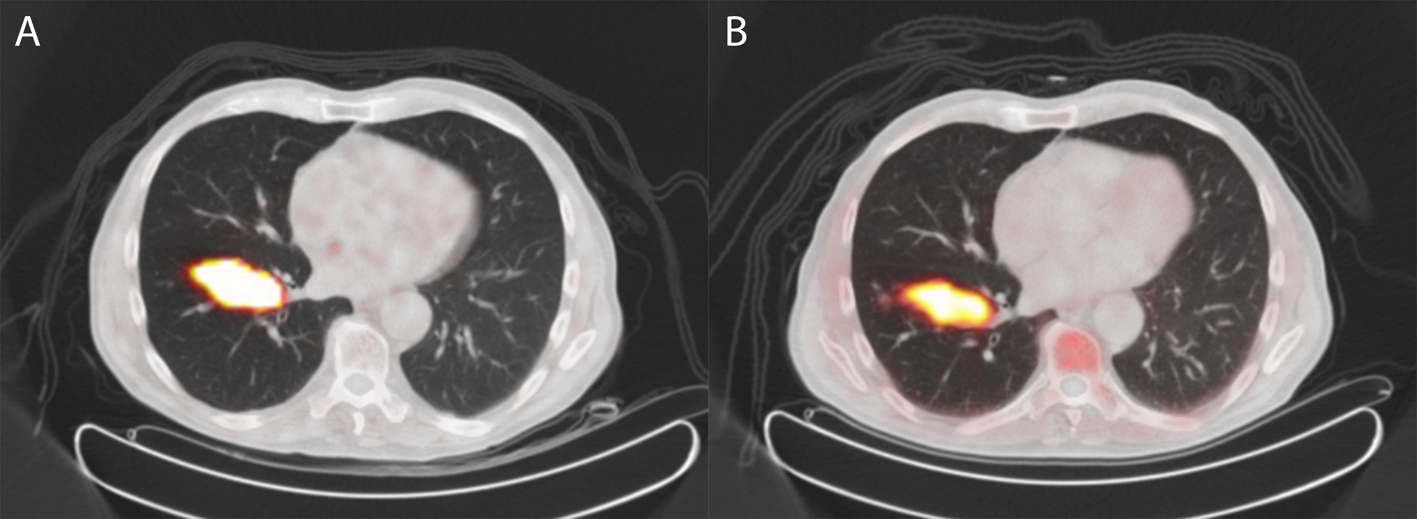 Click for large image | Figure 3. 68Gallium-DOTATATE PET/CT (A) and 18F-choline PET/CT (B) of a patient with an atypical carcinoid of the lung (case 2). The tumor shows increased somatostatin receptor expression on 68Gallium-DOTATATE PET/CT but also intense uptake of 18F-choline on 18F-choline PET/CT. |
Case 3
A 72-year-old man was referred for 68Ga-DOTATATE PET because of a high suspicion on a carcinoid in the right lower lobe revealing intense uptake in the lesion (Fig. 4a). Based on this finding and the results of previous patients, a choline PET was performed demonstrating increased uptake in this lung lesion, but less intense as observed in the 68Ga-DOTATATE PET (Fig. 4b). Histological examination after resection revealed an atypical carcinoid with a diameter of 2.5 cm, focal necrosis and mitotic activity of 2 per 2 mm2. Ki-67 was 10%, and chromogranin staining was 100%.
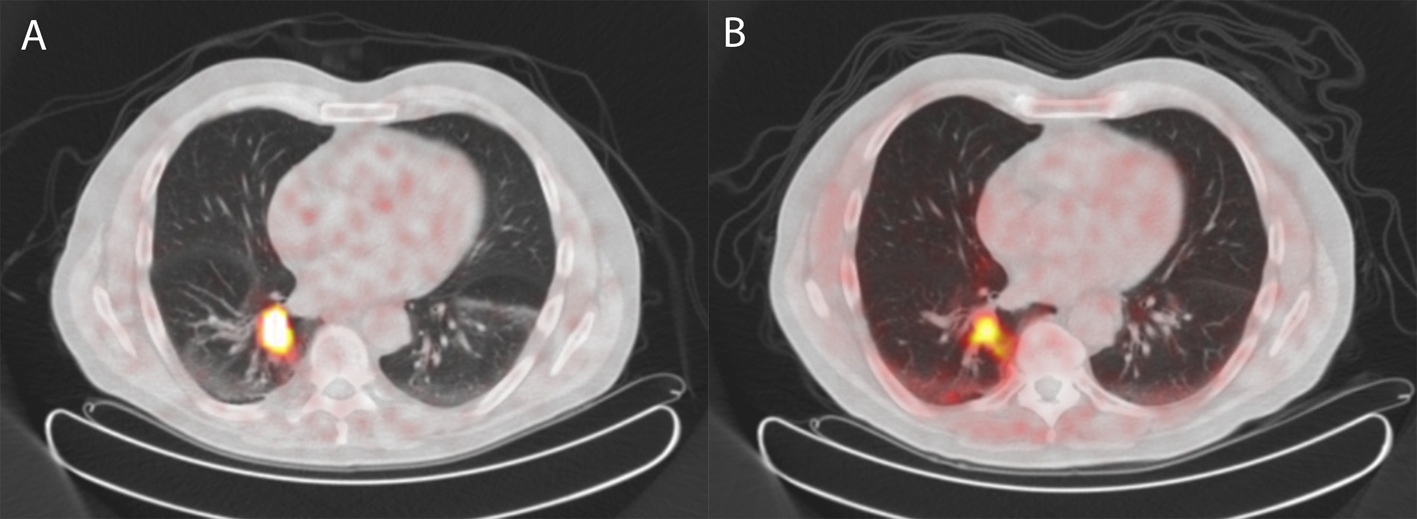 Click for large image | Figure 4. 68Gallium-DOTATATE PET/CT (A) and 18F-choline PET/CT (B) of a patient with an atypical carcinoid of the lung (case 3) revealing similar uptake of 68Gallium-DOTATATE as well as 18F-choline as compared to case 2. |
In these three cases, there was a strong discrepancy between the imaging and pathology parameters of the lung carcinoids; the high uptake of radiolabeled choline on PET/CT could not be explained by proliferation as demonstrated by the mitotic activity and supported by Ki-67 immunostaining. This led to the conclusion that another biological process can also contribute to upregulation of the choline pathway.
| Discussion | ▴Top |
Bronchopulmonary carcinoids arise from bronchial mucosal cells known as enterochromaffin cells or Kulchitsky cells, which are part of the diffuse neuroendocrine system (DNES). These well-differentiated neuroendocrine tumors account for only 0.4-3% of all lung cancers, but for approximately 25% of all carcinoids [4, 5]. Carcinoid tumors are classified by pathological features as typical carcinoid or atypical carcinoid, depending on the amount of mitosis/2 mm2 of viable tumor and the presence of necrosis or architectural disruption.
In clinical practice, computed tomography (CT) is used to identify and stage carcinoid tumors of the lung. Well-defined, centrally located tumors involving the airway with calcification, punctate or diffuse, are considered characteristic on CT [6]. Additional investigation and staging with 18F-fluoro-deoxyglucose (FDG) PET scanning is described in literature, but remains controversial. Due to the generally low metabolic activity and limited proliferation rate of carcinoid tumors, FDG uptake in PET scanning often demonstrates low uptake (equivocal in comparison to mediastinal uptake) [7-9]. In general, well-differentiated neuroendocrine tumors demonstrate high expression of somatostatin receptors on their cell surface. In this respect, non-invasive tumor characterization and staging with radiolabeled somatostatin analogs, such as 111Indium-octreotide or 68Gallium-DOTATATE, have shown good results. Therefore, these tracers are regarded as first choice for imaging in grade I and II tumors [10, 11].
Choline is a precursor for the biosynthesis of phospholipids, amongst others phosphatidylcholine (PtdCho), which are major components of the cell membrane [12]. After intracellular uptake, phosphorylation by choline-kinase is the first step in the biosynthesis of choline phospholipids such as PtdCho. Especially PtdCho is required for the build-up and maintenance of cell membranes. As a result, increased cell proliferation causes an increase of choline uptake, and this is the corner stone for PET scanning using radiolabeled choline in prostate cancer [13, 14]. So far, data in literature have shown promising results in restaging prostate cancer patients, and it was shown that 18F-choline PET yielded better results than 18F-FDG PET [15, 16].
Because of the very low proliferation rate of neuroendocrine tumors, the uptake of choline in present cases is probably not explained by an increased production of PtdCho. Other mechanisms may therefore be the explanation for the finding in these patients. It is known that choline is also involved in other metabolic pathways. For example in lung tissue, choline is an important precursor in the synthesis of surfactant, which is produced in alveolar cells, and for the production of acetylcholine (ACh) [17].
In a recent review, choline metabolite concentrations and enzyme expression in different human cancers were presented, demonstrating an increased choline-transport and choline-kinase (CK) activity in many of them [1]. In lung cancer, not only an increased CK activity was observed, but also an increased expression of choline transporters. The high-affinity choline transporter (CHT)1 which is expressed in neurons and is required for the synthesis of ACh, is not expressed by some lung tumors, amongst others small cell lung carcinoma (SCLC). Instead, these tumors express choline-transporter-like protein 1 (CTL1), by which the uptake and metabolism of choline is increased [18]. Whether this is also applicable to all lung neuroendocrine tumors is not clear and has to be elucidated.
Lung cancer cells create a cholinergic autocrine loop by syntheses and secretion of ACh and it responds to endogenous ACh. This loop has been best characterized in SCLC, a type of neuroendocrine lung tumor [19]. In cholinergic neurons, the neurotransmitter ACh is synthesized from choline and acetyl-CoA by choline acetyltransferase (ChAT) and is then translocated into synaptic vesicles by vesicular ACh transporter (VAChT). In contrast, the role of VAChT in lung cancer cells is unclear and the secretion of ACh is less tightly regulated and not necessarily vesicular [20]. In our first case, the electron microscope images of the neuroendocrine tumor in Figure 2 show numerous voluminous electron dense vesicles in the cytoplasm (C and D: black circles). Furthermore, active reticulo endoplasmic system (RES) organelles are present. These findings of neuroendocrine activity may indicate an overexpression of the cholinergic autocrine loop with a high turnover of intracellular vesicles probably requiring significant quantities of cell membrane components including choline. This may be a possible alternative mechanism of high choline uptake, not related to proliferation. These findings may lead to further investigation of new molecular imaging purposes for choline-PET.
ACh interacts with muscarinic and nicotinic ACh receptors. Data in literature have shown that choline may also interact directly as an agonist with the nicotinic acetylcholine receptors, and, consequently, 18F-choline PET scanning may be feasible to visualize increased expression of these receptors in non-proliferating tissues [21, 22].
These considerations also apply to functional imaging of benign secreting tissues that do not proliferate. After several incidental discoveries of functioning parathyroid gland adenomas [23-25], choline PET has been advocated as a new tool for the detection and localization of these hyperfunctioning lesions [2, 3, 26]. However, the biological processes behind the uptake of choline in parathyroid adenomas have not been evaluated or described. Based on the described theoretical considerations and the histopathological findings in our cases with NET, we assume that choline uptake in benign secreting tumors is also not related to proliferation but might be based on upregulation of the cholinergic autocrine loop, increased turnover of intracellular vesicles, and a corresponding increased expression of choline transporters. This hypothesis needs to be evaluated in further research.
Conclusion
Although the mechanism behind the enhanced uptake in well-differentiated, non-proliferating and secreting tumors is not yet fully understood, we propose that choline uptake might be related to an upregulation of the cholinergic autocrine loop and a corresponding increased expression of choline transporters. For clinical practice, this means that not every highly choline-avid lesion represents an aggressive cancer; the diagnosis of a functional neuroendocrine tumor however should be considered as well. These findings support attempts to apply 18F-choline PET for functional imaging of parathyroid adenomas and other benign non-proliferating secreting tissues.
Conflict of Interest
The authors declare that they have no conflict of interest.
| References | ▴Top |
- Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11(12):835-848.
doi - Michaud L, Burgess A, Huchet V, Lefevre M, Tassart M, Ohnona J, Kerrou K, et al. Is 18F-fluorocholine-positron emission tomography/computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J Clin Endocrinol Metab. 2014;99(12):4531-4536.
doi pubmed - Orevi M, Freedman N, Mishani E, Bocher M, Jacobson O, Krausz Y. Localization of parathyroid adenoma by (1)(1)C-choline PET/CT: preliminary results. Clin Nucl Med. 2014;39(12):1033-1038.
doi pubmed - Oberg K, Hellman P, Ferolla P, Papotti M. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii120-123.
doi - Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg. 2010;89(3):998-1005.
doi pubmed - Jeung MY, Gasser B, Gangi A, Charneau D, Ducroq X, Kessler R, Quoix E, et al. Bronchial carcinoid tumors of the thorax: spectrum of radiologic findings. Radiographics. 2002;22(2):351-365.
doi pubmed - Erasmus JJ, McAdams HP, Patz EF, Jr., Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol. 1998;170(5):1369-1373.
doi pubmed - Kruger S, Buck AK, Blumstein NM, Pauls S, Schelzig H, Kropf C, Schumann C, et al. Use of integrated FDG PET/CT imaging in pulmonary carcinoid tumours. J Intern Med. 2006;260(6):545-550.
doi pubmed - Daniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest. 2007;131(1):255-260.
doi pubmed - Herrmann K, Czernin J, Wolin EM, Gupta P, Barrio M, Gutierrez A, Schiepers C, et al. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: the referring physician's perspective. J Nucl Med. 2015;56(1):70-75.
doi pubmed - Darr R, Zophel K, Eisenhofer G, Abolmaali N, Gastmeier J, Wieczorek K, Jelinek V, et al. Combined use of 68Ga-DOTATATE and 18F-FDG PET/CT to localize a bronchial carcinoid associated with ectopic ACTH syndrome. J Clin Endocrinol Metab. 2012;97(7):2207-2208.
doi pubmed - Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood). 2006;231(5):490-504.
- Bhakoo KK, Williams SR, Florian CL, Land H, Noble MD. Immortalization and transformation are associated with specific alterations in choline metabolism. Cancer Res. 1996;56(20):4630-4635.
pubmed - Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Suda Y, Sekine T, Morishita Y. Increased choline kinase activity and elevated phosphocholine levels in human colon cancer. Jpn J Cancer Res. 1999;90(4):419-424.
doi pubmed - Leyton J, Smith G, Zhao Y, Perumal M, Nguyen QD, Robins E, Arstad E, et al. [18F]fluoromethyl-[1,2-2H4]-choline: a novel radiotracer for imaging choline metabolism in tumors by positron emission tomography. Cancer Res. 2009;69(19):7721-7728.
doi pubmed - Bauman G, Belhocine T, Kovacs M, Ward A, Beheshti M, Rachinsky I. 18F-fluorocholine for prostate cancer imaging: a systematic review of the literature. Prostate Cancer Prostatic Dis. 2012;15(1):45-55.
doi pubmed - Wessler IK, Kirkpatrick CJ. The Non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001;14(6):423-434.
doi pubmed - Wang T, Li J, Chen F, Zhao Y, He X, Wan D, Gu J. Choline transporters in human lung adenocarcinoma: expression and functional implications. Acta Biochim Biophys Sin (Shanghai). 2007;39(9):668-674.
doi - Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63(1):214-221.
pubmed - Song P, Spindel ER. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J Pharmacol Sci. 2008;106(2):180-185.
doi pubmed - Gahring LC, Vasquez-Opazo GA, Rogers SW. Choline promotes nicotinic receptor alpha4 + beta2 up-regulation. J Biol Chem. 2010;285(26):19793-19801.
doi pubmed - Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743.
doi pubmed - Mapelli P, Busnardo E, Magnani P, Freschi M, Picchio M, Gianolli L, Messa C. Incidental finding of parathyroid adenoma with 11C-choline PET/CT. Clin Nucl Med. 2012;37(6):593-595.
doi pubmed - Quak E, Lheureux S, Reznik Y, Bardet S, Aide N. F18-choline, a novel PET tracer for parathyroid adenoma? J Clin Endocrinol Metab. 2013;98(8):3111-3112.
doi pubmed - Cazaentre T, Clivaz F, Triponez F. False-positive result in 18F-fluorocholine PET/CT due to incidental and ectopic parathyroid hyperplasia. Clin Nucl Med. 2014;39(6):e328-330.
doi pubmed - van Raalte DH, Vlot MC, Zwijnenburg A, ten Kate RW. F18-Choline PET/CT: a novel tool to localize parathyroid adenoma? Clin Endocrinol (Oxf). 2015;82(6):910-912.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
