| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 5, Number 1-2, April 2015, pages 163-171
A Six-Week Home Exercise Program Improves Endothelial Function and CD34+ Circulating Progenitor Cells in Patients With Pre-Diabetes
Sabyasachi Sena, d, Sarah Witkowskib, Ann Lagoyc, Ashequl M. Islamc
aGeorge Washington University, Washington, DC, USA
bUniversity of Massachusetts, Amherst, MA, USA
cBaystate Medical Center, Springfield, MA, USA
dCorresponding Author: Sabyasachi Sen, George Washington University, School of Medicine and Health Sciences, Ross Hall, Suite 462, 2300 I Street, NW, Washington, DC 20037, USA
Manuscript accepted for publication April 13, 2015
Short title: Exercise Improves Endothelial Function
doi: http://dx.doi.org/10.14740/jem273w
| Abstract | ▴Top |
Background: Pre-diabetes is associated with endothelial dysfunction and affects endothelium-associated stem cells. Lifestyle modification has been shown to prevent the progression from pre-diabetes to overt type 2 diabetes; however, the effect of such interventions on CD34+ progenitor cells in pre-diabetes participants has not been tested. The purpose of this study was to determine whether a home-based 6-week exercise intervention improved vascular function, circulating number, function, and gene expression of circulating CD34+ progenitor cells in patients with pre-diabetes.
Methods: Patients (40 - 70 years, BMI of 25 - 39.9, n = 11) were enrolled in a 16-week randomized crossover study that consisted of 6 weeks each of an exercise (150 minutes/week) and non-exercise phase with a 4-week washout between phases. Adherence to the exercise regimen was monitored by accelerometry. All participants were encouraged to follow a low fat/low calorie diet throughout the 16 weeks. Endothelial function was assessed via brachial artery flow mediated dilatation (FMD) and CFU-Hill colony formation. CD34+ cell number, migratory function, gene expression, and serum inflammatory markers were evaluated.
Results: With the intervention, endothelial function improved (FMD, 5.7±0.6% to 11.2±0.9%, CFU-Hill, 3.3±0.2% to 8.2±0.6%, both P < 0.05). There was a significant reduction in cholesterol, triglyceride, insulin, leptin, interleukin-6 (IL-6), low density lipoprotein (LDL), apolipoprotein B (ApoB) and increase in apolipoprotein A1 (ApoA1) with the lifestyle intervention (all P < 0.05). An increase in circulating CD34+ cells (P < 0.005), decrease in CD34+ endothelin-1, IL-6, tumor necrosis factor (TNF)-α, p53, and p21 gene expression (all P < 0.05), and improved migration toward stromal cell derived factor (SDF)-1α occurred with the intervention.
Conclusion: The improvements in endothelial function and CD34+ circulating progenitor cells in patients with pre-diabetes indicate that pre-diabetes may be a clinical window of therapeutic opportunity for lifestyle interventions.
Keywords: Circulating angiogenic cells; Pre-diabetes; Endothelial function; Exercise
| Introduction | ▴Top |
Diabetes affects more than 11% of US adults and is projected to nearly double by 2025 [1]. Many patients with pre-diabetes are either overweight or obese. Both diabetes and obesity are associated with cardiovascular complications such as endothelial dysfunction, oxidative stress, endothelial cell inflammation, and cardiovascular pro-thrombotic states [2]. The presence of moderate hyperglycemia in addition to mild to moderate obesity may confer significant cardiovascular risk [3]. The American Diabetes Association reports that coronary artery disease and stroke are three times more common in pre-diabetics compared to non-diabetic patients [4]. Overt diabetes increases this risk five-fold.
Interventions at the pre-diabetes stage not only will delay or attenuate the progression of pre-diabetes to diabetes, but are also likely to reduce the cardiovascular disease (CVD) risk [2, 3]. The Diabetes Prevention Program (DPP) study [5, 6] and its 10-year follow-up [7] showed that a lifestyle intervention consisting of dietary changes and erobic exercise effectively prevented the progression of pre-diabetes to diabetes. However, the DPP study did not assess the effect of the program on endothelial health and endothelial-associated circulating progenitor stem cells in patients with pre-diabetes.
CD34+ progenitor cells are stem/progenitor cell precursors from the hematopoietic stem cell lineage [8] and are often referred to circulating progenitor cells. Given appropriate environmental conditions, CD34+ progenitors can perform beneficial angiogenic and vascular repair functions, including incorporation into the endothelium and release of growth factors and cytokines that promote repair [9, 10]. Cell sorting for CD34 positivity identifies stem like cells with endothelial/hematopoietic lineage markings [8]. While controversy over precise definitions of endothelial progenitor and other angiogenic cells has slowed progress in the field, it is generally agreed upon that CD34+ progenitor cells, whether through actions of particular endothelial lineage directed subsets or by acting as a pool for generic progenitors, perform proangiogenic actions that contribute to the maintenance of vascular endothelial integrity [11]. In addition, CD34+ cells have recently received a great deal of attention in the literature for their cell therapy applications [12].
CD34+ cells are susceptible to adverse in vivo environments related to cardiometabolic disease. Diabetes reduces the mobilization and circulating number of CD34+ cells [13]. Further, CD34+ cells isolated from patients with diabetes were unable to incorporate into damaged vessels and promote repair [14]. There is less known about CD34+ cells in pre-diabetic populations. Recently, CD34+ cells were reported to be lower in patients with pre-diabetes and were associated with hemoglobin A1C (HbA1C) [15]. However, alteration in CD34+ functional characteristics and the relationship with vascular function in this population is unknown. There is growing evidence that acute and chronic endurance exercise improves the impaired functional capacity of various circulating angiogenic cell populations in patients with CVD and risk factors [16]. In addition, 10 days of detraining resulted in significantly reduced circulating CD34+ cells in highly active older men [17]. Together, these data suggest that CD34+ circulating progenitors are influenced by physical activity. Therefore, the purpose of the current study was to evaluate the effect of a diet-controlled exercise intervention on CD34+ cells, gene expression, function, and vascular endothelial function in patients with pre-diabetes (Fig. 1).
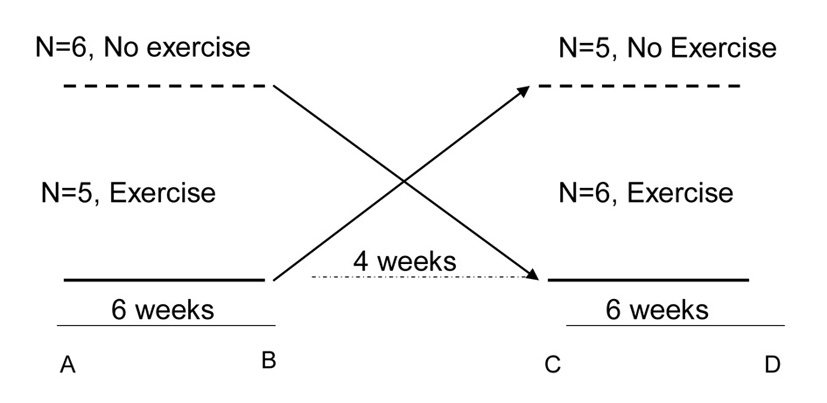 Click for large image | Figure 1. Flow chart of the study of 11 subjects with pre-diabetes. Dashed line: no exercise; solid line: exercise. A to B and C to D, are intervention periods (exercise or no exercise as the case may be) each of 6 weeks with 4 weeks of wash-out (between C to D). Six subjects started in no exercise phase and crossed over to exercise phase and five subjects started in exercise phase and crossed over to no exercise phase with 4 weeks of was-out in between. A, B, C and D were the measurement points for all outcome measures. |
| Materials and Methods | ▴Top |
Participants and screening
The protocol was approved by the Baystate Medical Center institutional review board. Adults aged 40 - 70, with a body mass index (BMI) ranging from 25 to 39.9, who were not participating in regular exercise were recruited from the local primary care physician patient pool and community. Participants were non-smokers, weight stable (less than 5% weight change over last 3 months), free of CVD or diabetes and not taking dietary supplements, or on medications such as glucocorticoids and hormonal preparations. Participants were not included in the study if they had any contraindication to moderate exercise; implanted devices (e.g., pacemakers) that may interact with Tanita scale, uncontrolled hypertension, defined as > 140/90 on three separate occasions, angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), previous coronary or cerebrovascular event, active or clinically significant coronary disease or peripheral vascular disease, premature familial coronary heart disease, high density lipoprotein (HDL) < 40 mg/dL, one or less Framingham risk; hemoglobinopathies with low hematocrit or abnormal complete blood count; diabetes (i.e., fasting blood glucose of 126 mg/dL and above, or 2-h post-challenge blood glucose of equal to or above 200 mg/dL); active smoking, pre-existing liver disease, or any new lipid lower agent started in the last 6 months, oral/injectable anti-diabetic medication, any form of steroid medication (oral, inhaled, injected or nasal) and no new medications started in the last 3 months. History or current moderate to severe kidney disease, history of or current pancreatitis, active non-healing wounds, recent surgery in the last 3 months, history or current anti-inflammatory disease, regular use of anti-inflammatory drugs, cancer, alcoholism, triglycerides > 400 mg/dL, hyper/hypothyroid disease, pregnancy or desire to become pregnant and postmenopausal women on hormone replacement therapy were exclusions of the study.
Participants were screened for cardiac risk factors, such as history of smoking, high blood pressure, resting tachycardia, and dyspnea on exertion as well as family history of premature cardiac events. Following successful screening of the patients, which included a detailed history, examination, sub-maximal exercise test to rule out EKG changes with exercise and screening blood tests. Eleven participants qualified and completed the study protocol.
Assessment of diabetes status
Pre-diabetes was defined as per American Diabetes Association (ADA) 2014 Clinical Recommendation guidelines [4] by one of the following: 1) impaired fasting glucose (IFG), defined as fasting glucose 100 - 125 mg/dL; or 2) HbA1C ≥ 5.7-6.4% (39 - 46 mmol/mol). Nine patients had fulfilled the HbA1C criterion, two patients fulfilled the IFG criterion, and three patients fulfilled both criteria.
Dietary control
Enrolled participants were referred to a dietitian and/or a certified diabetes educator (CDE) who advised subjects to consume a low fat/calorie diet, so that all participants consumed similar amount of calories with similar dietary macro-nutrient divisions of carbohydrate, fat and protein. Subjects were advised to follow healthy dietary habits following guidelines in ADA Standards of Diabetes Care. In general subjects were encouraged to consume a healthy diet comprising of low fat and low carbohydrate diet throughout the 16 weeks of the study. Adherences to dietary guidelines were assessed via 4-day self-reported food diaries. Body composition was assessed each visit via bioimpedance scale (Tanita, Arlington Heights, IL).
Lifestyle program and physical activity assessment
Subjects were randomized to the exercise or non-exercise control arm (6 weeks each with 4 weeks of wash-out) using a permuted block design. This approach ensured groups were appropriately balanced at any time during the study and at study completion. For the exercise arm, participants were prescribed 150 min/week of moderate to vigorous intensity aerobic physical activity (MVPA), spread over at least 3 days/week with no more than two consecutive days without exercise. This is similar degree of activity recommended by the ADA and American College of Sports Medicine [18]. For the first 3 weeks, participants were acclimatized to exercise duration. Participants increased their exercise duration from 70 min/week in the first week to 105 min/week in week 2 such that they achieved 150 min/week on week 3. Participants were contacted from time to time or as needed regarding their progress and maintenance of activity logs. All their activity was objectively measured by accelerometry (Actigraph model GT3X). Accelerometer data were collected in 60-s epochs and were downloaded every 2 weeks. ActiLife software (version 5.9.1) was used to evaluate ActiGraph acceleration outputs for minutes/day spent in MVPA using Freedson cut points [19].
Blood chemistry
Participants fasted at least 10 h prior to testing. Twenty milliliter fasting blood was obtained for serum measures of cholesterol, triglyceride, HDL, LDL, ApoB, and ApoA1. High sensitivity C-reactive protein (hsCRP), insulin, leptin and interleukin-6 (IL-6) were measured with standard laboratory methods.
Flow mediated dilation (FMD)
At each visit, resting blood pressure, pulse, and FMD of the non-dominant brachial artery were measured. Patients were tested following a 10-h fast from food and caffeine. Participants refrained from alcohol for 24 h prior to the test. For FMD measures following the exercise phase, there was at least 24 h between the measurements and last bout of exercise. FMD was measured by two observers and averaged to avoid observer bias and performed according to methods described previously [20]. Briefly, a pneumatic blood pressure cuff was applied on the upper portion of the non-dominant arm with the lower border of the cuff ending at least 3 cm above mid cubital fossa. The position of the cuff was measured to ensure proper cuff placement during each visit. The brachial artery was imaged using a Philips HD11XE Ultrasound in B-mode (2.5 - 12 MHz probe) and an insonation angle of 60°. The vessel was initially measured at baseline. The cuff was then inflated 20 mm Hg above systolic blood pressure for 5 min. After 5 min, the pressure in the cuff was released and the vessel diameter tracked for up to 2 min to determine the time to maximal diameter. Maximal vessel diameter occurred 30 - 45 s after release of the cuff and was recorded. The location of the probe was marked to replicate placement at baseline and following the hyperemic stimulus.
CFU-Hill colony and CD34+ progenitor cell assays
Approximately 60 mL of blood was collected from patients in EDTA tubes. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood via Ficoll centrifugation. PMBCs were counted and a subset was used for the CFU-Hill colony formation assay according to the manufacturer’s instructions (Stem Cell Technologies, Vancouver, BC). Colony unit formation was assessed 5 days following plating and has been positively correlated to CVD risk and vascular function [21].
To count CD34+ cells, the mononuclear cell (MNC) population was obtained from each sample and processed through a CD34 positive magnetic bead column (Miltenyi Biotec). CD34+ cells were counter stained with trypan blue and counted using cell cytometer (Nexelecom Biosciences). CD34+ selected cells were used for gene expression and migration assays.
CD34+ gene expression was assessed via reverse transcriptase PCR (RT-PCR). CD34+ mRNA was extracted and purified (Qiagen) and converted to cDNA (Biorad P-100). A list of genes that were hypothesized to be changed in CD34+ cells with exercise were analyzed using real time PCR (Applied Bio-Systems 7300) and inventoried probes. These included anti-oxidant gene expression (superoxide dismutase (SOD) 1, 2 and 3, catalase, glutathione peroxidase), apoptosis (p53, p21, Bcl2, caspase-3), endothelial function (eNOS, endothelin-1 (ET-1)), chemotaxis (vascular endothelial growth factor (VEGF)-A, stromal cell derived factor (SDF)-1α), inflammation (IL-6, TNF-α), and endothelial lineage cell surface markers (CD34, VEGFR2 (KDR), CD31). The gene expression of individual genes was normalized to 18S and GAPDH and expression was analyzed as cycle threshold (Ct) difference. Expression was calculated as the difference in Ct from baseline to final for the non-exercise phase and exercise phase.
To assess CD34+ migratory capacity, 100,000 cells were suspended in 300 μL of serum free media. Migration across 3 μm membrane inserts (Corning) to SDF-1α (0, 10, 100 ng/mL) and VEGF-A (20 and 50 ng/mL) was tested. Migrated cells were dissociated from the membrane and subsequently detected by CyQuant Dye (Invitrogen). Florescence was read with a fluorescence plate reader at 480 nm/520 nm. All tests were performed in triplicate.
Statistical analysis
Data are expressed as mean ± SE. Comparisons between the exercise phase and non-exercise phase were performed using paired and un-paired Student’s t-test. Statistical significance was accepted at P < 0.05. No statistical difference in outcomes was found between the beginning of the exercise and non-exercise phase.
| Results | ▴Top |
Subject characteristics and exercise
Participants were overweight, pre-diabetic and normotensive (Table 1). There was no significant weight loss noted between the non-exercise and exercise phase. Participants had significantly more minutes/week of MVPA during the 6-week exercise compared with the non-exercise phase (174 ± 25 min/week vs. 127 ± 34 min/week, P = 0.02). Analysis of daily exercise revealed that participants increased total time in all activity intensities and significantly decreased daily sedentary time (Table 2).
 Click to view | Table 1. Patient Characteristics (n = 11) |
 Click to view | Table 2. Physical Activity (n = 11) |
Blood biochemistry
Total cholesterol and triglyceride were significantly lower after the exercise compared with the non-exercise phase (Fig. 2a). ApoB was lower and ApoA1 was greater after the exercise phase. While HDL was slightly higher and LDL was lower following the exercise phase, they were not statistically significant. Both hsCRP and insulin values decreased following the exercise phase but was not statistically significant. IL-6 and leptin levels were significantly lower after the post exercise phase (Fig. 2b).
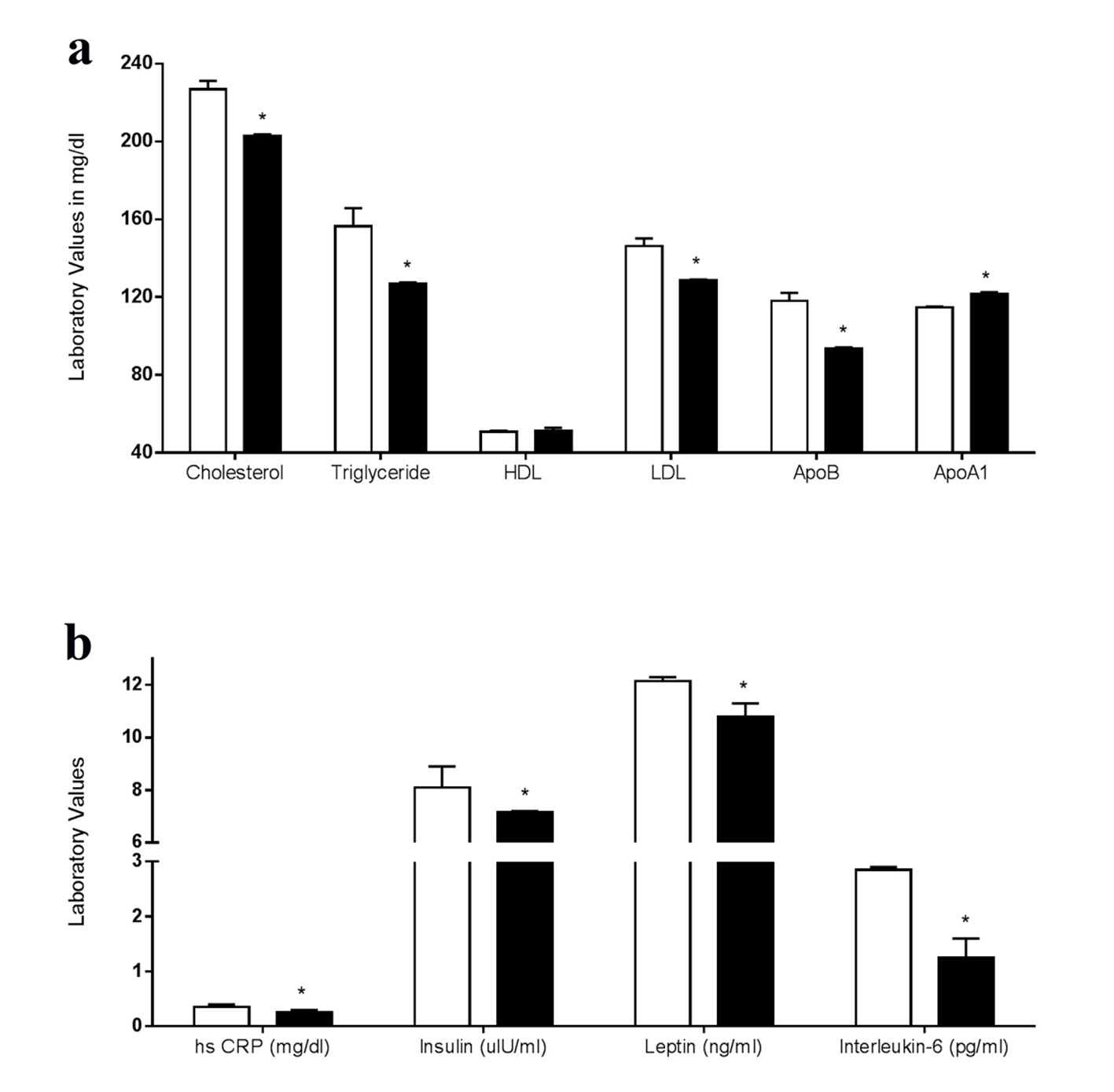 Click for large image | Figure 2. (a) Blood biochemistry and fasting lipid profile of subjects in the non-exercise phase (white bars) and post-exercise phase (black bars). *P < 0.05. (b) Blood biochemistry showing inflammatory markers, fasting insulin and fasting leptin of subjects in the non-exercise phase (white bars) and post-exercise phase (black bars). *P < 0.05. |
Endothelial and cellular characteristics
FMD of the brachial artery was significantly greater after the exercise compared with the non-exercise phase (Fig. 3). The EPC CFU-Hill colony formation, which is related to vascular function and CVD risk [21] was statistically greater following the exercise compared with the non-exercise phase (Fig. 4 2 - 4 vs. 8 - 9 colonies, P = 0.0017). The number of CD34+ cells increased following the exercise compared with the non-exercise phase (P = 0.002).
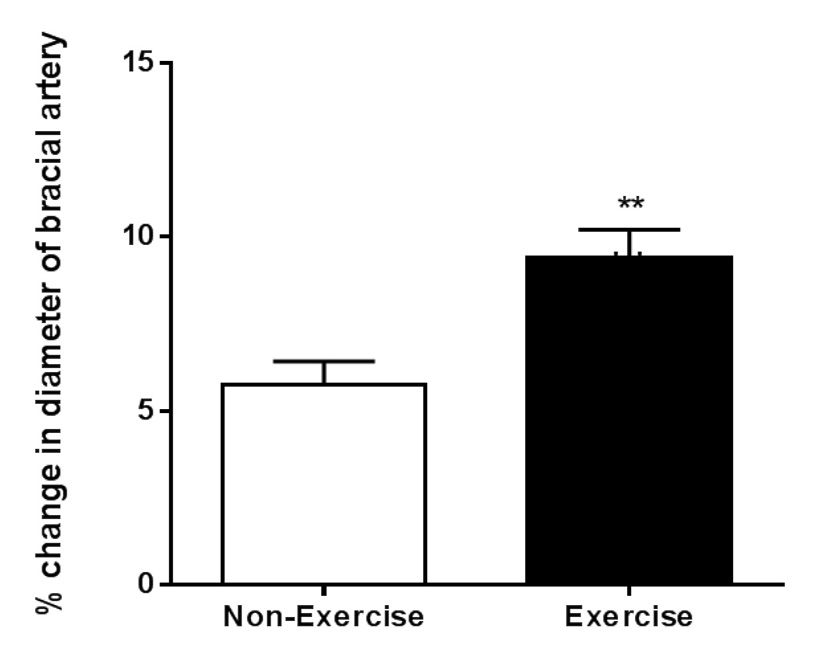 Click for large image | Figure 3. Brachial artery flow mediated dilatation in the non-dominant arm during the non- exercise (white bar) and post exercise (black bar) phase. **P = 0.0013. |
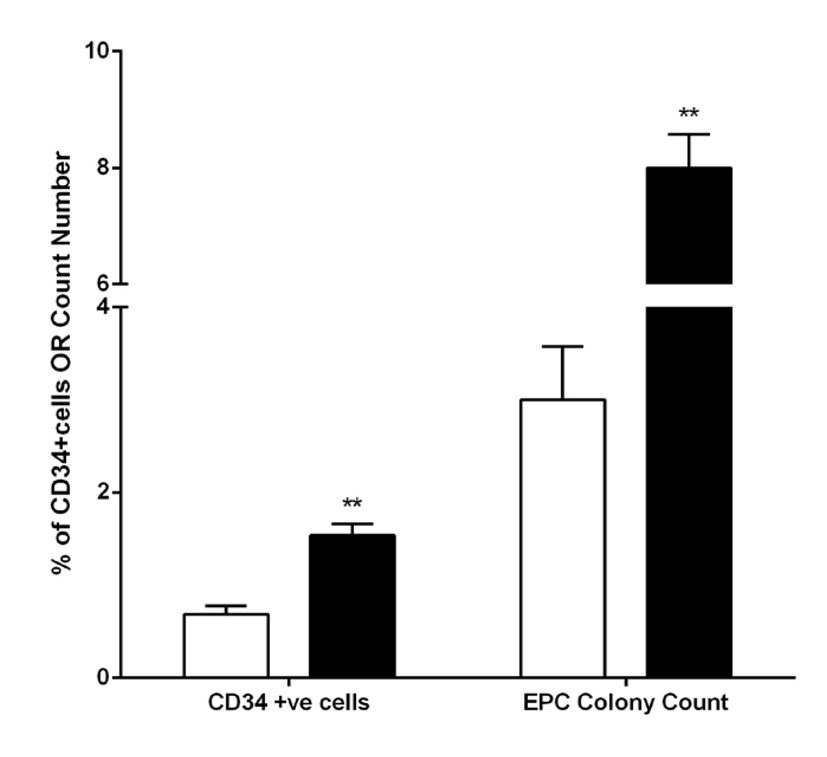 Click for large image | Figure 4. CD34+ cell number and CFU-Hill colony counts in the sedentary phase (white bars) and post exercise phase (black bars). **P < 0.005. |
We evaluated CD34+ cell migration in response to VEGF-A (0, 20 and 50 ng/mL) and SDF-1α (0, 10 and 100 ng/mL). The migratory response of CD34+ cells to VEGF 50 ng/mL was not significant between non-exercise and exercise phases (Fig. 5a 0.1841); however, migration to SDF-1α 10 ng/mL was significantly greater following the exercise phase (Fig. 5b 0.0206). CD34+ gene expression data revealed that TNF-α, IL-6 and ET-1 gene expression was significantly lower following the exercise phase. Interestingly, pro-apoptotic genes, p53 and p21 gene expression were also lower following the exercise phase (all P < 0.05, Fig. 6).
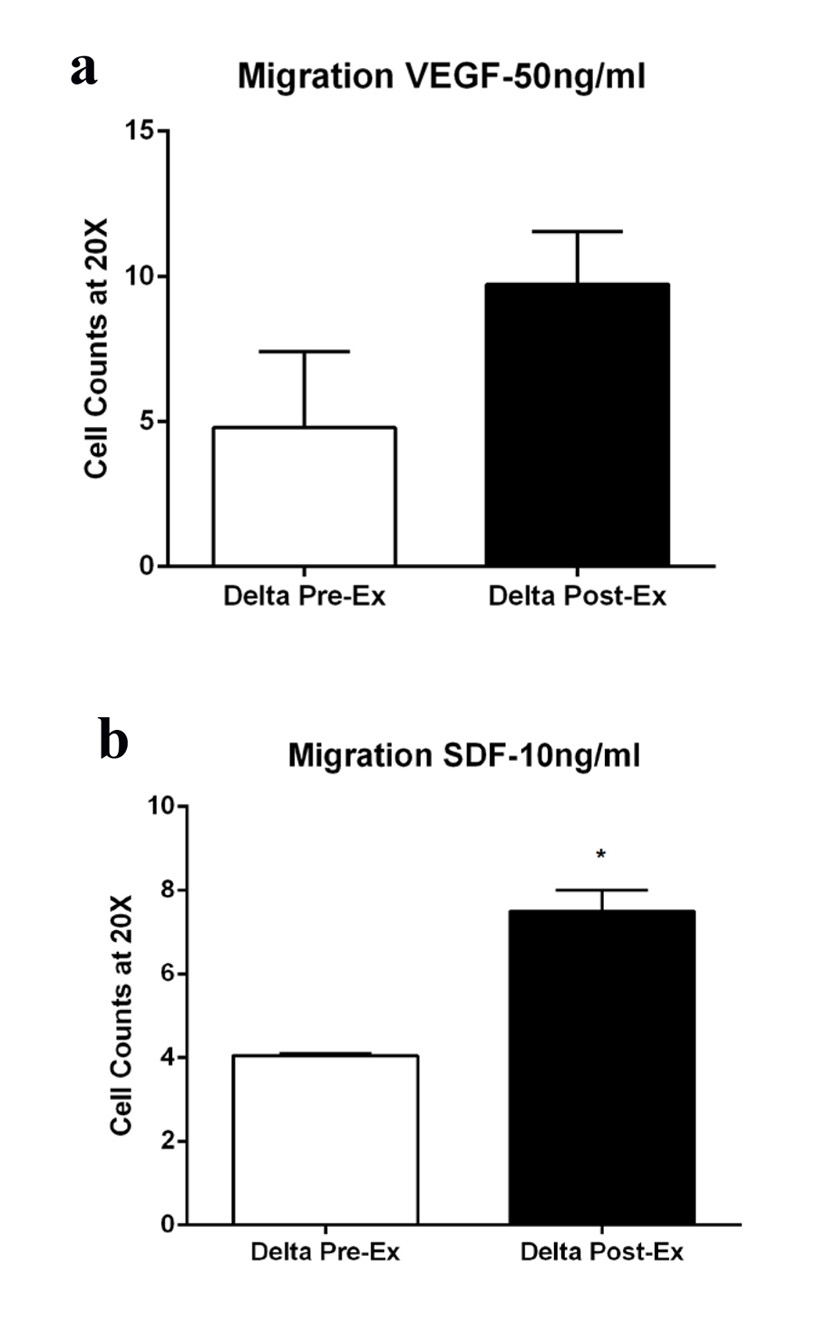 Click for large image | Figure 5. Migration of CD34+ cells in response to (a) VEGF-A (50 ng/mL), P = 0.1841 and (b) SDF-1 alpha (10 ng/mL), P = 0.0206 before (white bars) and after (black bars) exercise. Migration assay using VEGF-A as a chemotactic factor did not reach statistical significance. |
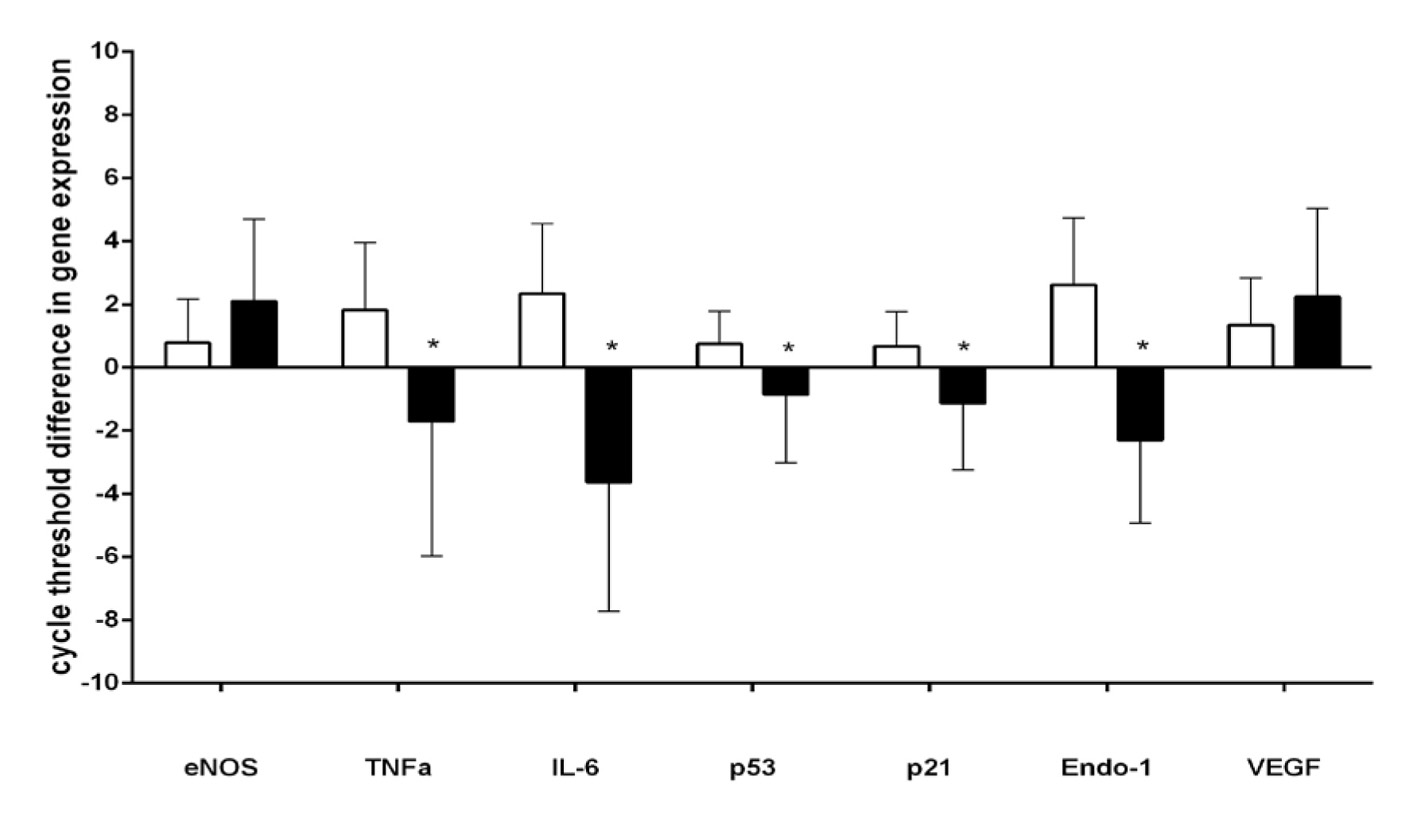 Click for large image | Figure 6. CD34+ gene expression before (white bars) and after (black bars) exercise. *P < 0.05. |
| Discussion | ▴Top |
This study demonstrates that a diet controlled, 6-week home-based physical activity intervention improved circulating CD34+ number, migratory function, apoptotic and inflammatory gene expression in patients with pre-diabetes. The changes in CD34+ cellular factors were accompanied by reduction in cardiovascular risk factors and improvement in brachial artery FMD, a measure of endothelial function. Importantly, these results were independent of weight loss or changes in body fat percentage. To our knowledge, this is the first study to report changes in CD34+ progenitor cells from an exercise intervention in participants with impaired glucose tolerance and pre-diabetes.
The influence of physical activity on CD34+ progenitor cells
Low circulating CD34+ progenitor cells are associated with impaired physical function [22] and cardiometabolic risk [23]. CD34+ progenitors in circulation are reduced in patients with pre-diabetes compared with healthy control participants [24]. Exercise training has well-known effects to improve insulin sensitivity and reduce cardiovascular risk in patients with diabetes and pre-diabetes [5, 6]. Our findings add to data from previous studies demonstrating exercise training induced increases in circulating CD34+ progenitor cells in non-diabetic populations [25]. During the exercise phase, our participants increased physical activity and achieved the prescribed 150 min/week of MVPA. Through objective activity monitoring we found modest daily increases in activity in addition to significant reductions in sedentary time. This study does not allow a determination of the independent effect of increased time in activity and reduction in sedentary behavior on outcomes; however, both likely contributed to the significant improvement in CD34+ progenitor cells, endothelial outcomes, and lipid profile as sedentary behavior is now recognized as a significant risk factor contributing to CVD [26, 27]. Most data from healthy individuals reveal that moderate to high intensity weight-bearing exercise training is necessary to elicit an increase in circulating CD34+ progenitor cells [28]. Our data indicate that in pre-diabetes patients, high intensity exercise is not necessary to increase circulating CD34+ progenitor cells and reduced sedentary time may be an important factor contributing to their appearance in circulation.
The prescribed exercise program also elicited important improvements in CD34+ cell function. Following the exercise phase, CD34+ cells from pre-diabetic patients had a better migratory response to the chemotactic factor SDF-1α. Impaired chemotaxis to SDF-1α was reported in circulating CD34+ cells from patients with type II diabetes and was related to reduced vasculogenic potential [29]. SDF-1α is essential for developmental and postnatal hematopoiesis and is a hypoxia-regulated chemokine that attracts cells to areas of vessel growth and repair [30]. The SDF-1α receptor, CXCR4 is located on endothelial cells and CD34+ progenitor cells [31]. Here we provide evidence that deficiency in CD34+ cell migration in prediabetes can be influenced by a diet and exercise lifestyle program.
Gene expression analysis of CD34+ cells revealed that the lifestyle program resulted in lower expression of inflammatory factors TNF-α and IL-6, vasocontrictor ET-1, and apoptosis markers p53 and p21. TNF-α, IL-6, and ET-1 are major factors associated with endothelial dysfunction [32] and plasma TNF-α and IL-6 are associated with insulin resistance and fasting glycemia [33]. One mechanism by which circulating progenitors are hypothesized to promote repair is through the release of various cytokines and growth factors [34, 35]. Inflammation is important for the recruitment of cells to areas of vascular repair, but persistent inflammation likely inhibits endogenous repair processes. TNF-α upregulates expression of adhesion molecules ICAM-1 and VCAM-1 on mature endothelial cells and some circulating progenitor cell populations [34] and is associated with atherosclerotic lesion formation [36]. ET-1 is a potent vasoconstrictor produced by endothelial cells. Greater ET-1 expression is associated with endothelial dysfunction [37]. Herein we report ET-1 gene expression from CD34+ circulating progenitors was lower following the exercise phase. From our data, it cannot be concluded that circulating progenitors are a source of ET-1; however, modulation of endothelial-related factors within circulating progenitors may be a novel mechanism by which exercise exerts effects on endothelial function. P53 and p21 are biomarkers for cellular senescence. Activation of the Akt/p53/p21 pathway has been reported in CD34+ circulating progenitor cells from diabetes patients and found to be an essential pathway modulating senescence in these cells [38]. Overall, our gene expression results indicate that the exercise program improved markers of endothelial dysfunction and senescence in CD34+ cells in pre-diabetes patients.
Physical activity and vascular function
In accordance with CD34+ cellular indicators of improved endothelial function, our data show improvement in FMD response with the lifestyle program. Endothelial dysfunction is an early manifestation of CVD and is predictive of CVD events [39]. Prior to exercise, FMD was similar to those observed in diabetic patients. Following the exercise phase, FMD improved to values similar to healthy individuals. These data were congruent with results from the CFU-Hill colony assay wherein we report significant increases in CFU-Hill colony counts. CFU-Hill colony number is associated with Framingham risk score and flow mediated brachial reactivity [21]. Overall our data indicate that prediabetes may be a window of therapeutic opportunity when vascular reactivity is still reversible.
Limitations
Our study is not without limitations. First, due to the limited participant number, this pilot study did not allow determination of differences in outcomes between participants with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). This is an important future direction as the etiology of these two conditions may account for differences in responses to lifestyle programs. Second, the protocol included an occlusion cuff that was placed above the ultrasound probe on the upper arm. Compared to a forearm location, this cuff placement is associated with a larger FMD response, but has been shown to be only partially NO dependent, involving other vasodilators and myogenic response [40]. To determine nitric oxide mediated vasodilation, other more invasive techniques such as dilation response to nitroglycerine would have to be employed; however, as this drug usually causes severe headache, poor patient compliance, and adherence to the study, it was therefore not utilized herein.
Conclusions
CD34+ cell dysfunction is hypothesized to contribute to poor vascular repair, increased risk of atherosclerosis, prolonged, and often-complicated recovery from ischemic events. Our data indicate that a diet-controlled, 6-week, home-based, objectively monitored exercise intervention elicited significant cellular, mechanical, and biochemical adaptations indicative of improved endothelial function and cardiovascular risk in patients with pre-diabetes. Greater vascular repair potential likely represents an important mechanism to reduce pre-diabetes-associated cardiovascular risk independent of reductions in body weight or body fat.
Acknowledgement
We would like to acknowledge help of Mr. Cyril Chou and Ms. Mary Young from Baystate Medical Center, Springfield, MA, in data acquisition and Department of Biostatistics for data analysis.
Funding
This work was funded by a Baystate Collborative Research Grant awarded to S. Sen and S. Witkowski.
Disclosures
There are no disclosures.
Abbreviations
HbA1C: hemoglobin A1C; BMI: body mass index; ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; DPP: Diabetes Prevention Program; MVPA: moderate to vigorous physical activity; HDL: high density lipoprotein; LDL: low density lipoprotein; ApoB: apolipoprotein B; ApoA1: apolipoprotein A1; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; FMD: flow mediated dilation; IL-6: interleukin-6; PBMC: peripheral blood mononuclear cell; RT-PCR: reverse transcriptase PCR; VEGF: vascular endothelial growth factor; ET-1: endothelin-1; NO: nitric oxide; SOD: superoxide dismutase; SDF: stromal cell derived factor; TNF: tumor necrosis factor; Ct: cycle threshold
| References | ▴Top |
- Department of Health, and Human Services National Institutes of Health, and National Center for Chronic Disease Prevention and Health Promotion National Diabetes Statistics: 2011 Fact Sheet. 2011.
- Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288(20):2579-2588.
doi - Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3(1):46-56.
doi pubmed - Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11-63.
doi pubmed - Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
doi pubmed - Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, Temprosa M. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28(4):888-894.
doi pubmed - Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677-1686.
doi - Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93-106.
doi pubmed - Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624-637.
doi pubmed - Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964-967.
doi pubmed - Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl). 2013;91(3):285-295.
doi pubmed - Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond). 2011;120(7):263-283.
- Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2013;36(4):943-949.
doi pubmed - Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56(4):960-967.
doi pubmed - Povsic TJ, Sloane R, Green JB, Zhou J, Pieper CF, Pearson MP, Peterson ED, et al. Depletion of circulating progenitor cells precedes overt diabetes: a substudy from the VA enhanced fitness trial. J Diabetes Complications. 2013;27(6):633-636.
doi pubmed - Witkowski S, Jenkins NT, Hagberg JM. Enhancing treatment for cardiovascular disease: exercise and circulating angiogenic cells. Exerc Sport Sci Rev. 2011;39(2):93-101.
doi pubmed - Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clin Sci (Lond). 2010;118(4):303-311.
doi pubmed - Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147-167.
doi pubmed - Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777-781.
doi pubmed - Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257-265.
doi - Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593-600.
doi pubmed - Povsic TJ, Sloane R, Zhou J, Pieper CF, Pearson MP, Peterson ED, Green JB, et al. Lower levels of circulating progenitor cells are associated with low physical function and performance in elderly men with impaired glucose tolerance: a pilot substudy from the VA Enhanced Fitness trial. J Gerontol A Biol Sci Med Sci. 2013;68(12):1559-1566.
doi pubmed - Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27(18):2247-2255.
doi pubmed - Fadini GP, Pucci L, Vanacore R, Baesso I, Penno G, Balbarini A, Di Stefano R, et al. Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia. 2007;50(10):2156-2163.
doi pubmed - Shalaby MN, Saad M, Akar S, Reda MA, Shalgham A. The Role of Aerobic and Anaerobic Training Programs on CD(34+) Stem Cells and Chosen Physiological Variables. J Hum Kinet. 2012;35:69-79.
doi pubmed - Shuval K, Finley CE, Barlow CE, Gabriel KP, Leonard D, Kohl HW, 3rd. Sedentary behavior, cardiorespiratory fitness, physical activity, and cardiometabolic risk in men: the cooper center longitudinal study. Mayo Clin Proc. 2014;89(8):1052-1062.
doi pubmed - Borodulin K, Karki A, Laatikainen T, Peltonen M, Luoto R. Daily Sedentary Time and Risk of Cardiovascular Disease: The National FINRISK 2002 Study. J Phys Act Health. 2014.
doi - De Lisio M, Parise G. Exercise and hematopoietic stem and progenitor cells: protection, quantity, and function. Exerc Sport Sci Rev. 2013;41(2):116-122.
doi pubmed - Jarajapu YP, Caballero S, Verma A, Nakagawa T, Lo MC, Li Q, Grant MB. Blockade of NADPH oxidase restores vasoreparative function in diabetic CD34+ cells. Invest Ophthalmol Vis Sci. 2011;52(8):5093-5104.
doi pubmed - Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, Katsura Y, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96(10):5663-5667.
doi pubmed - Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55(1):102-109.
doi pubmed - Sena CM, Pereira AM, Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216-2231.
doi pubmed - Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Sacca L, Ferrannini E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55(4):1133-1140.
doi pubmed - Zhang Y, Ingram DA, Murphy MP, Saadatzadeh MR, Mead LE, Prater DN, Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296(5):H1675-1682.
doi pubmed - Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733-742.
doi pubmed - Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18(5):842-851.
doi pubmed - Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76(1):8-18.
doi pubmed - Rosso A, Balsamo A, Gambino R, Dentelli P, Falcioni R, Cassader M, Pegoraro L, et al. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281(7):4339-4347.
doi pubmed - Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948-954.
doi pubmed - Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2-12.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
