| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Review
Volume 1, Number 2, June 2011, pages 47-56
Role of Vitamin D in Diabetes
Krishna G Seshadria, b, Bubblu Tamilselvana, Amarabalan Rajendrana
aDepartment of Endocrinology, Diabetes and Metabolism, Sri Ramachandra University, Chennai, Tamilnadu, India
bCorresponding author: Krishna G Seshadri, A1 Pvt Clinic, Department of Endocrinology, Diabetes and Metabolism, Sri Ramachandra University, Porur, Chennai – 600116, Tamilnadu, India
Manuscript accepted for publication May 26, 2011
Short title: Vitamin D and Diabetes
doi: https://doi.org/10.4021/jem23w
- Abstract
- Introduction
- Vitamin D
- Vitamin D and Type 1 Diabetes
- Vitamin D and Type 2 Diabetes
- Vitamin D and Glucose Transport
- Vitamin D and Gestational Diabetes
- Vitamin D Receptor Polymorphisms
- Vitamin D and Complications of Diabetes
- Conclusion
- References
| Abstract | ▴Top |
The role of vitamin D in the pathogenesis and prevention of diabetes has sparked widespread interest. Vitamin D receptors are present in both pancreatic beta-cells and immune cells. Beside its classical role as the major regulator for calcium absorption, vitamin D mediates the activity of beta-cell calcium-dependent endopeptidases promotes conversion of proinsulin to insulin and increases insulin output. In peripheral insulin target tissues, vitamin D enhances insulin action via regulation of the calcium pool. Vitamin D also acts as a potent immunosuppressor. It tends to down-regulate the transcription of various proinflammatory cytokine genes like Interleukin-2, Interlukin-12 and Tumor Necrosis Factor-alpha. It promotes the induction of regulatory T-lymphocytes, the production of anti-inflammatory cytokines and protects beta-cell from destruction. Vitamin D deficiency predisposes to type 1 diabetes in animal models and in humans. It is probable that a similar relationship exists for type 2 diabetes. Vitamin D deficiency impairs insulin secretion and induces glucose intolerance. Several vitamin D related genes are associated with different pathogenetic traits of the disease. Vitamin D supplementation has shown to reduce the risk of developing type 1 diabetes. Vitamin D has also been shown to reduce the risk of diabetes associated complications. Prospective clinical studies on vitamin D are required to firmly establish the role of vitamin D in the prevention and management of diabetes.
Keywords: Vitamin D; Diabetes; Immunosuppressor; Insulin; Glucose tolerance
| Introduction | ▴Top |
Diabetes is a metabolic disease that can affect nearly every organ system in the body. Diabetes continues to be a public health concern. It has been estimated that 380 million individuals would be affected with diabetes worldwide by the year 2025. In India alone 41 million individuals are affected by this deadly disease, and this is likely to go up to 70 million by the year 2025 [1]. Although important knowledge has been acquired on the etiology of diabetes its precise etiopathogenesis is still under discussion. Inflammatory factors, reactive oxygen species and autoimmune reactions have all strongly emerged as the major pathogenic effectors for diabetes. Recently, vitamin D has sparked widespread interest in the pathogenesis and prevention of diabetes. As the major regulator for calcium homeostasis, vitamin D directly and or indirectly improves insulin exocytosis via activating calcium-dependent endopeptidases. Vitamin D also improves glucose tolerance [2]. Vitamin D could also prevent type 2 diabetes through its role as an efficient antioxidant in. Additionally, the steroid hormone form of vitamin D promotes suppressor cell activity and inhibits the generation of cytotoxic (Tc), macrophages, delayed hypersensitivity type and natural killer (NK) cells [3]. Vitamin D also mediates several non-calcemic functions. It is a regulator of cellular proliferation, differentiation and replication, and mediator of autoimmune reactions, in a variety of organs and biological systems. The discovery of receptors for 1α, 25-dihydroxyvitamin D3 (1,25(OH)2D3), the activated form of vitamin D, in tissues with no direct role in calcium and bone metabolism (e.g., pancreatic beta-cells and cells of the immune system) has broadened our view of the physiological role of vitamin D [4].
| Vitamin D | ▴Top |
Vitamin D (VD) was first identified and characterized in 1923 by Goldblatt and Soames [5]. It is an essential vitamin naturally produced by the body on exposure to sunlight. The term vitamin D encompasses secosterols, ergocalciferol (VD2) and cholecalciferol (VD3). VD2 is produced commercially by irradiation of plant sterols (ergosterol), whereas VD3 is primarily manufactured in the skin from 7-dehydro cholesterol via photochemical synthesis using UV radiation from sunlight and can also be found in food of animal origin. The best food sources of VD are cod liver oil, fatty fish, and egg yolks.
Metabolism of vitamin D
Natural Vitamin D by itself has no hormonal activity. To become biologically active, vitamin D needs two successive hydroxylations in the liver (at carbon 25) and in the kidney (at a position of carbon 1). In the liver vitamin D is hydroxylated at carbon 25 to 25-hydroxy vitamin D (25(OH)D) (vitamin D2). Circulating 25(OH)D concentrations are considered an indicator of vitamin D status [6]. In the kidneys, 25-hydroxy vitamin D (25(OH)D) (vitamin D2) is converted to an activated (1,25-dihydroxy vitamin D; 1,25(OH)2D) (vitamin D3). This is the biologically active form of vitamin D [7] (Fig. 1).
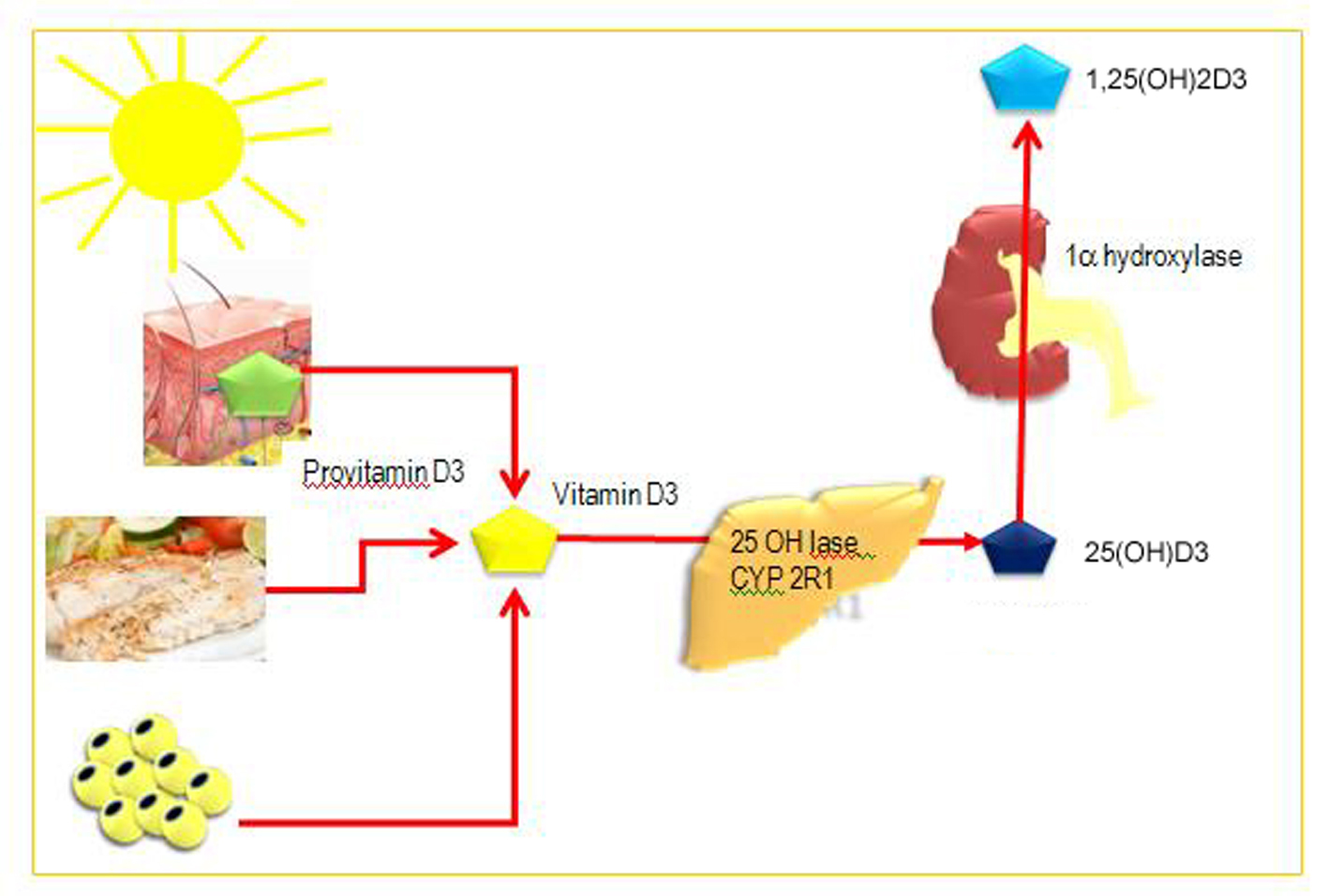 Click for large image | Figure 1. Synthesis and metabolism of vitamin D.Vitamin D can be obtained from food (vitamin D2 and D3) or by photobiogenesis in the skin (vitamin D3). In the blood, all vitamin D metabolites are bound to vitamin D-binding protein (DBP). Vitamin D3 is converted by two successive hydroxylations in the liver (25-hydroxylases) and kidney (1a-hydroxylase) into its active hormonal form, 1,25(OH)2D3. |
The production of 1,25(OH)2D3 in the kidney is regulated by several factors, particularly by levels of parathyroid hormone, although kidney 1α-hydroxylase is also subject to direct negative feedback inhibition by 1,25(OH)2D3.
The proximal renal tubule is the principal site of 1α-hydroxylation, although high levels of 1α-hydroxylase mRNA have also been found in human keratinocytes, dendritic cells and macrophages.
Another hydroxylation enzyme, 24-hydroxylase, initiates the catabolic cascade of 25-hydroxyvitamin D3 and 1,25(OH)2D3. In the circulation, all metabolites of vitamin D are bound to a carrier protein known as vitamin D-binding protein (DBP) [4].
Molecular action of 1,25-dihydroxyvitamin D3
The vitamin D hormone exerts its effects mainly by activating the nuclear vitamin D receptor (VDR), a member of the nuclear receptor super-family of ligand activated transcription factors. In humans the gene encoding the VDR is located on chromosome 12cen-q12. The binding of 1,25(OH)2D3 to the VDR leads to the transcription of genes regulated by 1,25(OH)2D3. The mechanism of this transcriptional regulation is very complex and is only just beginning to be unravelled. The cognate vitamin D response element (VDRE) to which the VDR binds consists of a hexanucleotide direct repeat spaced by three nucleotides (DR3-type VDRE). The VDR usually binds as a heterodimer with the retinoic X receptor (RXR), and the classical effects of 1,25(OH)2D3 are the result of interactions with this nuclear receptor (Fig. 2). With respect to its relevance in diabetes, the classical VDRE and other response sites are found within genes encoding proteins with important functions in beta-cells and within genes encoding proteins with key roles throughout the immune system, such as cytokines and transcription factors [8].
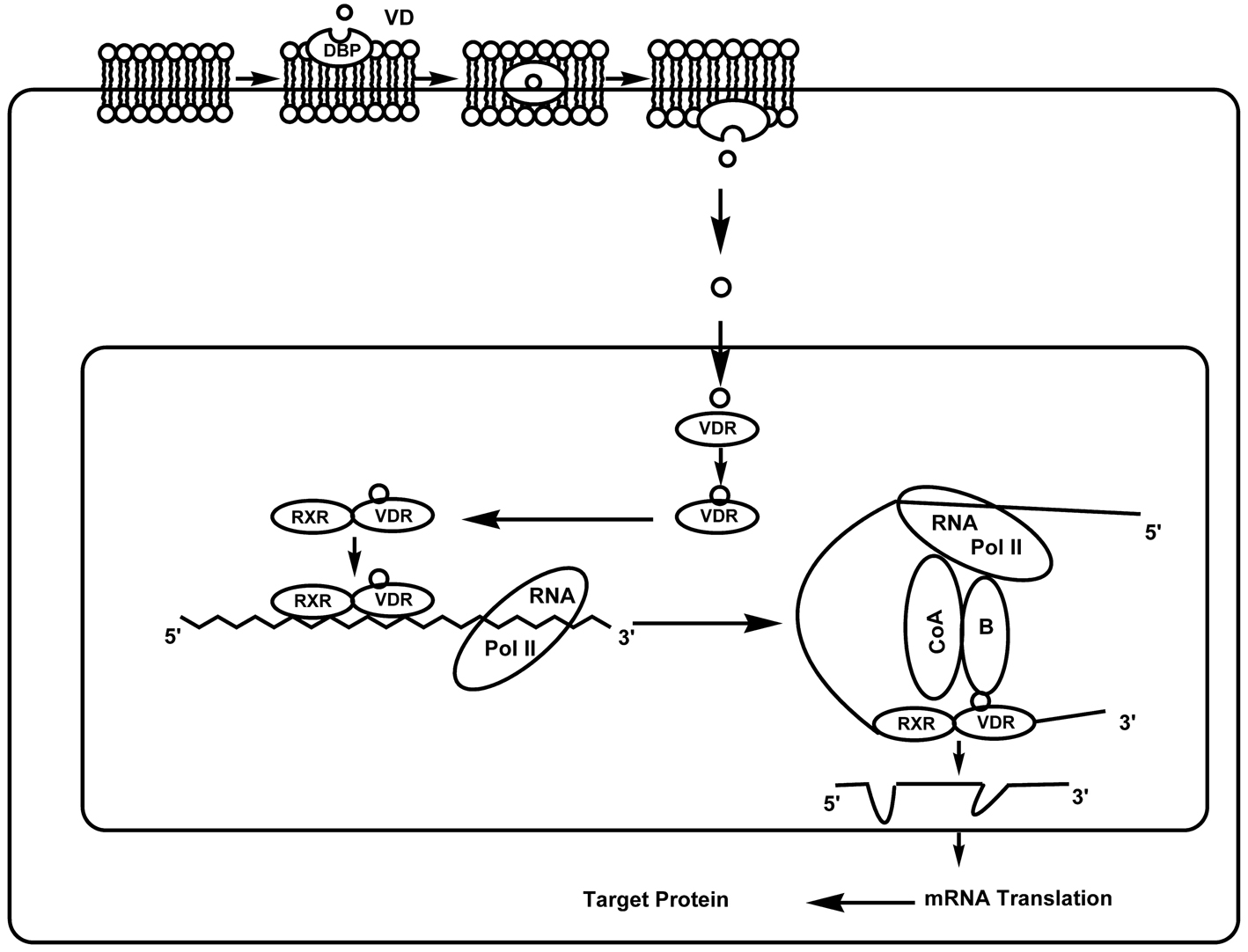 Click for large image | Figure 2. Genomic actions of 1,25-dihydroxyvitamin D3. Molecules of 1,25(OH)2D3 penetrate the plasma membrane with the help of DBP and exert their genomic effects by activating the VDR. Ligand binding to the VDR induces a conformational change in the receptor and subsequent heterodimerization with RXR. The RXR-VDR complex binds to the VDRE, which is located within the 5’ flanking region of target genes. Thereafter, co-repressor (CoR) proteins are released from the surface of the VDR, allowing interaction with co-activator (CoA) proteins. These molecules modulate chromatin structure and allow the interaction of the receptor with the RNA polymerase II transcriptional complex (POL II), thus activating transcription of the target gene. |
Vitamin D deficiency
Serum 25(OH)D2 is the best indicator of vitamin D body store levels. The desirable concentration of vitamin D in normal healthy adult should be greater than 100 nmol/l. Vitamin D deficiency is characterized by circulating levels of 25(OH)D2 less than 50 nmol/l. Concentrations ranging between 52 - 72 nmol/l are often considered insufficient [9].
Vitamin D deficiency is a common problem, and the clinical consequences are protean. Low vitamin D status can be caused by number of factors, including insufficient cutaneous synthesis (due to limited sunlight exposure or aging), inadequate intake and absorption of vitamin D, obesity or darker skin. Low blood levels of its main metabolite, 25(OH)D, have been linked to poor health outcomes such as fractures, poor physical function, sarcopenia, diabetes, osteoporosis, cancer, cardiovascular, neurodegenerative, autoimmune and infectious diseases [10].
| Vitamin D and Type 1 Diabetes | ▴Top |
Type 1 diabetes is characterized by autoimmune destruction of insulin producing beta-cells in the pancreas [11]. Several epidemiological studies have described an intriguing correlation between geographical latitude and the incidence of type 1 diabetes, and an inverse correlation between monthly hours of sunshine and the incidence of diabetes. A seasonal pattern of disease onset has also been described for type 1 diabetes [4], once again suggesting an inverse correlation between sunlight and the disease. Vitamin D is an obvious candidate as a mediator of this sunshine effect. Administration of 1,25(OH)2D3 had significantly reduced the incidence of diabetes to 80% in non-obese diabetes (NOD) type 1 mice. Conversely, mice subjected to vitamin D deprivation had greater susceptibility [12]. In humans, a 33% reduction in the risk of developing childhood-onset diabetes type 1 has been demonstrated in children who received vitamin D supplementation compared with non-supplemented children. An intake of 2,000 IU of vitamin Dduring the first year of life diminished the risk of developing type 1 diabetes. The incidence of childhood diabetes is three times higher in subjects with suspected rickets. In contrast to the previous studies that conclusively confirmed the potent activity of vitamin D supplementation in reducing the risk of diabetes type 1, a study by Stene and Joner confirmed that neither maternal use of cod liver oil nor vitamin D supplements during pregnancy could apparently reduce the risk of the offspring subsequently developing type 1 diabetes [11]. The reason for such a conflict between the results may probably be due to genetic variants of the VDR gene among different populations.
Mechanisms of action of vitamin D in Type 1 diabetes
Vitamin D as an immune modulator
Type 1 Diabetes is associated with an imbalance of pro-/anti-inflammatory cytokines, e.g., transforming growth factor beta1 (TGF-b1), interferon gamma (INF-g), interleukin-1 receptor antagonist (IL-1ra), interleukin-1 alpha (IL-1a), interleukin-1b (IL-1b), IL-4, IL-6, IL-12, and, tumor necrosis factor (TNF)-a [2]. The main source of these cytokines and other inflammatory mediators is the immunesystem, particularly activated T and B lymphocytes, dendritic cells (DCs), natural killer (NK) cells, and macrophages. The prevention of such imbalances in these inflammatory mediators could prevent or reduce risk of diabetes. The identification of VDRs on almost all cells of the immune system, especially antigen-presenting cells (macrophages and dendritic cells) and activated T lymphocytes, prompted investigation of 1,25(OH)2D3 as a potential immunomodulator [4]. Immune cells, particularly, activated macrophages and dendritic cells also contain the enzyme 1α-hydroxylase, which is involved in the conversion of vitamin D2 to vitamin D3, the metabolically active molecule [13]. A unique feature of 1,25(OH)2D3 is that not only does it interact with T cells, but also targets the antigen-presenting cell, the central cell in the immune cascade. In vitro, 1,25(OH)2D3 stimulates the phagocytosis and killing of bacteria by macrophages but suppresses the antigen-presenting capacity of these cells and dendritic cells. It is noteworthy that the expression of MHC-II molecules and adhesion molecules necessary for full T cell stimulation is suppressed. Cytokines secreted by antigen-presenting cells for the recruitment and activation of T cells are also directly influenced by 1,25(OH)2D3 (VD3). VD3 and its analogues also inhibit the secretion of IL-2, a key cytokine in the immune system. This protein, which is produced by macrophages and dendritic cells, is the major determinant of the direction in which the immune system will be activated, since it stimulates the development of CD4 T-helper type 1 (Th-1) cells and inhibits the development of CD4 Th-2 lymphocytes. This inhibition is observed in vitro, but IL-12 suppression and a shift from Th1 to Th2 predominance can also be observed after in vivo administration of VD3 or its analogues. Inhibition of IL-12 is achieved through a direct interaction between 1,25(OH)2D3 bound to the VDR (as a heterodimer with RXR) and nuclear factor κB (NF-κB), which interferes with the NF-κB-induced transcription of IL-12. The secretion of other cytokines by macrophages/dendritic cells is also influenced by 1,25(OH)2D3. Prostaglandin E2, a suppressive cytokine, is stimulated, while the monocyte recruiter granulocyte macrophage-colony-stimulating factor (GM-CSF) is suppressed. Suppression of GM-CSF is achieved via binding of ligand-bound monomers of the VDR to a DNA element in the promoter region of the gene encoding GM-CSF in the absence of RXR. Several T cell cytokines are direct targets of 1,25(OH)2D3 and its analogues. The secretion of IL-2 is suppressed through the inhibition of nuclear factor of activated T cells (NFAT) complex formation by ligand-bound VDR-RXR heterodimers through binding to the distal NFAT binding site in the human IL-2 promoter. Another key T cell cytokine, IFN-γ, is downregulated by 1,25(OH)2D3. The promoter region of the gene encoding this protein contains a classical Vitamin D Responsive Element (VDRE) (DR3 type), and binding of the ligand-bound VDR-RXR heterodimers causes direct suppression of transcription. Taken together, these observations suggest a physiological role for 1,25(OH)2D3 in the immune system, with a tightly regulated secretion of 1,25(OH)2D3 by macrophages and dendritic cells upon immune stimulation on the one hand and a direct inhibitory effect of the molecule onantigen presentation and T cell proliferation and cytokine secretion on the other hand. These immune effects are typically mediated through the VDR [4].
Vitamin D and the beta-cell
Beta-cell damage by cytokines and other inflammatory agents might play an important role in the pathogenesis of type 1 diabetes. Vitamin D deficiency clearly impairs secretion of insulin and induces glucose intolerance. Islets isolated from vitamin D deficient animals show impaired insulin release when cultured in vitro and challenged with glucose, whereas these abnormalities can be prevented by culturing the islets in the presence of high concentrations of vitamin D. Moreover, studies in VDR null mice also showed impaired insulin secretion and glucose intolerance. The inhibition of beta-cell function (insulin synthesis and insulin secretion) induced by IL-1β or IFN-γ in vitro is prevented by 1,25(OH)2D3 and its analogues, MC903 and KH1060. In contrast, Mauricio et al observed no effect of 1,25(OH)2D3 on IL-1β-induced beta-cell dysfunction. This discrepancy may be due to the fact that an incubation time of 24 h was used in the latter study, whereas incubation periods ranging from 48 to 72 h were used in the studies describing protection. In addition to the alteration of the effects of cytokines on beta-cell function, 1,25(OH)2D3 blocks the induction of surface markers by these cytokines. The upregulation of expression of MHC-II molecules and intercellular adhesion molecule-1 on rat islets after exposure to IFN-γ was markedly decreased by co-incubation with 1,25(OH)2D3 or an analogue (ZXY1106). Recently, Riachy et al demonstrated that 1,25(OH)2D3 might preserve the insulin content of human islets and prevent MHC-I expression, IL-6 production and NO release. Moreover, it was reported that 1,25(OH)2D3 induced and maintained high levels of A20, an anti-apoptotic protein, in rat RINm5F cells and human islets after exposure to inflammatory cytokines. Roger Bouillon et al [14] recently performed a comparative study of different cell systems (whole rat islets, FACS-purified beta-cells and INS-1E cells) and did not observe direct protection by 1,25(OH)2D3 against cytokine-induced beta-cell death, but demonstrated decreased expression of chemokines by beta-cells treated with 1,25(OH)2D3 [14].
| Vitamin D and Type 2 Diabetes | ▴Top |
Type 2 diabetes is characterized by insulin resistance and altered insulin secretion. The role of vitamin D in type 2 diabetes is suggested by a seasonal variation in glycemic control reported in patients with type 2 diabetes being worse in the winter [4], which may be due to prevalent hypovitaminosis D as a result of reduced sunlight in winter. Several studies have demonstrated a link between vitamin D and the incidence of type 2 diabetes. In a recent study, de Boer et al [15], examined the effect of calcium plus vitamin D supplementation on the incidence of drug-treated diabetes in postmenopausal women and concluded that, calcium plus vitamin D3 supplementation did not reduce the risk of developing diabetes over seven years of follow-up in this randomized, placebo controlled trial. However, they suggested that, higher doses of vitamin D might be required to affect diabetes risk. In support of this argument, in Nurses Health Study – a large prospective, observational cohort, women with the highest calcium and vitamin D intake (> 1200 mg and > 800 IU daily, respectively) had a 33% lower risk of incident type 2 diabetes mellitus than women with the lowest calcium and vitamin D intake (< 600 mg and < 400 IU daily, respectively) [16]. Another large cohort study from Finland indicated inverse association between serum 25(OH)D3 and risk of type 2 diabetes. These results were consistent with those from the Nurses’ Health Study by Pittas et al [2], where an inverse association was observed for the intake of vitamin D supplements. However, these studies were not able to differentiate whether the results were due to the effect of vitamin D deficiency on beta-cell function or on insulin resistance. Several reports have ascribed an active role to vitamin D in the functional regulation of the endocrine pancreas, particularly the beta-cells. Not only are receptors for 1,25(OH)2D3 found in beta-cells, but the effector part of the vitamin D pathway is also present in the form of vitamin D-dependent calcium-binding protein, also known as calbindin-D28k. The expression of calbindin-D28Khas been shown to protect beta-cells from cytokine-mediated cell death [4], thereby reducing the risk of type 2 diabetes.
Mechanisms of action of vitamin D on Type 2 diabetes
Vitamin D and the beta-cell
There are several lines of evidence supporting a role for vitamin D in pancreatic beta-cell function (Table 1). Sheena et al [17] examined the cross-sectional association between vitamin D and beta-cell dysfunction in subjects at risk for type 2 diabetes and showed a positive association between vitamin D and beta-cell function. A high prevalence of hypovitaminosis D was noted among women with type 2 diabetes. Hyper responsive insulin secretion after a glucose challenge has been found in older men with hypovitaminosis D [18]. Vitamin D may act in two possible pathways; vitamin D may act directly to induce beta-cell insulin secretion by increasing the intracellular calcium concentration via non-selective voltage-dependent calcium channels or it may mediate activation of beta-cell calcium-dependent endopeptidases to produce the cleavage that facilitates the conversion of proinsulin to insulin. In peripheral insulin-target tissues, vitamin D might directly enhance insulin action through stimulation of the expression of insulin receptors and regulation of insulin-mediated intracellular processes via regulation of the calcium pool [4].
 Click to view | Table 1. Potential Mechanisms and Beneficial Effects of Vitamin D on Diabetes |
Insulin resistance
Insulin resistance is a recognized precursor for the development of type 2 diabetes. Vitamin D may have a beneficial effect on insulin action either directly, by stimulating the expression of insulin receptors thereby enhancing insulin responsiveness for glucose transport [7], or indirectly via its role in regulating extracellular calcium ensuring normal calcium influx through cell membranes and adequate intracellular cytosolic calcium [Ca2+]i pool (Table 1). Calcium is essential for insulin-mediated intracellular processes in insulin-responsive tissues such as skeletal muscle and adipose tissue with a very narrow range of [Ca2+]i needed for optimal insulin-mediated functions. Changes in [Ca2+]i in primary insulin target tissues may contribute to peripheral insulin resistance via impaired insulin signal transduction leading to decreased GLUT-4 activity [7]. Associations between low vitamin D level and decreased insulin sensitivity have been reported in cross-sectional studies. A recent study by Enju Liu et al [9] examined the association between vitamin D status and insulin resistance in non-diabetic individuals and showed that higher vitamin D status was inversely associated with fasting markers of insulin resistance. A positive correlation of 25(OH)D concentration with insulin sensitivity and a negative effect of hypovitaminosis have been reported by Ken C Chiu et al [19]. Some observational [15, 19] studies have shown an inverse association between vitamin D-calcium status and insulin resistance. Results from randomized trials on the effect of vitamin D and/or calcium supplementation on insulin resistance show either improvement [19-21] or no effect [22] of insulin action with supplementation.
Inflammation
Type 2 Diabetes is associated with systemic inflammation. Systemic inflammation has been linked primarily to insulin resistance but elevated cytokines may also play a role in beta-cell dysfunction by triggering beta-cell apoptosis. Vitamin D may improve insulin sensitivity and promote beta-cell survival by directly modulating the generation and effects of cytokines (Table 1). There are very limited and conflicting data from human studies that have directly examined the relationship between vitamin D or calcium status and systemic inflammation in relation to type 2 Diabetes [3].
| Vitamin D and Glucose Transport | ▴Top |
Insulin stimulates glucose metabolism in its target tissues via recruitment of transporters from a large intracellular pool to the plasma membrane. The functional activity of these transporters has been shown to be impaired in diabetes [23]. As vitamin D modulates insulin secretion, it is reasonable that vitamin D deficiency may in part contribute to the altered expression of these transporters. Although the role of vitamin D in diabetes is well recognized, its relation to glucose transport is not well studied. Consuelo et al [24] observed the effect of vitamin D on glucose transport in adipocytes in non-diabetic and streptozotocin induced diabetic rats. Treatment with 1,25 D3 to non-diabetic rats did not alter basal and insulin stimulated glucose transport in adipocytes from these animals. The treatment with vitamin D to streptozotocin induced diabetic rats, improved the decreased basal glucose transport by 107% and the insulin stimulated glucose transport in adipocytes by 71% from these diabetic animals. The Possible mechanism is likely to be the indirect effect of vitamin D. Vitamin D contributes to normalization of extracellular calcium ensuring normal intracellular calcium pool as elevated intracellular calcium impairs insulin receptor phosphorylation leading to impaired insulin signal transduction and decreased GLUT-4 activity. Peeyush et al [25] examined the effect of vitamin D supplementation on preventing the altered expression of GLUT-3 in STZ-induced diabetic rats leading to imbalanced glucose transport in the neurons of cerebellum. They observed that treatment with vitamin D and insulin, stabilized the glucose transport mechanism mediated through GLUT-3 in the cerebellum. They concluded that supplementation with vitamin D to STZ-induced diabetic rats has beneficial effects in reducing the alterations in GLUT-3 and imbalanced glucose utilization in cerebellum. However large, well-controlled, randomized studies are required to define the relationship between vitamin D and glucose transport.
| Vitamin D and Gestational Diabetes | ▴Top |
Gestational diabetes (GDM) is a condition in which women without previously diagnosed diabetes exhibit high blood glucose levels during pregnancy. Pregnant women with diabetes and their fetus are known to be at greater risk of reduced vitamin D levels [26]. In GDM, vitamin D concentrations remain lower in comparison with those of normal pregnant women. Hollis and Wagner demonstrated that hypovitaminosis D is not an important factor contributing to gestational diabetes at 30 weeks gestation [26]. A cross-sectional study conducted on 741 pregnant women, showed a total prevalence of vitamin D deficiency (< 25 nmol/l) in 70.6% of pregnant women, and also demonstrated that the prevalence of severe vitamin D deficiency in GDM patients was higher than in normoglycemic pregnancies (< 12.5) [27]. Rudnicki et al [28] observed that intravenous administration of vitamin D to pregnant women with gestational diabetes transiently decreased fasting glucose levels, surprisingly, the level of insulin also decreased. This suggests that the effect of vitamin D on glucose metabolism is not only through an effect on insulin secretion but may also be mediated by an increase in the cellular absorption of glucose, either directly or by an increase in insulin sensitivity. This shows a positive correlation of 25(OH) vitamin D concentrations with insulin sensitivity. Furthermore, vitamin D deficiency could be a confirmative sign for insulin resistance.
| Vitamin D Receptor Polymorphisms | ▴Top |
The vitamin D receptor (VDR) is a ligand-activated transcription factor and has the efficiency to mediate the genomic effects of 1,25-dihydroxyvitamin D in a wide variety of tissues. Multiple polymorphisms have been identified. Polymorphisms are induced via hereditary mutations in the vitamin D-responsive gene. To date, more than 25 different polymorphisms have been described to the VDR locus [2]. As vitamin D modulates insulin secretion, it is possible that genetic variants of the VDR gene may contribute to the development of diabetes mellitus. It has been proposed that genetic changes or alterations in the VDR might contribute to the pathogenesis of type 2 diabetes mellitus by at least four different pathways: alteration in calcium metabolism, modulation of adipocyte function, modulation of insulin secretion and modification of cytokine expression [29]. Four common allelic variants or polymorphisms of the VDR gene have been identified and described in detail: FokI, BsmI, ApaI and TaqI [3]. The role of these VDR polymorphisms has been thoroughly studied in patients with diabetes. Hitman et al [30] showed an association between the ApaI polymorphism (homozygosis for the a allele) and lower insulin secretion in a healthy Bangladeshi Asian population at risk of type 2 diabetes living in London (UK) who, have a high prevalence of vitamin D deficiency. A correlation between ApaI polymorphism and fasting plasma glucose and glucose intolerance has also been observed in a community based study of older adults without known diabetes. Ogunkolade et al corroborated these data and alsoshowed a positive association between the TaqI (genotype TT) and the BsmI (genotype bb) polymorphisms with reduced insulin secretory capacity in the samepopulation. Speer et al [31] reported that patients with diabetes and obesity with the BB genotype of the BsmI allele in the VDR gene presented higher levels of postprandial serum C-peptide, which points to a possible role in the pathogenesis of type 2 diabetes. It has also been reported that in subjects with 25(OH)D3 insufficiency, TaqI polymorphism is a determinant of insulin secretion. Though there is strong evidence of association between VDR polymorphism and diabetes, there is great conflict between results among different population. In Chile, Angel et al [32] did not demonstrate the contribution of VDR alleles in the etiology of type 1 diabetes. In a large case-control series (more than 2000 controls and about 1000 patients) from the Finnish population, that studied the effect of three vitamin D receptor gene polymorphisms (VDRA, VDRB, VDRF) on susceptibility to type 1 diabetes concluded that these are not associated with diabetes type 1 [33]. Furthermore, single nucleotide polymorphisms of the VDR gene are unlikely to contribute significantly to diabetes type 1 susceptibility in the Portuguese population. In Polish population, VDR gene polymorphisms did not constitute a risk factor for the incidence of type 2 diabetes.
| Vitamin D and Complications of Diabetes | ▴Top |
Diabetes is associated with complications such as cardiovascular disease, renal impairment, and peripheral neuropathies. Studies in the past have explored the relationship of vitamin D concentrations to these complications. The earliest evidence for the relationship of vitamin D concentration to cardiovascular disease began with diabetic patients who had end stage renal disease. It was found that in persons having dialysis the cardiovascular mortality was 10 to 20 times higher than the general population. The severe hypertension in diabetic patients was attributed to the reduced synthesis of calcitriol by the kidneys. When patients were given 1 alpha-vitamin D and the vitamin D analogue paricalcitol, it was noted that the mortality rate for cardiovascular disease was significantly reduced [34]. A study on 825 US hemodialysis patients to determine the relationship between vitamin D levels and mortality reported that 78% of the patients were vitamin D deficient and that this was associated with an increased early mortality [35]. Zhang et al [36] reported that receptor-mediated vitamin D actions may be protective of kidneys in rats with diabetic nephropathy. This suggests that vitamin D may be useful and preventative for the kidneys. In a study to determine the relationship between 25(OH)D and kidney functions on NHANES III participants, the level of 25(OH)D was significantly lower in persons with severely decreased glomerular filtration rate when compared with those with normal kidney function [37]. Diabetes has been associated with several neurological disorders including reduced locomotor activity which in turn is associated with low concentration of vitamin D [24]. A study by K.T. Peeyush [25] reported the altered expression of cholinergic and dopaminergic receptors in the central nervous system of STZ-induced diabetic rats. They further suggested that the altered expression of these receptors was brought back to control by the treatment with vitamin D. A small, clinical study of 51 patients with type 2 diabetes (37 female) evaluated neuropathic complaints such as pain, burning, tingling, numbness, and throbbing sensations. Patients who were vitamin D deficient at base line were given cholecalciferol (D3) and re-evaluated at 3-month follow-up. Serum concentrations increased 67.4% from 18 to 30 ng/mL, and were associated with significantly lower pain scores [38]. These findings provide a confirmatory evidence for neuroprotective role of vitamin D and represent a novel possibility for the better management of diabetic mediated complications.
| Conclusion | ▴Top |
The published literature supports a possible role of vitamin D in the pathogenesis and prevention of diabetes. Vitamin D deficiency appears to be detrimental to beta-cell function, and leads to glucose intolerance in animal models and humans, consequently type 2 diabetes. Vitamin D deficiency in early life predisposes NOD mice and humans to the later development of autoimmune diabetes. Several vitamin D related genes have shown association with different pathogenetic traits of the disease. Vitamin D and its related metabolic and immune pathways may be involved in the pathogenesis of diabetes at environmental and genetic levels. Studies on vitamin D supplementation on prevention of diabetes are inconclusive. However robust clinical data is required to support a role for vitamin D supplementation in the prevention of diabetes.
Conflict of Interest
None.
| References | ▴Top |
- Sicree R, Shaw J, Zimmet P. Prevalence and projections. In: Diabetes Atlas 3rd ed. Brussels, Belgium: International Diabetes Federation, 2006:16-104.
- Tuorkey MJ, Abdul-Aziz KK. Strategies for diabetes and pathways of vitamin D. Diabetes and Metabolic Syndrome: Clinical Research and Reviews 2010;4(2):101-110.
doi - Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92(6):2017-2029.
pubmed doi - Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia 2005;48(7):1247-1257.
pubmed doi - DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80(6 Suppl):1689S-1696S.
pubmed - Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010;2010:351385.
pubmed - Mathieu C, Gysemans C. Vitamin D and diabetes. Av Diabetol 2006;22(3):187-193.
- Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 2006;147(12):5542-5548.
pubmed doi - Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, Jacques PF. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 2009;139(2):329-334.
pubmed doi - Milaneschi Y, Shardell M, Corsi AM, Vazzana R, Bandinelli S, Guralnik JM, Ferrucci L. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab 2010;95(7):3225-3233.
pubmed doi - Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004;79(3):362-371.
pubmed - Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child 2008;93(6):512-517.
pubmed doi - Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab 2005;16(6):261-266.
pubmed doi - Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29(6):726-776.
pubmed doi - de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 2008;31(4):701-707.
pubmed doi - Peechakara SV, Pittas AG. Vitamin D as a potential modifier of diabetes risk. Nat Clin Pract Endocrinol Metab 2008;4(4):182-183.
pubmed doi - Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, Perkins BA, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33(6):1379-1381.
pubmed doi - Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care 2001;24(8):1496.
pubmed doi - Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79(5):820-825.
pubmed - Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res 2004;12(4):582-590.
pubmed doi - Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30(4):980-986.
pubmed doi - Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1994;59(5):1083-1087.
pubmed - Karnieli E, Armoni M. Regulation of glucose transporters in diabetes. Horm Res 1990;33(2-4):99-104.
pubmed doi - Calle C, Maestro B, Garcia-Arencibia M. Genomic actions of 1,25-dihydroxyvitamin D3 on insulin receptor gene expression, insulin receptor number and insulin activity in the kidney, liver and adipose tissue of streptozotocin-induced diabetic rats. BMC Mol Biol 2008;9:65.
pubmed - Peeyush KT, Savitha B, Sherin A, Anju TR, Jes P, Paulose CS. Cholinergic, dopaminergic and insulin receptors gene expression in the cerebellum of streptozotocin-induced diabetic rats: functional regulation with Vitamin D3 supplementation. Pharmacol Biochem Behav 2010;95(2):216-222.
pubmed doi - Hollis BW, Wagner CL. Vitamin D deficiency during pregnancy: an ongoing epidemic. Am J Clin Nutr 2006;84(2):273.
pubmed - Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev 2008;24(1):27-32.
pubmed doi - Rudnicki PM, Molsted-Pedersen L. Effect of 1,25-dihydroxycholecalciferol on glucose metabolism in gestational diabetes mellitus. Diabetologia 1997;40(1):40-44.
pubmed doi - Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008;10(3):185-197.
pubmed doi - Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K, North BV, et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002;51(7):2294-2300.
pubmed doi - Speer G, Cseh K, Winkler G, Vargha P, Braun E, Takacs I, Lakatos P. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur J Endocrinol 2001;144(4):385-389.
pubmed doi - Angel B, Santos JL, Carrasco E, Albala C, Perez-Bravo F. Vitamin D receptor polymorphism and susceptibility to type 1 diabetes in Chilean subjects: a case-parent study. Eur J Epidemiol 2004;19(12):1085-1087.
pubmed doi - Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes Metab 2005;31(4 Pt 1):318-325.
pubmed doi - Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol 2006;92(1):39-48.
pubmed doi - Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 2007;72(8):1004-1013.
pubmed doi - Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 2008;73(2):163-171.
pubmed doi - Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int 2007;71(2):134-139.
pubmed doi - Lee P, Chen R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Intern Med 2008;168(7):771-772.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
