| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 3, Number 4-5, October 2013, pages 119-123
Effects of an Inhibitor of 3β-Hydroxysteroid Dehydrogenase on Glucose, Lipid Metabolism and Adipose Tissue in a Patient With Type 2 Diabetes
Hidekatsu Yanaia, b, d, Hiroki Adachia, Akahito Sakoa, b, Yuji Hirowataric
aDepartment of Internal Medicine, National Center for Global Health and Medicine Kohnodai Hospital, Chiba, Japan
bClinical Research Center, National Center for Global Health and Medicine Kohnodai Hospital, Chiba, Japan
cBioscience Division, Tosoh Corporation, Kanagawa, Japan
dCorresponding author: Hidekatsu Yanai, Department of Internal Medicine and Clinical Research Center, National Center for Global Health and Medicine Kohnodai Hospital, 1-7-1 Kohnodai, Chiba 272-8516, Japan
Manuscript accepted for publication August 14, 2013
Short title: Effects of 3β-Hydroxysteroid Dehydrogenase
doi: https://doi.org/10.4021/jem180w
| Abstract | ▴Top |
Elevated serum levels of glucocorticoids promote visceral obesity, hyperlipidemia, insulin resistance, as observed in patients with Cushing’s syndrome. Trilostane inhibits the synthesis of cortisol by competitive and reversible blocking of 3β-hydroxysteroid dehydrogenase, and is used in the treatment of Cushing’s syndrome. We studied effects of trilostane on glucose, lipid metabolism and adipose tissue in a patient with type 2 diabetes who was complicated with Cushing’s syndrome. Trilostane significantly reduced visceral fat, serum lipoproteins including atherogenic lipoproteins, and also increased serum adiponectin in this patient, proposing a new approach for the treatment of obesity and metabolic syndrome.
Keywords: Adiponectin; Cushing’s syndrome; Serum lipoproteins; Type 2 diabetes; Trilostane
| Introduction | ▴Top |
Glucocoroticoids play a crucial role in modulating adipose tissue metabolism and distribution. Elevated serum levels of glucocorticoids promote visceral obesity, hyperlipidemia, insulin resistance, as observed in patients with Cushing’s syndrome [1, 2]. Trilostane is an inhibitor of 3β-hydroxysteroid dehydrogenase (3β-HSD) used in the treatment of Cushing’s syndrome [3]. Here we show effects of trilostane on glucose, lipid metabolism and adipose tissue in a patient with type 2 diabetes who was complicated with Cushing’s syndrome.
| Case Report | ▴Top |
A 67-year-old woman was admitted to our hospital due to muscle weakness in May, 2012. She was diagnosed as having type 2 diabetes in 1996, and also Cushing’ syndrome due to left adrenal adenoma in December, 2011. She and her family refused left adrenalectomy. On admission, her body weight, height, and body mass index were 47.3 kg, 152.0 cm, and 20.3 kg/m2, respectively, and abdominal circumference was 103.1 cm, showing central obesity. Plasma glucose was 82 mg/dL and hemoglobin A1c was 5.8% by using sitagliptin (50 mg/day), and serum potassium level was reduced to 2.5 mmol/L. Daily urinary cortisol secretion was remarkably increased (86.2 µg/day). We started to use trilostane (240 mg/day) on June 14, 2012, and we reduced dose of trilostane from 240 mg/day to 120 mg/day since July 14. We measured subcutaneous and visceral fat area by abdominal computed tomography, and also investigated daily urinary cortisol secretion, plasma glucose, lipoprotein fractions by the newly developed anion-exchange high-performance liquid chromatography at before the start of treatment, at 3 weeks after the treatment using trilostane (240 mg/day) started, and at 4 weeks after reduction of daily dose of trilostane (from 240 to 120 mg/day) [4]. Further, we studied serum levels of lipoprotein (a), small-dense low-density lipoprotein (LDL), oxidized LDL and adiponectin.
Three weeks-use of trilostane decreased daily urinary cortisol secretion to 54.7 µg/day, and also reduced body weight by 3 kg (Fig. 1). At 4 weeks after reduction of daily dose of trilostane (from 240 to 120 mg/day), daily cortisol secretion was still suppressed (Fig. 1). While visceral fat area significantly decreased from 156 to 101.9 cm2, subcutaneous fat area increased from 126.3 to 138.4 cm2, by using trilostane (240 mg/day) (Fig. 1). At 4 weeks after reduction of daily dose of trilostane, body weight did not change, and visceral fat area significantly increased, however, subcutaneous fat area still decreased (Fig. 1). Plasma glucose levels decreased from 92 - 149 to 67 - 123 mg/dL by using trilostane (240 mg/day), and increased (89 - 192 mg/dL) by reduction of daily trilostane dose.
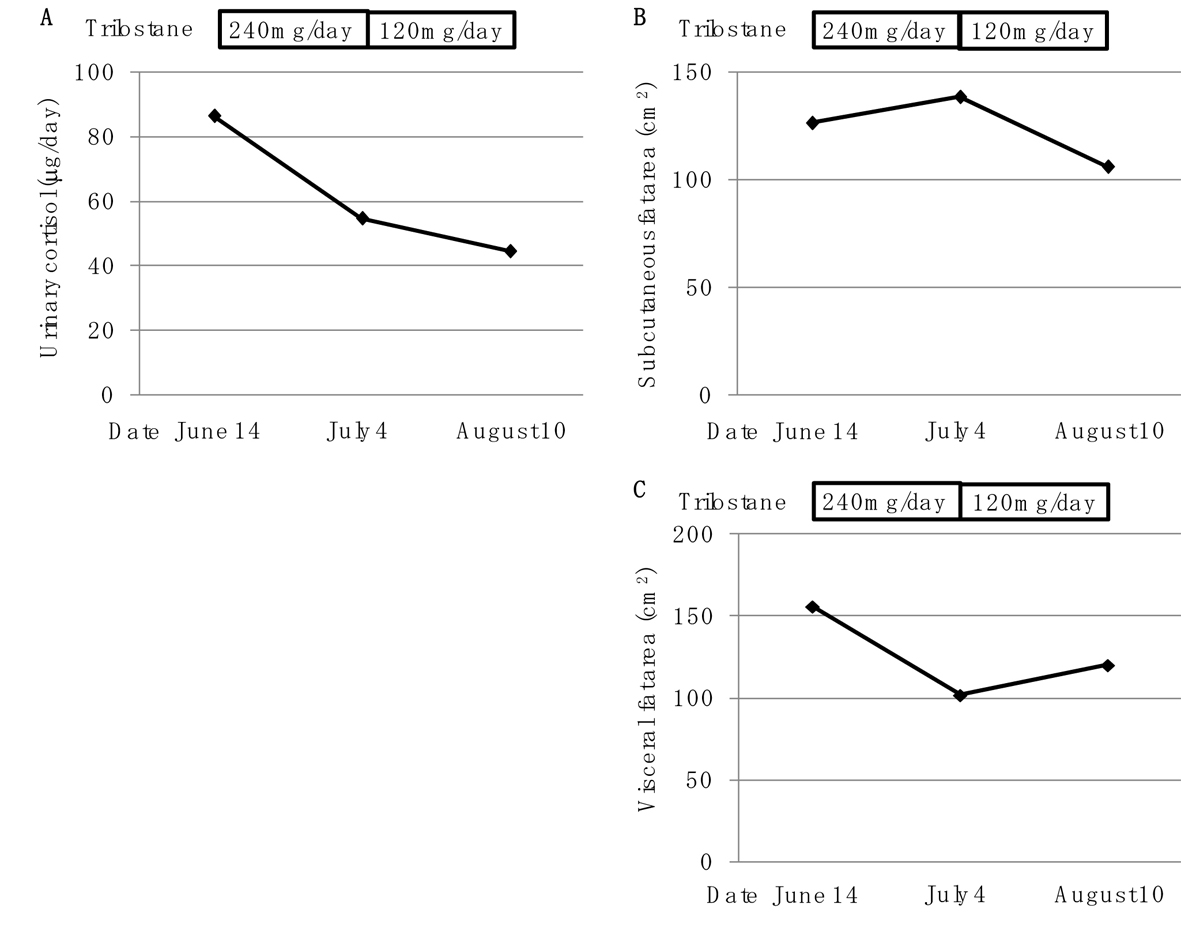 Click for large image | Figure 1. Changes of urinary cortisol concentration (A), subcutaneous fat area (B) and visceral fat area (C) by using trilostane (240 mg/day and 120 mg/day). |
Trilostane (240 mg/day) significantly decreased serum levels of high-density lipoprotein (HDL)-C from 33.7 to 12.8 mg/dL, LDL-cholesterol (LDL-C) from 126.4 to 48.5 mg/dL, intermediate-density lipoprotein (IDL)-C from 21.8 to 12.8 mg/dL, very LDL (VLDL)-C from 21.7 to 13.3 mg/dL, chylomicron (CM)-C from 0.8 to 0.4 mg/dL (Fig. 2). The reduction of trilostane dose significantly increased serum levels of HDL-C, LDL-C, IDL-C, VLDL-C and CM-C (Fig. 2). Serum levels of lipoprotein (a), small-dense LDL and oxidized LDL decreased from 2.6 to 1.5 mg/dL, from 33.8 to 23.8 mg/dL and from 173.0 to 127.0 mg/dL, respectively, by using trilostane (240 mg/day) (Fig. 3). The reduction of trilostane dose increased serum levels of lipoprotein (a), however, small dense LDL and oxidized LDL levels were still suppressed (Fig. 3). Trilostane (240 mg/day) increased serum adiponectin concentration from 9.5 to 10.9 µg/mL, and serum adiponectin concentration was reduced by reduction of daily trilostane dose (Fig. 3).
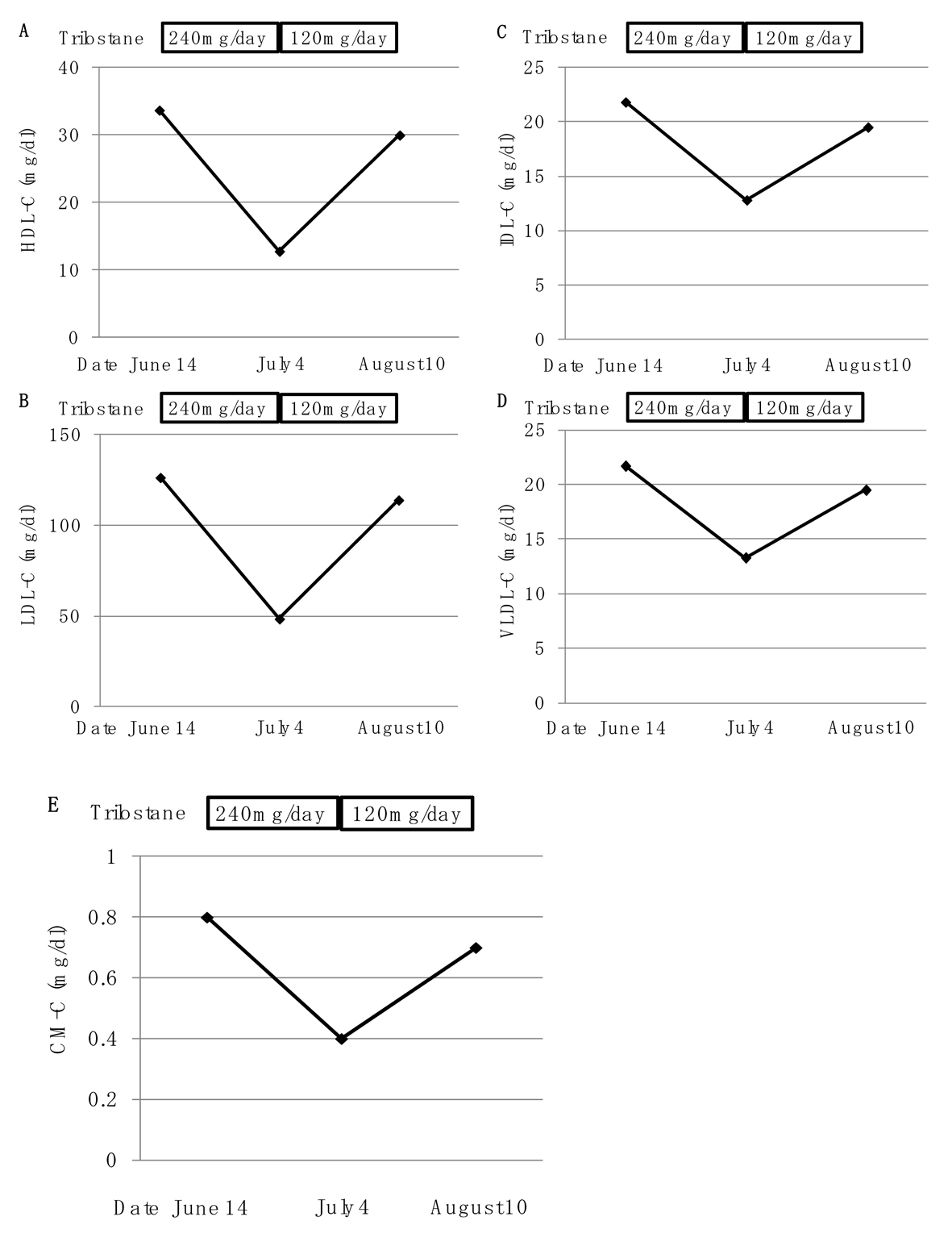 Click for large image | Figure 2. Changes of serum levels of high-density lipoprotein-cholesterol (HDL-C) (A), low-density lipoprotein-cholesterol (LDL-C) (B), intermediate-density lipoprotein-cholesterol (IDL-C) (C), very low-density lipoprotein-cholesterol (VLDL-C) (D) and chylomicron-cholesterol (CM-C) (E) by using trilostane (240 mg/day and 120 mg/day). |
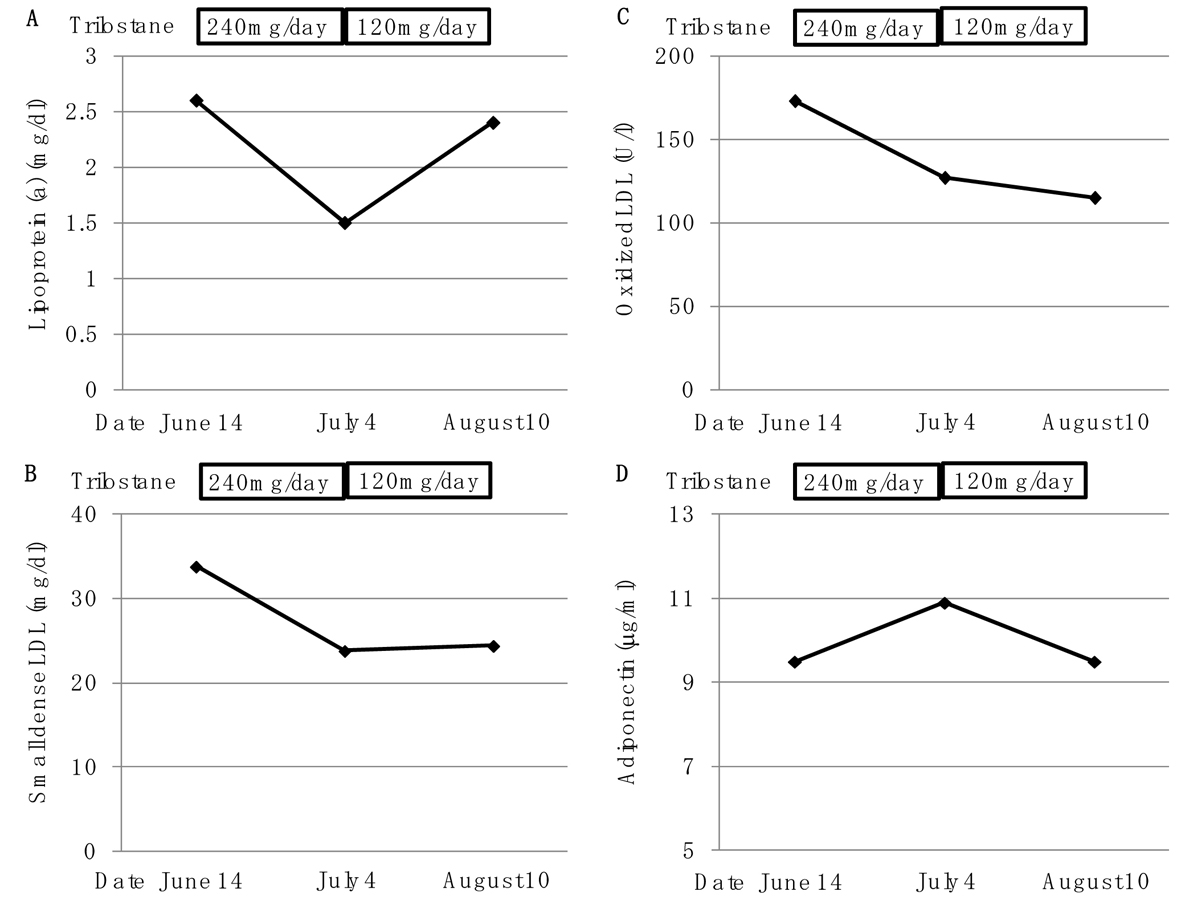 Click for large image | Figure 3. Changes of serum levels of lipoprotein (a) (A), small dense low-density lipoprotein (LDL) (B), oxidized low-density lipoprotein (LDL) (C) and adiponectin by using trilostane (240 mg/day and 120 mg/day). |
| Discussion | ▴Top |
Glucocorticoids play an important role in modulating adipose tissue distribution, metabolism and function. Increased serum glucorticoids levels, which are observed in patients with Cushing’s syndrome, induce visceral obesity, insulin resistance and lipid metabolism abnormalities [1, 2], 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) locally converts inactive glucorticoids into bioactive forms such as cortisol in humans [5], inducing amplification of glucocorticoids action in adipose tissue [6, 7]. Inhibition of 11β-HSD1 has been reported to ameliorate metabolic disorders in diabetic and obese animals. Inhibition of 11β-HSD1 reduces hepatic triacylglycerol content and plasma lipids, and also protects against diet-induced obesity [8, 9]. Transgenic mice with adipocytes overexpressing 11β-HSD1 developed a visceral obesity that was exacerbated by a high-fat diet [10].
The 3β-HSD has been considered to be one of candidates to have potentially important roles in obesity, abdominal fat accumulation and the metabolic syndrome as well as 11β-HSD1 [11], however, effects of 3β-HSD on metabolic parameters and obesity remain unknown. Trilostane inhibits the synthesis of cortisol by competitive and reversible blocking of 3β-HSD, and is used in the treatment of Cushing’s syndrome [3]. To our knowledge, effects of inhibition of 3β-HSD on metabolic parameters in humans have not ever discussed anywhere. In our study, inhibition of 3β-HSD reduced visceral fat and increased subcutaneous fat, indicating an important role of 3β-HSD for adipose tissue distribution. Further, trilostane decreased serum HDL-C, LDL-C, IDL-C, VLDL-C, CM-C and lipoprotein (a) in a dose-dependent manner. Visceral obesity is associated with elevated VLDL and reduced HDL, and weight loss reduces VLDL and increases HDL [12]. Our study showed that trilostane reduced all lipoprotein fractions, suggesting that trilostane possibly affects synthesis of cholesterol as precursor for cortisol as well as synthesis of cortisol. Interestingly, trilostane suppressed atherogenic lipoproteins such as small dense LDL and oxidized LDL, in a dose-independent manner. Adiponectin has been reported to decrease with the accumulation of visceral adipose tissue [13]. In our study, changes in serum adiponectin levels by trilostane corresponded to changes in visceral fat area, indicating that reduction of visceral fat by trilostane induces an increase of adiponectin.
In conclusion, our study showed that trilostane significantly ameliorates metabolic parameters in a patient with type 2 diabetes who was complicated with Cushing’s syndrome, proposing a new approach for the treatment of obesity and metabolic syndrome.
Acknowledgments
This work was supported by a grant from the National Center for Global Health and Medicine.
Conflict of Interest
No conflict of interest.
Grant Support
This work was funded by a grant from the National Center for Global Health and Medicine.
| References | ▴Top |
- Wolf G. Glucocorticoids in adipocytes stimulate visceral obesity. Nutr Rev. 2002;60(5 Pt 1):148-151.
pubmed - Boscaro M, Barzon L, Fallo F, Sonino N. Cushing's syndrome. Lancet. 2001;357(9258):783-791.
doi - Komanicky P, Spark RF, Melby JC. Treatment of Cushing's syndrome with trilostane (WIN 24,540), an inhibitor of adrenal steroid biosynthesis. J Clin Endocrinol Metab. 1978;47(5):1042-1051.
doi pubmed - Hirowatari Y, Yoshida H, Kurosawa H, Doumitu KI, Tada N. Measurement of cholesterol of major serum lipoprotein classes by anion-exchange HPLC with perchlorate ion-containing eluent. J Lipid Res. 2003;44(7):1404-1412.
doi pubmed - Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142(4):1371-1376.
doi - Masuzaki H, Flier JS. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)—a promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3(4):255-262.
doi - Stewart PM. Tissue-specific Cushing's syndrome, 11beta-hydroxysteroid dehydrogenases and the redefinition of corticosteroid hormone action. Eur J Endocrinol. 2003;149(3):163-168.
doi pubmed - Berthiaume M, Laplante M, Festuccia W, Gelinas Y, Poulin S, Lalonde J, Joanisse DR,
et al . Depot-specific modulation of rat intraabdominal adipose tissue lipid metabolism by pharmacological inhibition of 11beta-hydroxysteroid dehydrogenase type 1. Endocrinology. 2007;148(5):2391-2397.
doi pubmed - Liu J, Wang L, Zhang A, Di W, Zhang X, Wu L, Yu J,
et al . Adipose tissue-targeted 11beta-hydroxysteroid dehydrogenase type 1 inhibitor protects against diet-induced obesity. Endocr J. 2011;58(3):199-209.
doi pubmed - Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166-2170.
doi pubmed - Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34(11-12):737-745.
doi pubmed - Nikolic D, Katsiki N, Montalto G, Isenovic ER, Mikhailidis DP, Rizzo M. Lipoprotein subfractions in metabolic syndrome and obesity: clinical significance and therapeutic approaches. Nutrients. 2013;5(3):928-948.
doi pubmed - Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24(1):29-33.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
