| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 2, Number 6, December 2012, pages 220-227
Pentraxin 3 Levels as a Marker of Chronic Inflammation in Patients With Metabolic Syndrome
Muhammed Kizilgula, c, Mehmet Uzunlulua, Melike Karacakayaa, Aysun Semercia, Banu Isbilenb, Ferruh K. Ismanb
aDepartment of Internal Medicine, Medeniyet University, Goztepe Training and Research Hospital, Istanbul, Turkey
bDepartment of Biochemistry, Medeniyet University, Goztepe Training and Research Hospital, Istanbul, Turkey
cCorresponding author: Muhammed Kizilgul, Merdivenkoy mah. Sahika sok. Guner apt. Kat:7 D:16 Kadikoy, Istanbul, Turkey
Manuscript accepted for publication December 28, 2012
Short title: Pentraxin 3 Levels as a Marker
doi: https://doi.org/10.4021/jem142w
| Abstract | ▴Top |
Background: Pentraxin 3 (PTX3), an acute phase protein, is involved in chronic inflammation and does increase in atherosclerotic cardiovascular diseases. Metabolic syndrome (MetS) is characterized by chronic inflammation. We aimed to test the hypothesis of whether pentraxin 3 levels are high and do involve in chronic inflammation in patients with MetS. For that purpose, we investigated the association of PTX3 levels with chronic inflammation and its relation with metabolic parameters in patients with MetS compared to the patients with rheumatoid arthritis (RA) and healthy control cases.
Methods: We studied 22 non-diabetic adults ≥ 20 years of age. Patient population consisted of 27 cases with MetS diagnosed according to the IDF (International Diabetes Federation) definition (mean age 47.70 ± 8.92). There were two control groups. The first group consisted of diseased control cases with rheumatoid arthritis (n= 28, mean age 45.93 ± 13.18), and the second one consisted of healthy control cases (n = 27, mean age 46.18± 9.27). Study groups were compared with demographic, anthropometric and biochemistry data, and serum PTX3 levels. Correlation analysis was used for determining the relation between PTX3 levels and cardiometabolic parameters. PTX3 levels were measured by ELISA method using Human Pentraxin-3/TSG-14 kit.
Results: Mean serum PTX3 levels were 0.73 ± 0.53 ng/mL, 0.70 ± 0.67 ng/mL and 0.78 ± 0.65 ng/mL in the patients, diseased control, and healthy control groups, respectively. PTX3 levels were similar between the groups (P > 0.05). PTX3 levels and cardiometabolic parameters were not significantly correlated (P > 0.05).
Conclusion: These results do not confirm that PTX3 levels do increase as a marker of chronic inflammation in patients with MetS.
Keywords: Metabolic syndrome; Rheumatoid arthritis; Pentraxin 3
| Introduction | ▴Top |
Metabolic Syndrome (MetS) is a cluster of risk factors including abdominal obesity, hypertension, atherogenic dyslipidemia, hyperglycemia, prothrombotic and proinflammatory status, and an important risk factor for atherosclerotic cardiovascular diseases (CVD) [1]. MetS is characterized with chronic low grade inflammation, and inflammatory markers such as C-reactive protein (CRP), which is released from abdominal fatty tissue, involve in this process [2]. Pentraxin 3 (PTX3) is a member of the complex superfamily of multifunctional proteins characterized by cyclic multimeric structure. The pentraxin family consists of short pentraxins, namely C-reactive protein (CRP) and serum amyloid P component, and the long one, PTX3. PTX3 is an acute-phase protein in man and mouse, and does increase rapidly during a range of inflammatory and infectious conditions [3]. PTX3 is produced and released by a variety of cell types, including in particular, dendritic cells, mononuclear phagocytes, fibroblasts, endothelial cells, smooth muscle cells, adipocytes, synovial cells, chondrocytes, renal and alveolar epithelial cells [4]. PTX3 concentrations are high in systemic inflammatory response syndrome, septic shock, chronic renal insufficiency, myocardial infarction, heart insufficiency, atherosclerosis, vasculitis, pulmonary infections, acute pulmonary damage, lung cancer, eclampsia, rheumatoid arthritis (RA) and psoriasis[5-7].
Circulating PTX3 levels are consistently reported to be high in CVD and suggested as a predicting factor for CVD in general population [8]. The hypothesis of whether PTX3 levels are high and do involve in chronic inflammation in patients with MetS is tested in this study. For that purpose, the relation of PTX3 levels with chronic inflammation and metabolic parameters in patients with MetS are compared to the patients with RA and healthy control cases.
| Materials and Methods | ▴Top |
We enrolled patients with MetS or RA who attended at Internal Medicine or rheumatology outpatient clinics, respectively, with Goztepe Training and Research Hospital and healthy individuals who presented for a routine health check and who provided written informed consent to participate in the study. This study was approved by the Local Ethics Review Board (Date: 05. 04. 2011, Decree No:11/A). All participants are informed about the investigation and written informed consent was obtained from all of them before the study.
Inclusion criteria
MetS group; patients diagnosed with MetS according to the International Diabetes Federation (IDF) criteria. Diseased control group; patients diagnosed as RA by a rheumatology consultant. Healthy control group; healthy individuals who presented for a routine health check and do not have a known disease history, and do have normal laboratory values.
MetS diagnosis
The IDF criteria were used (waist circumference > 94 cm (male) or > 80 cm (female)) and at least two of the following criteria: blood pressure ≥ 130/85 mmHg (or taking antihypertensive medication; fasting plasma glucose≥ 100 mg/dL (or taking antidiabetic medication); fasting triglycerides (≥150 mg/dL or taking medication for high blood triglycerides; HDL-cholesterol < 40 mg/dL (male) or < 50 mg/dL (female) or taking medication for low HDL-cholesterol [9].
Rheumatoid arthritis diagnosis
The Revised American College of Rheumatolog was used [10].
Exclusion criteria
Type 1 or type 2 diabetes mellitus, liver, kidney, or heart insufficiency, metabolic disturbances (hypo-hyperthyroidism, and so on), acute infections, pregnancy, ascites or mass in the abdomen, autoimmune diseases except for RA, concomitant medications affecting insulin resistance (lipid lowering agents, metformine, glitazones, and so on).
Study design
Demographic characteristics, concomitant diseases, smoking and alcohol drinking habits, concomitant medication usage, anthropometric and biochemistry data of the eligible patients who has given informed consent were recorded. Groups were compared with respect to demographics, anthropometric and biochemistry data and serum PTX3 levels. The relation between PTX3 and clinical parameters (age, body mass index (BMI), gender, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), uric acid, total cholesterol (total-C), LDL-cholesterol (LDL-C), triglyceride (TG), HDL-cholesterol (HDL-C), TG/HDL-C, total-C/HDL-C, non-HDL-C, insulin, homeostasis model assessment insulin resistance (HOMA-IR), erythrocyte sedimentation rate (ESR), and CRP evaluated by correlation analysis.
Anthropometric measures
Body weight, waist circumference, and height were measured using standard measures by the same person. Waist circumference was measured midway between the arcus costarium and spina iliaca anterior superior, at the narrowest diameter during mild expirium. BMI was calculated as patient’s weight in kilograms divided by height in meters squared (kg/m2). Blood pressure was measured with an appropriate mercury sphygmomanometer based on Korotkoff phase I and phase V sounds with the subject in a sitting position at rest at least for ten minutes. A second measurement was taken from the arm had higher value. Mean systolic and diastolic blood pressure values calculated from the two measurements, at least three minutes apart.
Biochemistrical assays
Venous blood samples were taken after 8 - 12 hours of fasting into tubes with EDTA and without anticoagulant, and centrifuged at 1,500 g for 10 minutes. Obtained serum and plasma samples were stored at -20 °C until being analyzed. FPG levels were measured by hexokinase method, uric acid, total-C, HDL-C, LDL-C and TG were measured by enzymatic colorimetric method with Olympus AU2700 analyzer (Olympus Inc, USA), insulin levels were measured by chemiluminescence immunoassay with UniCell DxI 800 (Beckman Coulter Inc, USA) immunoassay analyzer. CRP levels were analyzed by nephelometric method (Immage, Beckman-Coulter Inc., USA). ESR was studied by ESR analyzer (Berkhun SDM 60, Turkey). Insulin resistance was calculated by HOMA-IR model [11].
Plasma PTX3 levels were measured by ELISA method with Human Pentraxin-3/TSG-14 kit produced by R&D Systems Inc (Minneapolis, USA). Mean lower limit of detection of the test was 0.025 ng/mL. Intra-assay coefficient of variances (CVs) of the test for three different concentrations were 3.8 (mean level: 2.61, SD: 0.01, n = 20), 3.7 (mean level: 7.72, SD: 0.28, n = 20) and 4.4 (mean level: 14.1, SD: 0.62, n = 20), and inter-assay CVs of the test for three different concentrations were 6.1 (mean level: 2.75, SD: 0.17, n = 40), 5.0 (mean level: 7.94, SD: 0.39, n = 40) ve 4.1 (mean level: 14.5, SD: 0.60, n = 40).
Statistical analysis
NCSS (Number Cruncher Statistical System) 2007&PASS 2008 Statistical Software (Utah, USA) program was used. In addition to the definitive analytic methods (mean, standart deviation), One-way Anova test was used for comparing quantitative data between groups for parameters with normal distribution, and Tukey HSD test was used for determination of the group causing the difference. Kruskal Wallis test was used for comparing data between groups for parameters without normal distribution, and Mann Whitney U test was used for determination of the group causing the difference. Spearman’s rho correlation coefficient was used for analyzing the relation between the parameters. Chi-square test was used for comparing qualitative data. Significance was assessed at P < 0.05 level.
| Results | ▴Top |
We enrolled 82 participants (19 male, 63 female, mean age 46.59 ± 10.57) in the study. MetS group consisted of 27 cases (7 male, 20 female, mean age 47.70 ± 8.92), RA group consisted of 28 cases (5 male, 23 female, mean age 45.93 ± 13.18), control group consisted of 27 cases (7 male, 20 female, mean age 46.18 ± 9.27).
Table 1 shows the demographic characteristics of the groups. Smoking frequency was significantly higher in the control group than MetS and RA groups (P < 0.01), proportion of hypertensive patients was significantly higher in the MetS group than RA and control groups (P < 0.01).
 Click to view | Table 1. Demographic Characteristics |
Table 2 shows the anthropometric characteristics of the groups. Mean BMI was significantly higher in the MetS group than the RA and control groups (P = 0.048; P = 0.001, respectively), mean waist circumference was significantly higher in females of MetS group and RA groups than the control group (both P < 0.01), mean waist circumference was significantly higher in males of MetS group than the RA and control groups (P = 0.048; P =0.001, respectively), mean systolic and diastolic blood pressures were significantly higher in the MetS group than the RA and control groups (both P < 0.01).
 Click to view | Table 2. Anthropometric Characteristics (Mean ± SD) |
Mean levels of FBG, triglycerides, uric acid, insulin, and total cholesterol/HDL-cholesterol and triglyceride/HDL-cholesterol ratios were significantly higher in the MetS group than the RA and control groups (P < 0.01 for all); mean non-HDL-cholesterol levels were significantly higher in the MetS group than the RA group (P = 0.016); mean HDL-cholesterol levels were significantly lower in the MetS group than the RA and control groups (both P =0.001). Mean ESR and CRP values were significantly higher in the MetS and RA groups than the control group (both P < 0.01). PTX3 levels were similar between the groups (P > 0.05) (Table 3, 4, Fig. 1).
 Click to view | Table 3. Biochemical Characteristics (Mean ± SD) |
 Click to view | Table 4. Post-Hoc Results of the Significantly Different Parameters |
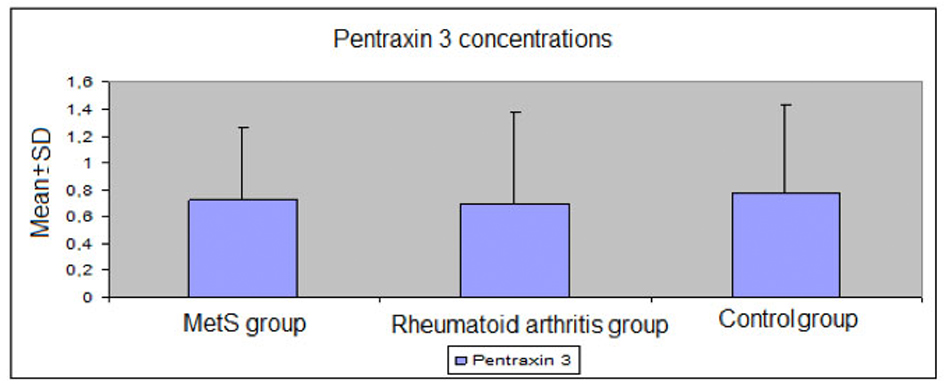 Click for large image | Figure 1. Plasma levels of pentraxin 3 in the study groups. |
Table 5 shows the correlation analysis for the relationship between PTX 3 and the clinical parameters. PTX3 levels were not significantly correlated with age, BMI, waist circumference, SBP, DBP, FBG, uric acid, total cholesterol, LDL-cholesterol, triglycerides, HDL-cholesterol, triglyceride/HDL-cholesterol, total cholesterol/HDL-cholesterol, non-HDL-cholesterol, insulin, HOMA-IR, ESR, or CRP (P > 0.05).
 Click to view | Table 5. Correlation Between PTX 3 and the Clinical Parameters for All Cases |
| Discussion | ▴Top |
Serum PTX3 levels of the patients with MetS were not significantly different from the control group in this study.
PTX3 is a new marker for acute phase inflammatory response and does have an important role in natural immunity. PTX3 is a member of the pentraxin family, just like CRP and serum amyloid P component.
Circulating PTX3 levels increase in several pathological conditions, such as autoimmune, infectious, and degenerative disturbances. One of its main differences from CRP, which is produced only in liver, its rapid increase in serum, is depending on production and release by a variety of cell types. PTX3 is the only independent predictor for acute myocardial infarction compared to the established risk factors such as CRP. Circulating PTX3 level is a predicting factor for cardiovascular and all-cause mortalities in elderly without CVD, independent from CRP and cardiovascular risk factors [12]. MetS is characterized a clustering of cardiometabolic risk factors related to chronic inflammation, and it is a known fact that inflammatory markers have a role in this process, such as adhesion molecules (P-selectin, E-selectin, intra-cellular adhesion molecule-1), cytokines (IL-1, IL-6, IL-10, TNF-α) and acute phase reactants (fibrinogen, hsCRP) [13]. We have evaluated PTX3 levels related to chronic inflammation and its correlation with metabolic parameters in patients with MetS. Therefore, we have compared PTX3 levels of the patients with MetS to patients with RA, which is characterized with chronic inflammation, and healthy controls. PTX3 levels were found similar between the groups. There are conflicting reports about PTX3 levels in patients with MetS. For instance, in a study assessing PTX3 levels in a healthy population, PTX3 was reported as lower in MetS patients than the others, and had a negative correlation with triglycerides and BMI [14]. On the other hand, Zanetti M et al reported higher PTX3 levels in MetS patients with subclinic atherosclerosis and high PTX3 levels were significantly correlated with low HDL-cholesterol and high triglycerides [15]. Ogawa et al. reported lower PTX3 levels and higher CRP levels in males with obesity and/or MetS compared to the control group. In this study, it was suggested that lower PTX3 levels might accelerate chronic inflammation and atherosclerosis in obese and/or MetS population because of tissue-protective and antiprotective roles of PTX3 [16].
RA is a chronic inflammatory disease characterized by tenderness and swelling of the joints and synovial joint destruction and resulting in serious physical disability and premature mortality [17]. Luchetti et al. reported that patients with RA has higher immunoreactive PTX3 levels in their synovial fluid compared to osteoarthritis patients and that endothelial cells and synoviocytes found in synovial tissue of RA patients were positively stained by PTX3 immunohistochemically [18]. In another study, PTX3 levels were found higher in patients with active RA than silent disease [19]. We found similar levels of PTX3 in RA and control groups in our study. This finding might be explained with antiinflammatory and immunosuppressive treatment received by these RA patients and remission of their disease. On the other hand, similar CRP levels found in these RA patients and the control group support this finding. Since, steroid and immunosuppressive therapies influence IL-1β and TNFα production, which triggers PTX3 production. This supports that antiinflammatory and immunosuppressive treatments control the signals inducing PTX3 production. In conclusion, PTX3 levels might be related to disease activity and treatment response [19].
PTX3 levels are reported to be positively correlated with age and pulse pressure, and negatively correlated with total- and LDL-cholesterol in subjects with insulin resistance [20]. PTX3 levels were not significantly correlated with insulin resistance determined by HOMA-IR or the other metabolic parameters in our study.
CRP levels are high in patients with MetS, and higher CRP levels are associated with higher cardiovascular risk in patients with MetS [21]. CRP levels were higher in patients with MetS compared to the control group in our study.
Conclusion
These results do not confirm that PTX3 levels do increase as a marker of chronic inflammation in patients with MetS.
Study Center
Medeniyet University, Goztepe Training and Research Hospital, Department of Internal Medicine and Rheumatology, Istanbul, Turkey.
| References | ▴Top |
- Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47(6):1093-1100.
doi pubmed - Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
doi pubmed - Bottazzi B, Bastone A, Doni A, Garlanda C, Valentino S, Deban L, Maina V, et al. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006;79(5):909-912.
doi pubmed - Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28(1):1-13.
doi pubmed - Suliman ME, Qureshi AR, Carrero JJ, Barany P, Yilmaz MI, Snaedal-Jonsdottir S, Alvestrand A, et al. The long pentraxin PTX-3 in prevalent hemodialysis patients: associations with comorbidities and mortality. QJM. 2008;101(5):397-405.
doi pubmed - He X, Han B, Liu M. Long pentraxin 3 in pulmonary infection and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1039-1049.
doi pubmed - Diamandis EP, Goodglick L, Planque C, Thornquist MD. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin Cancer Res. 2011;17(8):2395-2399.
doi pubmed - Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110(16):2349-2354.
doi pubmed - Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-480.
doi pubmed - Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315-324.
doi pubmed - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419.
doi pubmed - Hollan I, Bottazzi B, Cuccovillo I, Forre OT, Mikkelsen K, Saatvedt K, Almdahl SM, et al. Increased levels of serum pentraxin 3, a novel cardiovascular biomarker, in patients with inflammatory rheumatic disease. Arthritis Care Res (Hoboken). 2010;62(3):378-385.
doi pubmed - Ikonomova K. Inflamation and Metabolic Syndrome. Turkish Journal of Endocrinology and Metabolism. 2004;3:85-89.
- Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47(4):471-477.
doi pubmed - Zanetti M, Bosutti A, Ferreira C, Vinci P, Biolo G, Fonda M, Valente M, et al. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: evidence for association with atherogenic lipid profile. Clin Exp Med. 2009;9(3):243-248.
doi pubmed - Ogawa T, Kawano Y, Imamura T, Kawakita K, Sagara M, Matsuo T, Kakitsubata Y, et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity (Silver Spring). 2010;18(9):1871-1874.
doi pubmed - Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
doi pubmed - Luchetti MM, Piccinini G, Mantovani A, Peri G, Matteucci C, Pomponio G, Fratini M, et al. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin Exp Immunol. 2000;119(1):196-202.
doi pubmed - Fazzini F, Peri G, Doni A, Dell'Antonio G, Dal Cin E, Bozzolo E, D'Auria F, et al. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44(12):2841-2850.
doi - Jylhava J, Haarala A, Kahonen M, Lehtimaki T, Jula A, Moilanen L, Kesaniemi YA, et al. Pentraxin 3 (PTX3) is associated with cardiovascular risk factors: the Health 2000 Survey. Clin Exp Immunol. 2011;164(2):211-217.
doi pubmed - Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836-843.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
