| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 2, Number 4-5, October 2012, pages 190-194
Pituitary Disease in Chronic Hepatitis C Infection and Interferon-alpha Related Therapy: Two Case Reports
Huy A Trana, c, d, Patricia A Crockb, c, Glenn EM Reevesa, c
aHunter Area Pathology Service, Locked Bag Number 1, Hunter Mail Region Centre, Newcastle, New South Wales 2310, Australia and University of Newcastle, Newcastle, New South Wales, Australia
bDepartment of Paediatric Endocrinology and Diabetes, John Hunter Children Hospital, Locked Bag 1, Hunter Region Mail Centre, Newcastle, New South Wales 2310, Australia and University of Newcastle, Newcastle, New South Wales, Australia
cAll authors contributed equally to this work
dCorresponding author: Huy A Tran, Hunter Area Pathology Service, Locked Bag Number 1, Hunter Mail Region Centre, Newcastle, New South Wales 2310, Australia and University of Newcastle, Newcastle, New South Wales, Australia
Manuscript accepted for publication August 17, 2012
Short title: Pituitary Disease
doi: https://doi.org/10.4021/jem117w
| Abstract | ▴Top |
Pituitary dysfunction in chronic hepatitis C infection treated with interferon-α is a rare condition with 4 case reports world wide. We hereby report two cases of pituitary dysfunction in HCV patients, with and without interferon-α therapy. Case 1: A 34-year-old man co-infected with HIV and HCV presented with a 3 month history of lethargy, listlessness and a general lack of energy. Past medical histories include inactive neurosyphillis, chronic schizophrenia and seizure. His HCV is genotype 1 without cirrhosis and he completed a 48-week course of combination IFN-α and RBV for 48 weeks uneventfully 3 months prior. Examination and investigation found him to isolated ACTH deficiency. His condition improved markedly with corticosteroid replacement therapy. Case 2: A 45 year-old and treatment naive man with chronic HCV infection presented with a 20 kg weight loss, lack of energy and the occasional dizziness. Examination and investigation found him to have panhypopituitarism. Replacement therapy was initiated including hydrocortisone, testosterone and hydrocortisone. He made a slow but steady recovery and regained about 15 kg of weight but unfortunately was lost to follow up. It concluded that hepatitis C infection on its own or in conjunction with interferon-α based therapy can result in pituitary failure. The condition is readily treatable and hence should be considered in the appropriate clinical setting.
Keywords: Pituitary; Hypophysitis; Hepatitis C; Interferon-alpha
| Introduction | ▴Top |
Pituitary pathology associated with interferon-α (IFN-α) therapy is an uncommon condition which so far has been poorly described and reported. Most are anecdotal with few unconvincing case reports. The mechanism as a result then is poorly understood but perhaps and similar to IFN-α related thyroid disease, immune-modulation is the major underlying pathogenesis. We hereby describe two cases of hepatitis C and IFN-α related disease where pituitary failure developed.
| Case Report | ▴Top |
Case 1
A 34-year-old man co-infected with Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) presented with a 3 month history of lethargy, listlessness and a general lack of energy. Past medical histories include treated and inactive neurosyphilis, chronic schizophrenia and seizure. His HCV is genotype 1 without cirrhosis and he completed a 48-week course of combination IFN-α and RBV for 48 weeks uneventfully 3 months prior.
Clinically he was unwell with BP of 110/70 sitting and 100/60 standing and PR of 89 beats per minute (bpm). General examination was unremarkable and there was no pigmentation. A baseline serum cortisol was 36nmol/L at 07:05 hrs with Adrenocorticotropic (ACTH) level of 3.3 pmol/L (Reference Range (RR), < 10). His Thyrotropin (TSH) level was 0.96 mIU/L (RR, 0.4 - 4.0), free tetra-iodothyronine (fT4) of 19.1 pmol/L (RR, 10.8 - 21.0), Luteinising Hormone (LH) 13.8 IU/L (RR, 5.5 - 11.5), Follicular Stimulating Hormone (FSH) 7.7 IU/L (RR, 2.1 - 8.0), Testosterone 13.9 nmol/L (RR, 8.0 - 25.9), Growth Hormone (GH) < 0.2mIU/L, Insulin-like Growth Factor 1 (IgF-1) 0.73 U/mL (RR, 0.5 - 2.0), Prolactin 402 mIU/L (RR, < 410). A 250 µg Synacthen stimulation test showed a rise from baseline of 70 to 304 nmol/L at 60 minutes. His electrolytes were normal with Na of 137 and K 4.1 mmol/L. His pituitary Magnetic Resonance Imaging was normal. Pituitary antibodies were not available.
The patient was started on Hydrocortisone with marked improvement. Mineralocorticoid replacement therapy was not indicated as this is likely to be secondary adrenal insufficiency. The patient was to be followed up for an assessment of possible pituitary recovery.
Case 2
A 45-year-old man presented with cachexia, unintentional 25 kg weight loss over 6 months, recurrent nausea and vomiting on a background of chronic hepatitis C infection which he had acquired 20 years before from intravenous drug use. His past medical history included type 2 diabetes which recently became labile. He also developed recurrent hypoglycaemia without any major changes in his routine dietary and oral intake. There was no change or non-compliance with his oral hypoglycaemic regimen. Clinically he was unwell, cachectic with weight of 56.2 kg and height of 1.65 m, body mass index of about 20 kg/m2. His BP was 120/70 sitting and 100/60 standing with pulse rate of 88 bpm. He appeared hypo-androgenic with sparse body hair distribution and an absence of pubic and axillary hair. His testes were 6 and 8 mL in size bilaterally. Further investigations are as follow: TSH 1.58 mU/L, fT4 9.1 pmol/L, fT3 4.7 pmol/L, ACTH < 1.1 pmol/L, Cortisol 326 nmol/L, LH 0.6 IU/L, FSH 0.3 IU/L, Testosterone < 0.7 nmol/L, Prolactin 252 mIU/L. A short-synacthen test revealed a rise from 326 to 430 nmol/L over 60 minutes consistent a sub-optimal response. On the basis of these results, no dynamic stimulation test was warranted.
The patient was given triple replacement therapy including thyroxine, cortisone acetate and testosterone isocaproate (Sustanon) injections. He made a rapid recovery and great symptomatic improvement. His hypoglycaemic crises resolved. Indeed, he became hyperglycaemic. He gained about 5 kg and was referred for treatment consideration with IFN-α therapy. However, he did not return for review and was subsequently lost to follow up.
| Discussion | ▴Top |
These two cases highlight the immuno-modulating effects of the hepatitis C viral particles and IFN-α therapy individually, especially in regards to pituitary pathology. Due to its rarity, the condition is poorly understood [1]. Theoretically however, it is thought that prolactin plays a major part in the pathogenesis of the condition [2]. The presence of HCV particles and IFN-α, both of which are potent immuno-modulators, further inflame the condition. The three combine to act through a similar pathway which was previously proposed for IFN-α related thyroid disease [3]. Prolactin is thought to activate the JAK/STAT pathways which lead to the activation of Interferon Regulatory Factor 1 (IRF1). Prolactin also activates TH1 and TH2 cytokine activities which lead to the development of autoimmunity. In addition, the T helpers are further in turn regulated by T regulator cells (Treg). The latter function is dampened in the presence of IFN-α therapy, amplifying the PRL response, leading to the clinical expression of anterior pituitary deficiency [3]. The PRL hypothesis is probably more relevant in post-partum nursing mothers where hyperprolactinaemia predominates. However, only 50% of the discussed cases are females and none was breastfeeding. Genetic predisposition must play a part, as is the vascular supply. The anterior pituitary has an extensive vascular supply, exposing the pituitary cells to the HCV particles, IFN-α and associated antibodies, Figure 1. It remains unknown if the condition is reversible, especially once the virus has been terminated or cured with IFN-α therapy. The extermination of the HCV particles also reduces the stimulating effect helping the reversibility of the condition.
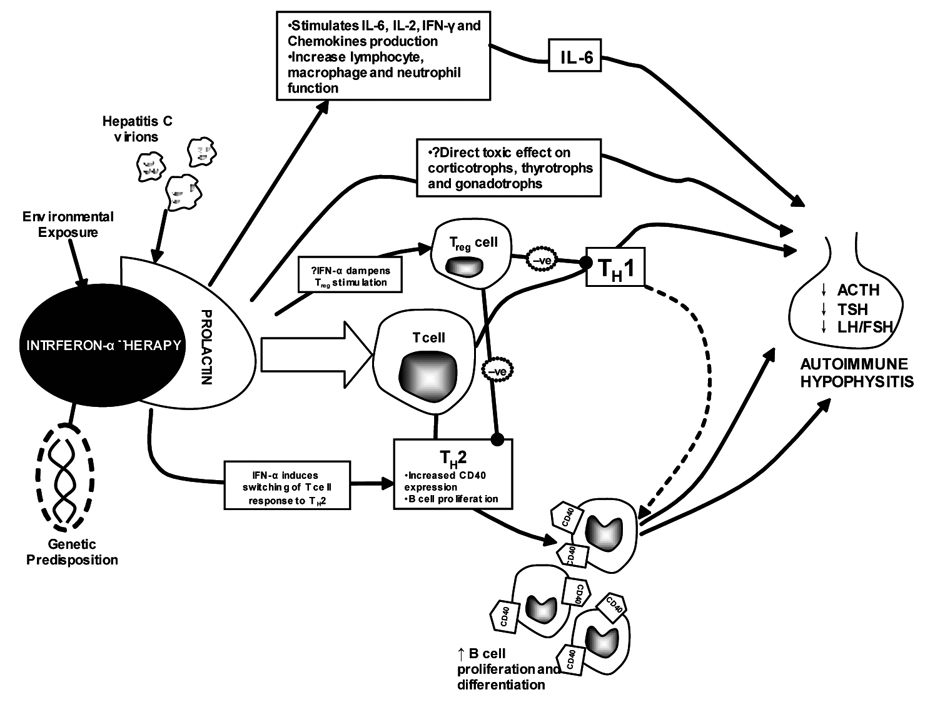 Click for large image | Figure 1. The proposed hypothesis for the development of autoimmune hypophysitis in HCV infection and IFN-based therapy. IL-6: Interleukin-6; IFN: Interferon; MHC-II: major histocompatability complex-II; TH: T helper. |
Previous published cases in the literature were sparse. The first case was described by Sakane et al [4] in 1995 in which the endocrinopathies developed 2 months (out of six) after stopping IFN therapy. This case was shown to have pituitary antibodies against GH3 cells, a rat pituitary tumor cell line that secretes growth hormone and prolactin. Fortunately, the condition was reversible. In 2003, Concha et al [5] reported a second similar case. The proposed panhypopituitarism was detected 1 year after the completion of therapy although there was no evidence of antipituitary antibodies. Chan et al [6] described a case of panhypopituitarism but in the presence of hepatitis B infection. The patient developed amenorrhoea whilst on treatment and displayed permanent panhypopituitarism thereafter. Ridruejo et al [7] in 2006 reported a possible case of reversible or spontaneously recovered hypophysitis whilst on combination IFN and RBV therapy. The diagnosis was clinically based in all cases using the temporal relationship with treatment, pituitary hormonal profile, pituitary magnetic resonance imagings, all of which are normal or non-contributory, and the absence of thyroid and other autoimmune markers. Except for case 3, all demonstrated the typical sequence of deficiencies in autoimmune hypophysitis where ACTHis the first to be affected, followed by TSH and then LH/FSH [8]. Antipituitary antibodies are also not available in most case as these remain poorly defined and the test is not routinely available in practice [8]. Contrary to de novo cases, none developed headache and/or visual disturbance. These few published cases are summarized in Table 1.
 Click to view | Table 1. Summary of Our Cases and Available Published Reports, Please Note Case 3 Involved Hepatitis B Infection |
In addition, INF-α can unmask previously undetected pituitary Sheehan’s syndrome or syndrome of inappropriate antidiuresis [9-11] which can be fatal if unrecognized.
The prevalence of pituitary dysfunction in relation to hepatitis C infection and IFN-α therapy is poorly known and appears very rare. Our previous report in postmortem cases found no evidence of pituitary involvement in untreated HCV cases [12]. This is not surprising given the rarity of clinical panhypopituitarism in relation to HCV infection and suggests that the condition occurs in an ad hoc fashion, presumably in genetically susceptible individuals. Surveillance therefore is not recommended. However, the diagnosis of pituitary dysfunction, either partial or complete should be considered in HCV patients with the appropriate clinical symptomatology. Similarly and not evidence based, patients should be followed up to assess for reversibility of the condition.
Conclusion
Hepatitis C infection and IFN-α associated pituitary dysfunction is rare but should be considered in the appropriate clinical setting. The condition is readily treatable and is potentially reversible.
Disclosure Statement
All authors have no conflict of interests relevant to this work.
| References | ▴Top |
- Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26(5):599-614.
doi pubmed - De Bellis A, Ruocco G, Battaglia M, Conte M, Coronella C, Tirelli G, Bellastella A, et al. Immunological and clinical aspects of lymphocytic hypophysitis. Clin Sci (Lond). 2008;114(6):413-421.
doi pubmed - Tran HA, Reeves GE. The Spectrum of Autoimmune Thyroid Disease in the Short to Medium Term Following Interferon-alpha Therapy for Chronic Hepatitis C. Int J Endocrinol. 2009;2009:241786.
- Sakane N, Yoshida T, Yoshioka K, Umekawa T, Kondo M, Shimatsu A. Reversible hypopituitarism after interferonalfa therapy. Lancet. 1995;345(8960):1305.
doi - Concha LB, Carlson HE, Heimann A, Lake-Bakaar GV, Paal AF. Interferon-induced hypopituitarism. Am J Med. 2003;114(2):161-163.
doi - Chan WB, Cockram CS. Panhypopituitarism in association with interferon-alpha treatment. Singapore Med J. 2004;45(2):93-94.
pubmed - Ridruejo E, Christensen AF, Mando OG. Central hypothyroidism and hypophysitis during treatment of chronic hepatitis C with pegylated interferon alpha and ribavirin. Eur J Gastroenterol Hepatol. 2006;18(6):693-694.
doi pubmed - Howlett TA, Levy MJ, Robertson IJ. How reliably can autoimmune hypophysitis be diagnosed without pituitary biopsy. Clin Endocrinol (Oxf). 2010;73(1):18-21.
- Kanda K, Kayahara T, Seno H, Yamashita Y, Chiba T. Conversion from latent to symptomatic Sheehan's syndrome by pegylated interferon therapy for chronic hepatitis C. Intern Med. 2008;47(10):939-941.
doi pubmed - Mabe K, Shinzawa H, Yamatani K, Takeda T, Ishibashi M, Yamada N, Misawa H, et al. Case report: interferon induced coma in Sheehan's syndrome. J Gastroenterol Hepatol. 1997;12(7):551-553.
doi pubmed - Tanaka M, Kamoi K, Takahashi T. Interferon-alpha is a predisposing risk factor for carbamazepine-induced hyponatremia: A case of syndrome of inappropriate antidiuresis caused by interferon-alpha therapy. Int J Gen Med. 2008;1:21-25.
pubmed - Tran HA, Reeves GE, Lyons TJ, Attia JR. Histopathologic findings of autoimmunity in thyroid, pituitary, and adrenal diseases in chronic hepatitis C postmortem cases. Endocr Pract. 2010;16(4):566-569.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.
