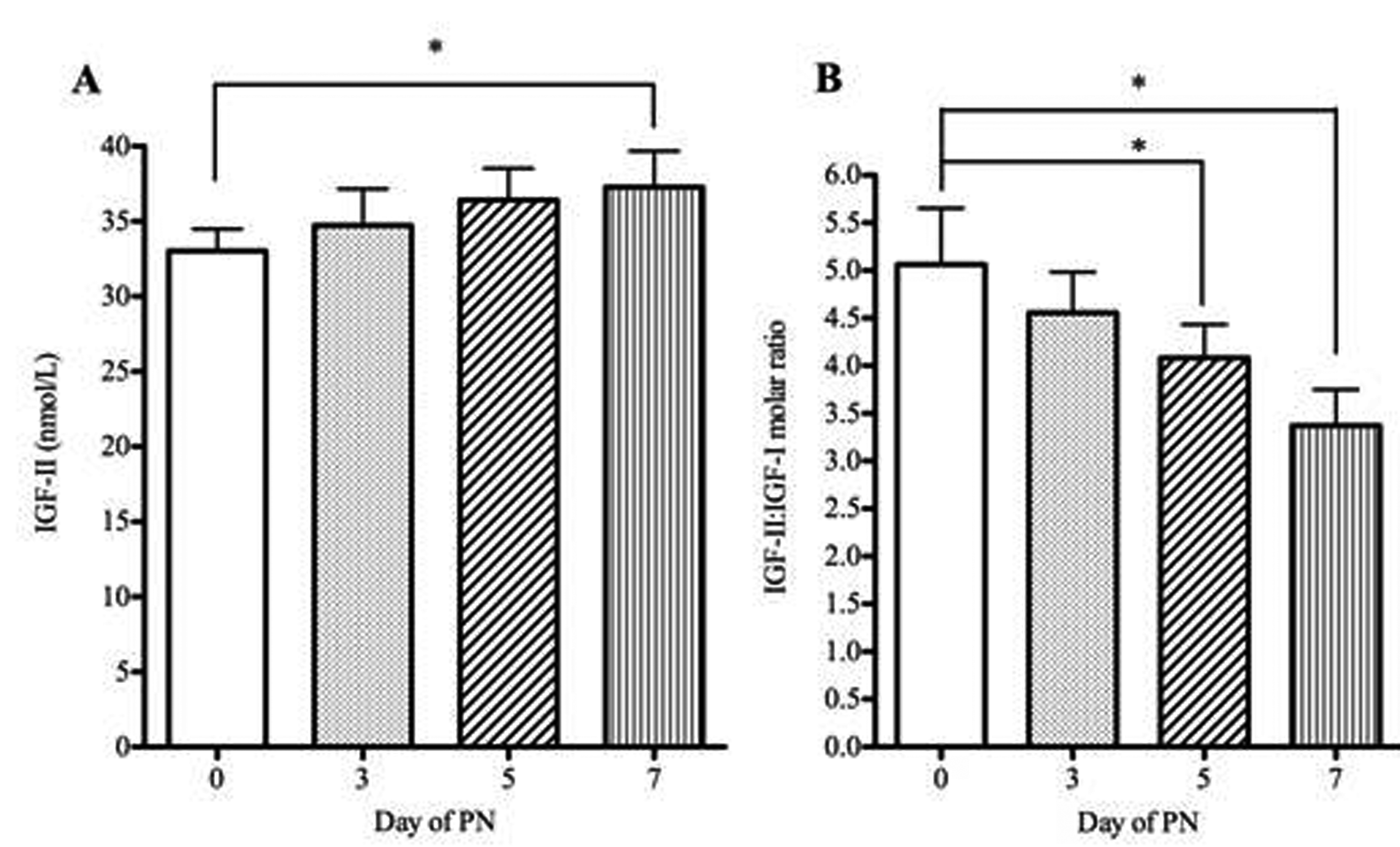
Figure 1. IGF-II concentrations measured by RIA and IGFII-IGF-I molar ratio in patients receiving parenteral nutrition. Serum IGF-II (A), IGF-II:IGF-I molar ratio (B) in patients at baseline (day 0) and days three, five and seven of PN administration. Serum IGF-II and the IGF-II: IGF-I molar ratio were tested for significance using repeated measures ANOVA with Dunnett’s multiple comparison tests. * P < 0.05. Error bars are +SEM.
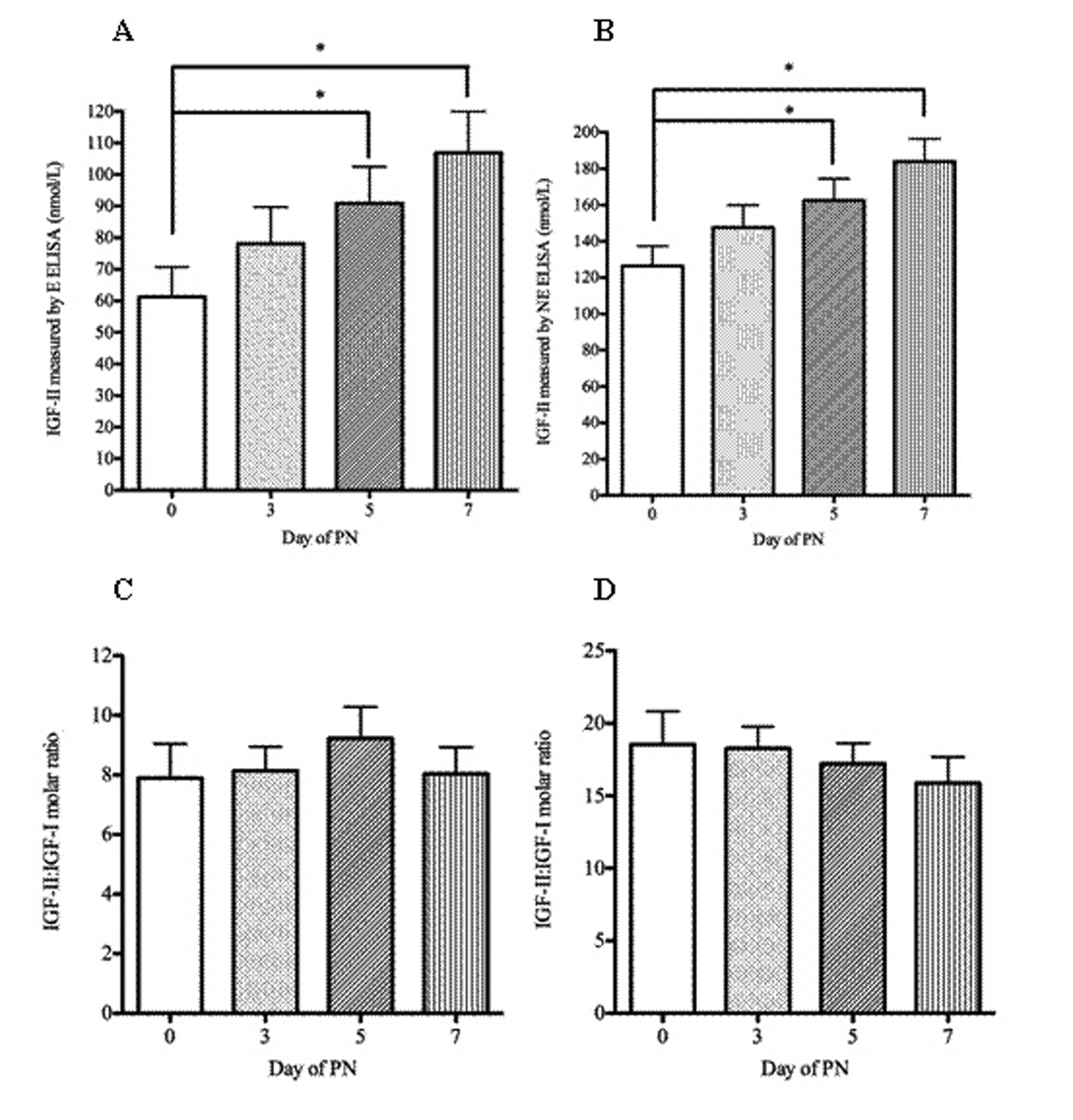
Figure 2. IGF-II concentrations measured by ELISA and IGFII-IGF-I molar ratio in patients receiving parenteral nutrition. Serum IGF-II (A, B), IGF-II: IGF-I molar ratio (C, D) in patients at baseline (day 0) and days three, five and seven of PN administration. A and C were measured by extraction ELISA and B and D by non-extraction ELISA). Serum IGF-II and the IGF-II: IGF-I molar ratio were tested for significance using repeated measures ANOVA with Dunnett’s multiple comparison tests. * P < 0.05. Error bars are +SEM.
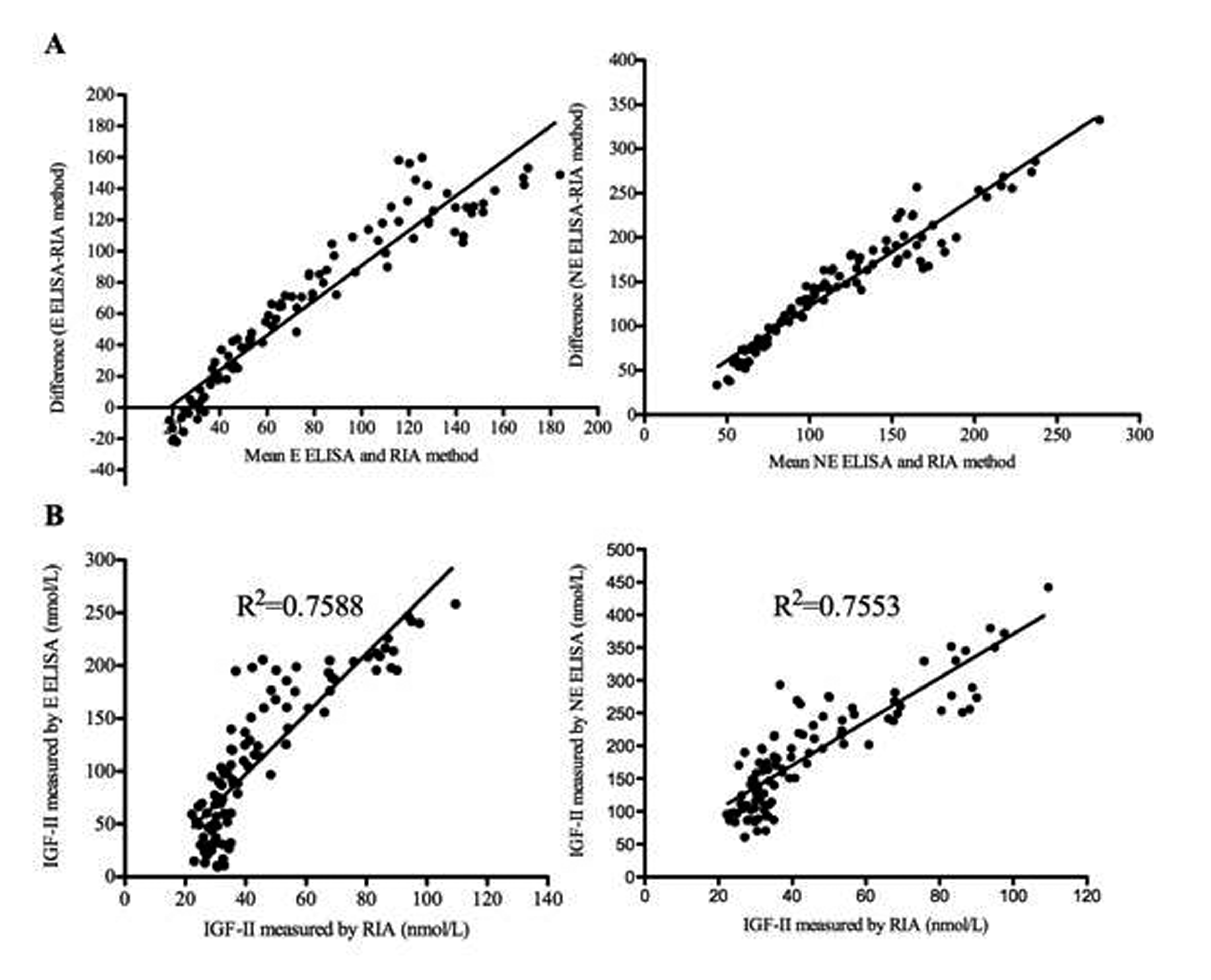
Figure 3. A. Bland Altman plots comparing IGF-II extraction (E) and non-extraction (NE) ELISA assays to RIA. B. Correlation analysis comparing IGF-II extraction and non-extraction ELISAs to the RIA, using the Pearson correlation coefficient, R2. Results are shown from IGF-II levels measured on a total of 23 healthy volunteers and 79 patient specimens.


