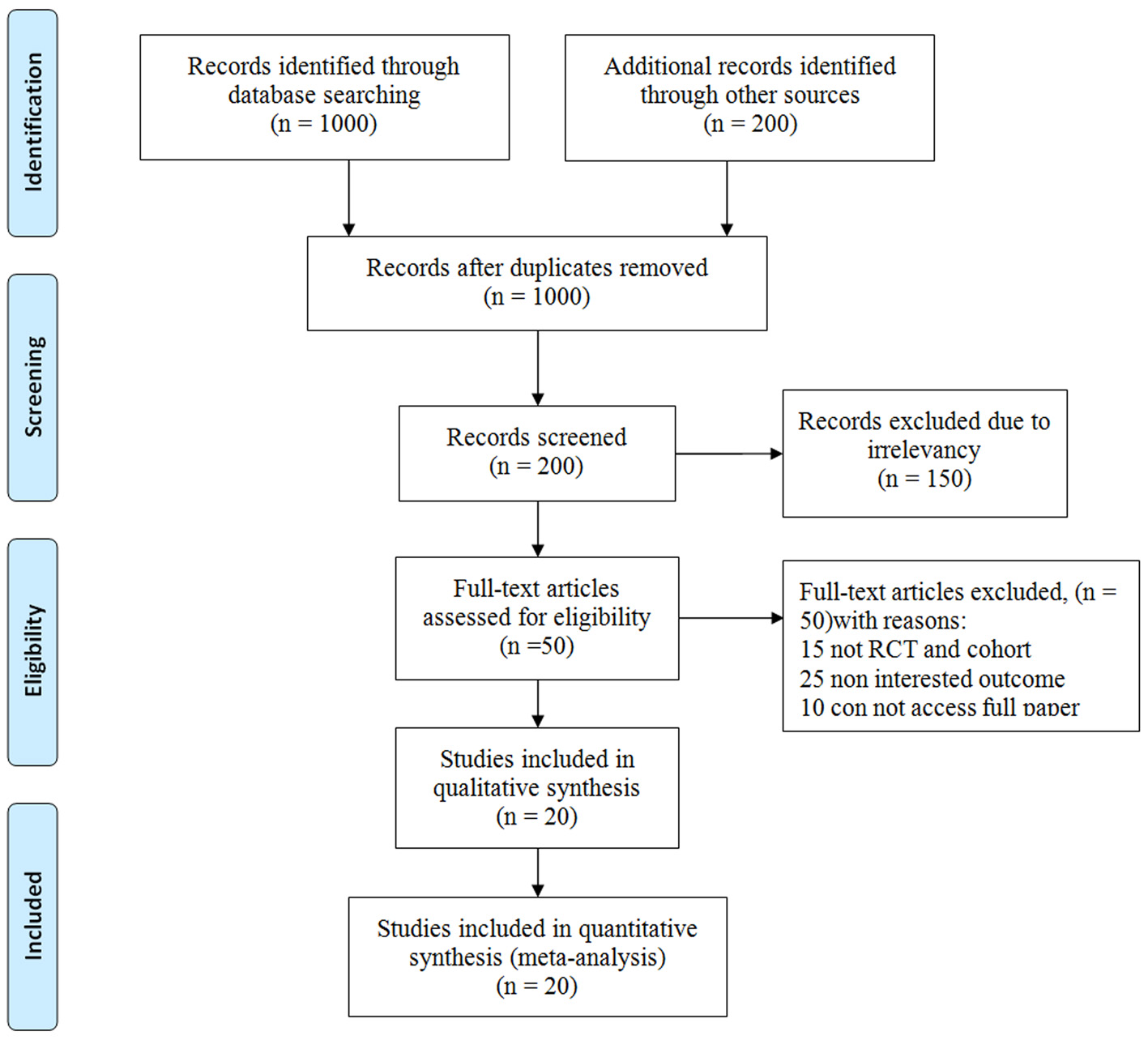
Figure 1. The flowchart stages of entering the articles into meta-analysis.
| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Review
Volume 7, Number 2, April 2017, pages 45-54
The Effect of Growth Hormone Treatment on Adult Height of Children With Idiopathic Short Stature: A Systematic Review and Meta-Analyses
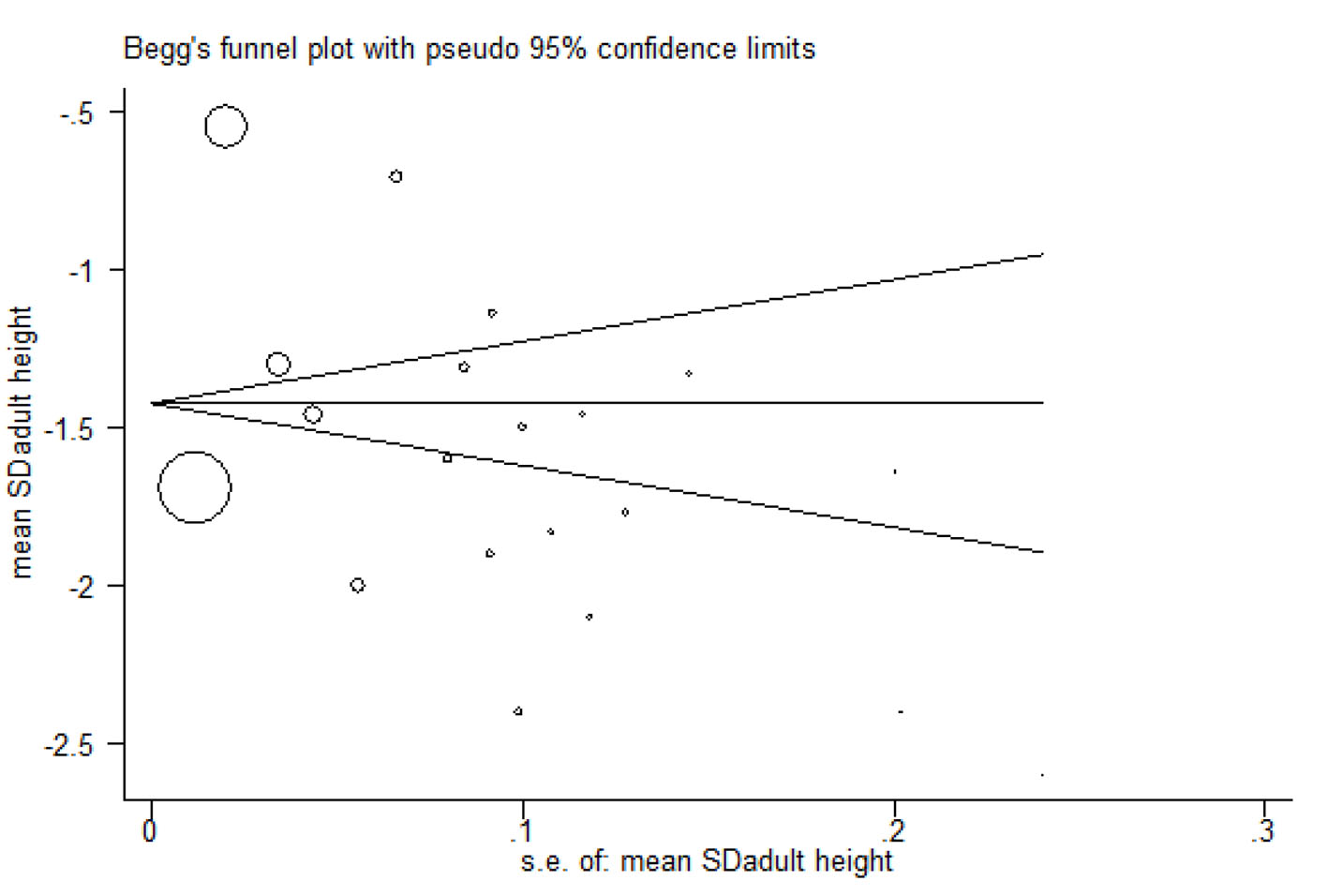
Figures

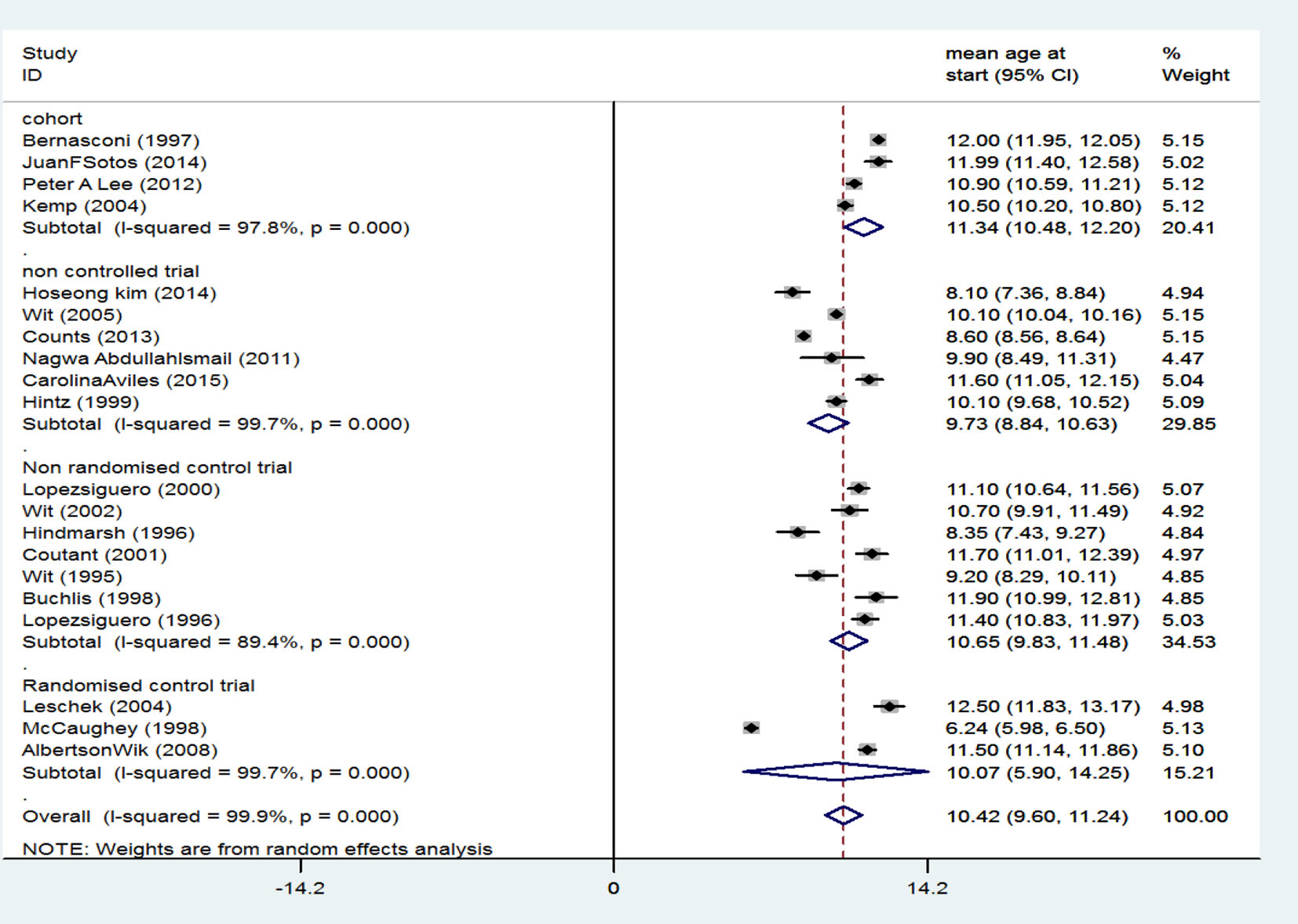
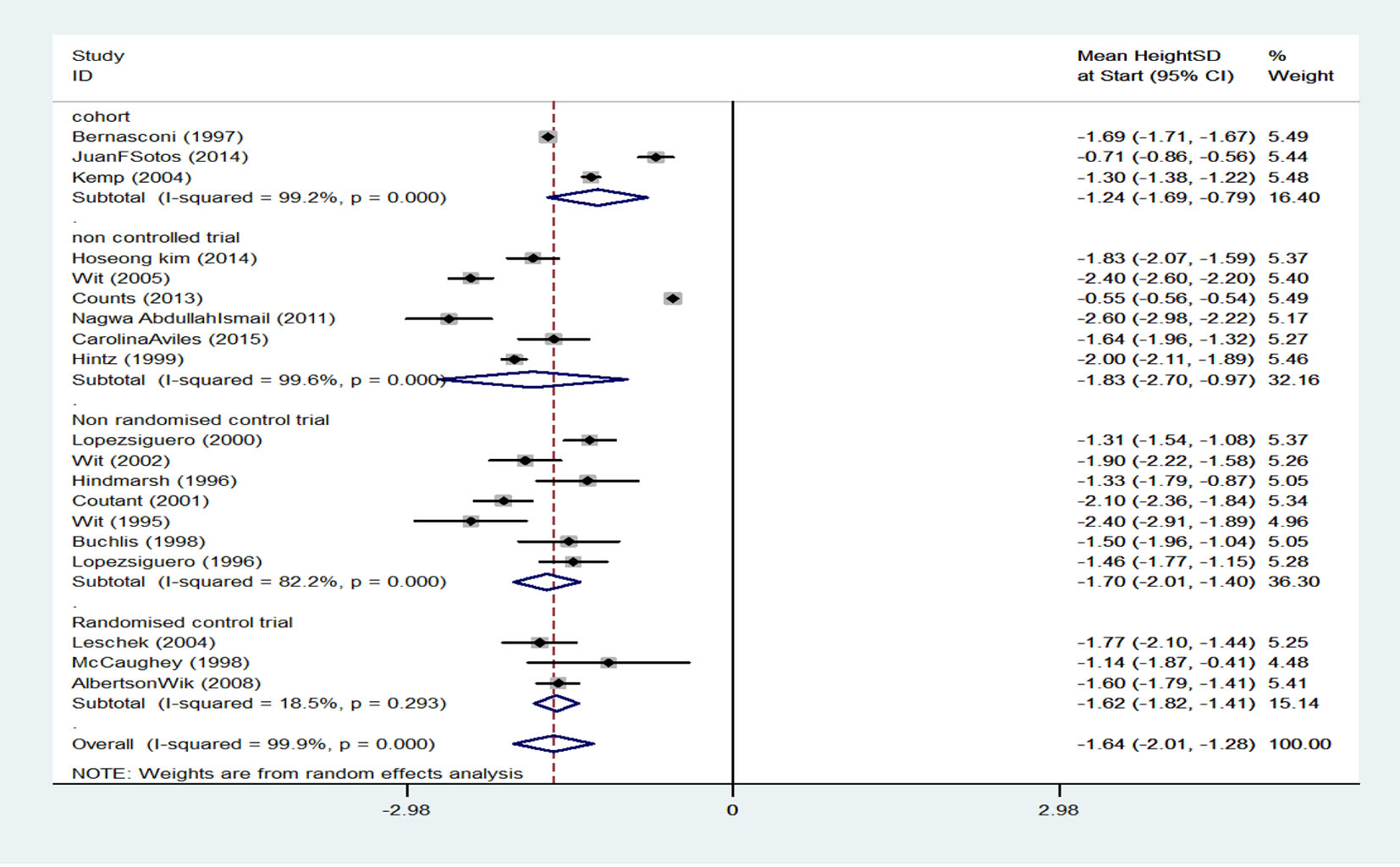
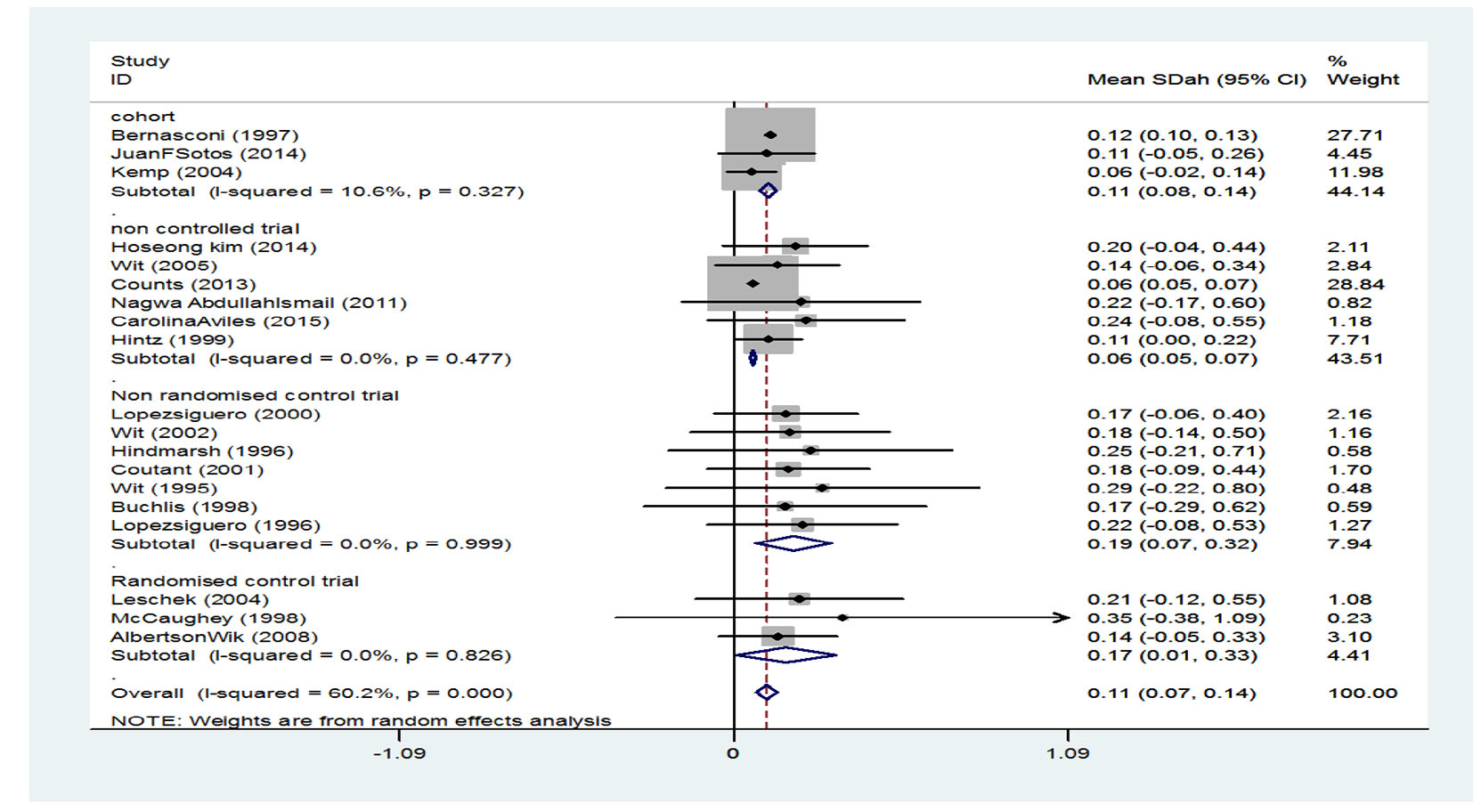
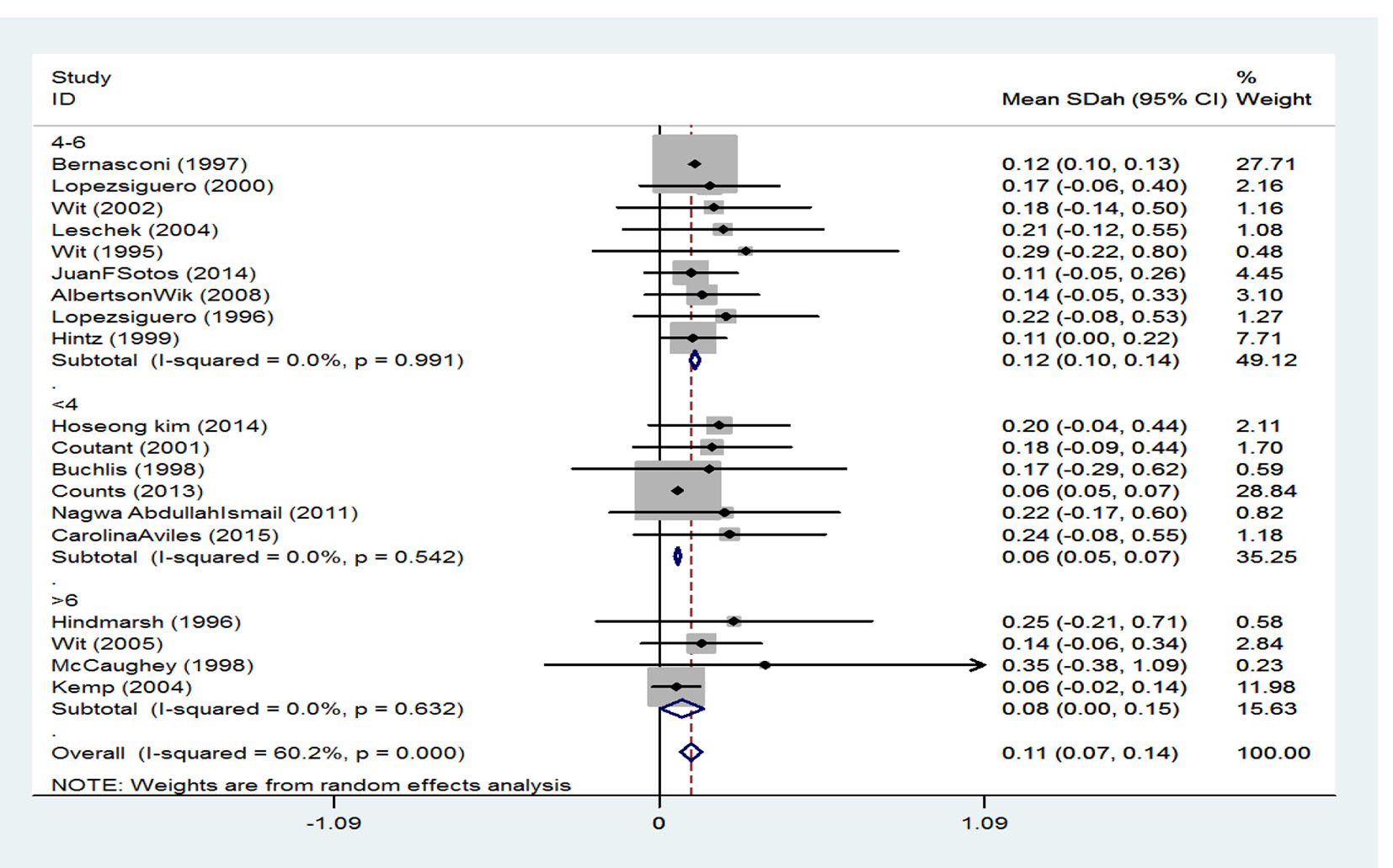
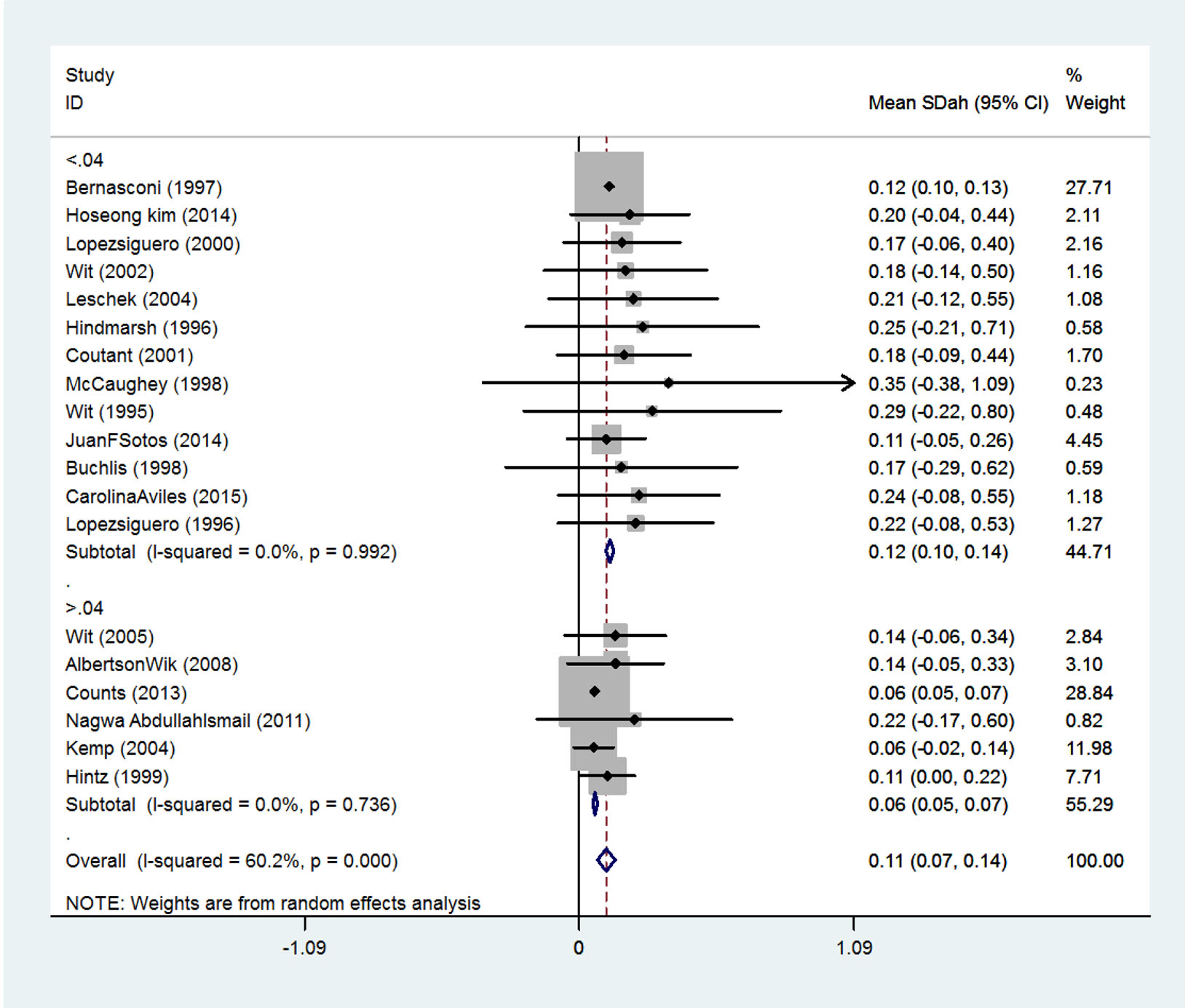
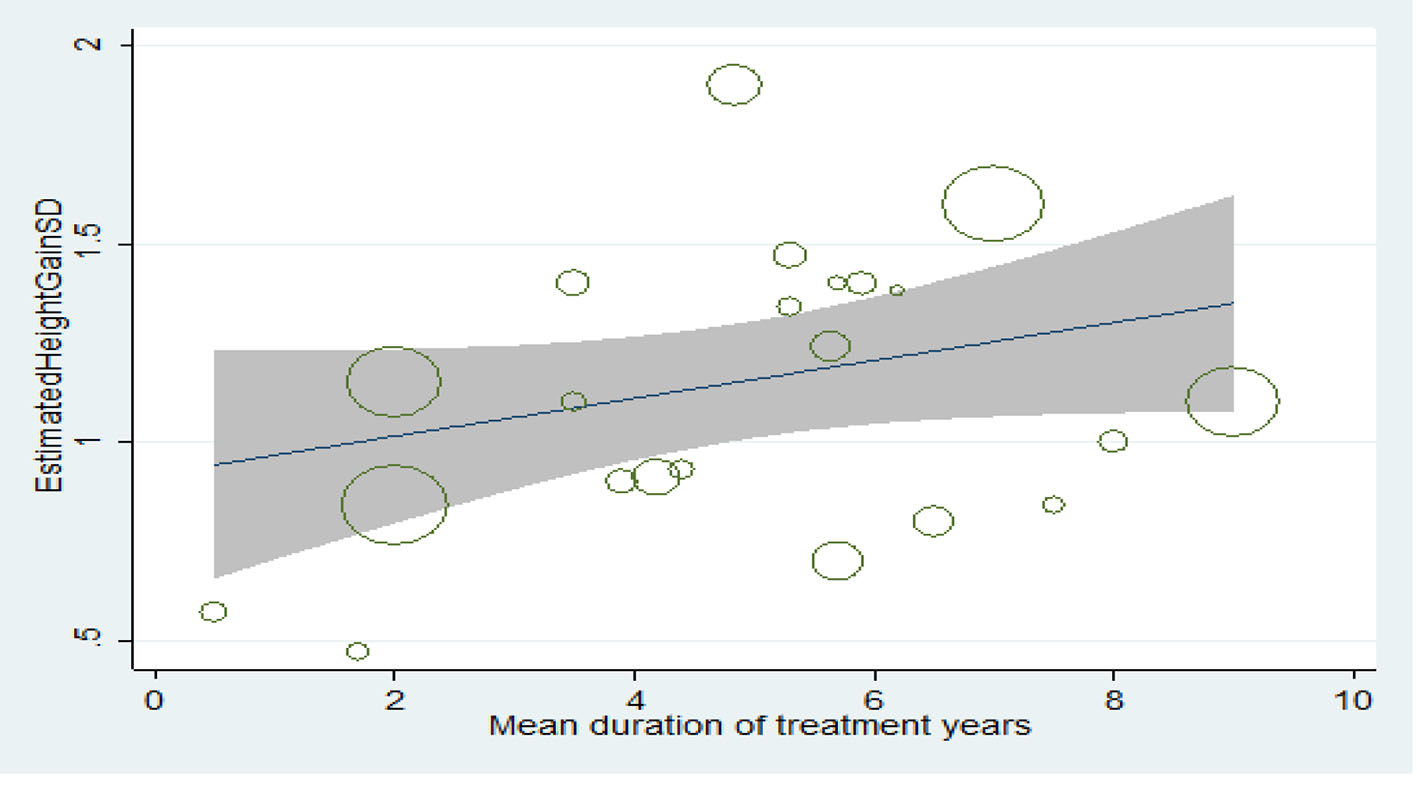
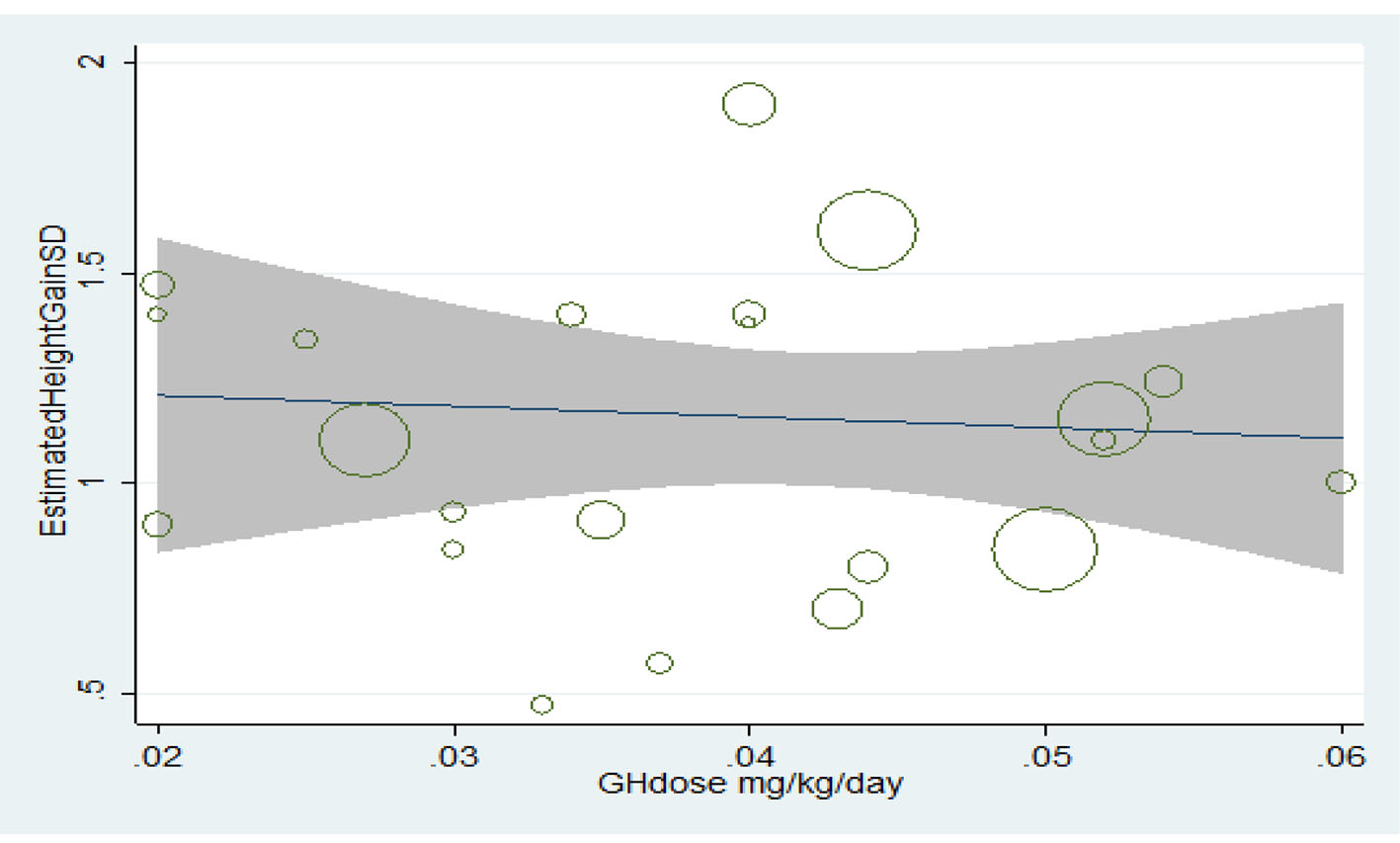
Table
| Author, publication year, reference | Type design | Country | Continent | No. of patient | Mean age start | SD of age | Mean height start | SD of height | Growth hormone dose | Mean duration | Adult height MSD | Adult height SD | Estimate height gain | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bernasconi et al, 1997 [15] | Cohort | Italy | Europe | 71 | 12 | 0.2 | -2.6 | 0.1 | 0.035 | 4.2 | -1.69 | 0.07 | 0.91 | 0.05 |
| Hintz et al, 1999 [16] | Non-controlled trial | USA | North America | 80 | 10.1 | 1.9 | -2.7 | 0.5 | 0.043 | 5.7 | -2 | 0.5 | 0.7 | - |
| Kemp et al, 2005 [17] | Cohort | USA | North America | 303 | 10.5 | 2.7 | -2.9 | 0.6 | 0.044 | 7 | -1.3 | 0.7 | 1.6 | - |
| Wit et al, 2005 [11] | Non-controlled trial | Netherland | Europe | 50 | 10.1 | 0.21 | -3.2 | 0.7 | 0.044 | 6.5 | -2.4 | 0.72 | 0.8 | 0.025 |
| Counts et al, 2013 [18] | Non-controlled trial | USA | North America | 267 | 8.6 | 0.34 | -1.7 | 0.33 | 0.052 | 2 | -0.55 | 0.08 | 1.15 | 0.0001 |
| Kim et al, 2014 [19] | Non-controlled trial | South Korea | Asia | 25 | 8.1 | 1.9 | -2.4 | 0.54 | 0.37 | 0.5 | -1.83 | 0.6 | 0.57 | 0.05 |
| Sotos and Tokar, 2014 [20] | Cohort | USA | North America | 88 | 11.99 | 2.83 | -2.6 | 0.62 | 0.04 | 4.8 | -0.71 | 0.74 | 1.9 | 0.0001 |
| Aviles Espinoza, et al 2016 [21] | Non-controlled trial | Chile | South America | 18 | 11.6 | 1.2 | -2.1 | 0.85 | 0.033 | 1.7 | -1.64 | 0.69 | 0.47 | 0.0001 |
| Ismail et al, 2011 [22] | Non-controlled trial | Egypt | Africa | 21 | 9.9 | 3.3 | -3.7 | 1.1 | 0.052 | 3.5 | -2.6 | 0.9 | 1.1 | 0.0001 |
| Lee et al, 2012 [23] | Cohort | USA | North America | 334 | 10.9 | 2.9 | -2.3 | 0.8 | 0.05 | 2 | -1.64 | 0.84 | 0.001 | |
| McCaughey et al, 1998 [24] | Randomized control trial | UK | Europe | 8 | 6.24 | 0.38 | -2.5 | 0.26 | 0.04 | 6.2 | -1.14 | 1.06 | 1.38 | 0.008 |
| Leschek et al, 2004 [25] | Randomized control trial | USA | North America | 22 | 12.5 | 1.6 | -2.7 | 0.6 | 0.03 | 4.4 | -1.77 | 0.8 | 0.93 | 0.04 |
| Albertsson-Wikland et al, 2008 [10] | Randomized control trial | Sweden | Europe | 49 | 11.5 | 1.3 | -2.8 | 0.56 | 0.054 | 5.6 | -1.6 | 0.68 | 1.24 | 0.001 |
| Wit et al, 1995 [26] | Non-randomized control trial | Netherland | Europe | 12 | 9.2 | 1.6 | -2.8 | 0.7 | 0.02 | 5.7 | -2.4 | 0.9 | 1.4 | 0.002 |
| Hindmarsh and Brook, 1996 [27] | Non-randomized control trial | UK | Europe | 16 | 8.35 | 1.88 | -2.2 | 0.58 | 0.03 | 7.5 | -1.33 | 0.94 | 0.84 | 0.03 |
| Lopez-Siguero et al, 1996 [28] | Non-randomized control trial | Spain | Europe | 20 | 11.4 | 1.3 | -2.8 | 0.52 | 0.025 | 5.3 | -1.46 | 0.7 | 1.34 | - |
| Buchlis et al, 1998 [29] | Non-randomized control trial | USA | North America | 36 | 11.9 | 2.8 | -2.9 | 0.6 | 0.04 | 3.5 | -1.5 | 1.4 | 1.4 | 0.001 |
| Lopez-Siguero et al, 2000 [30] | Non-randomized control trial | Spain | Europe | 35 | 11.1 | 1.4 | -2.9 | 0.5 | 0.02 | 5.3 | -1.31 | 0.7 | 1.47 | 0.04 |
| Coutant et al, 2001 [31] | Non-randomized control trial | France | Europe | 32 | 11.7 | 2 | -3 | 0.67 | 0.02 | 3.9 | -2.1 | 0.76 | 0.9 | 0.01 |
| Wit and Rekers-Mombarg, 2002 [32] | Non-randomized control trial | Netherland | Europe | 30 | 10.7 | 2.2 | -3.3 | 0.5 | 0.034 | 5.9 | -1.9 | 0.9 | 1.4 | 0.04 |